Abstract
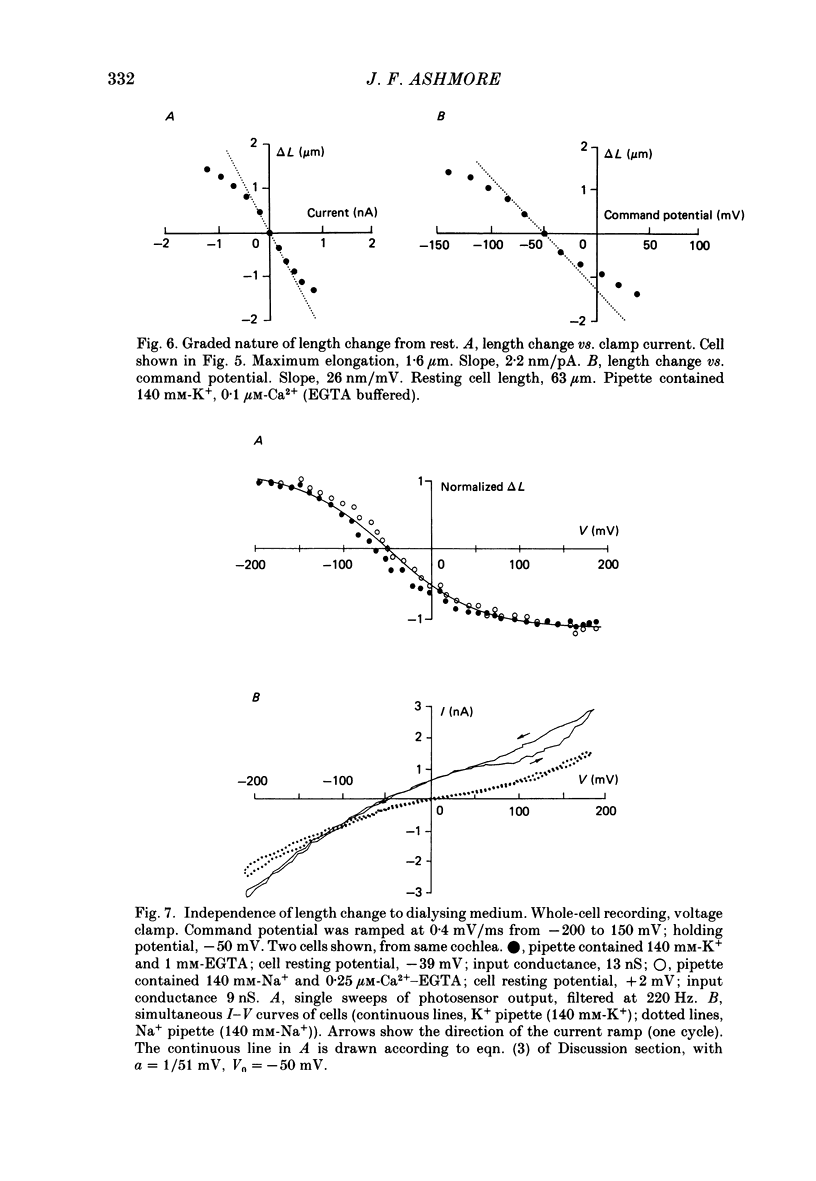
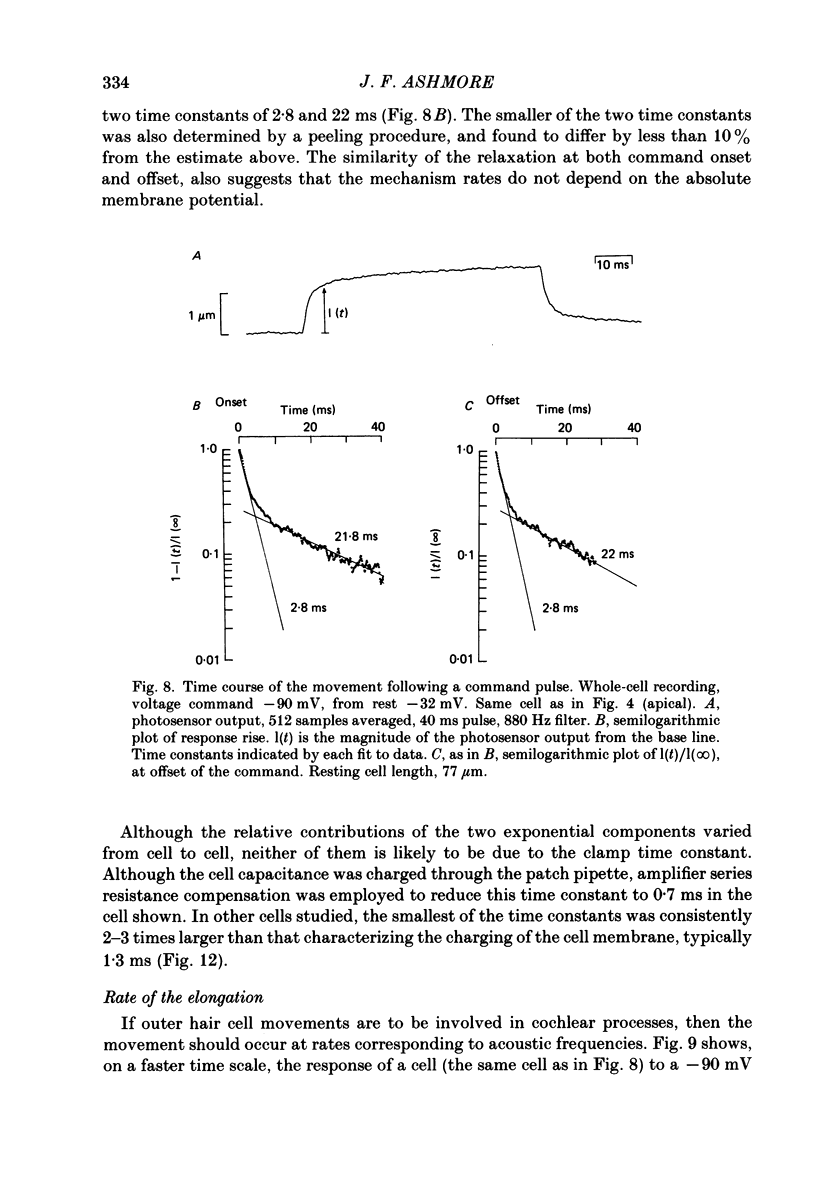
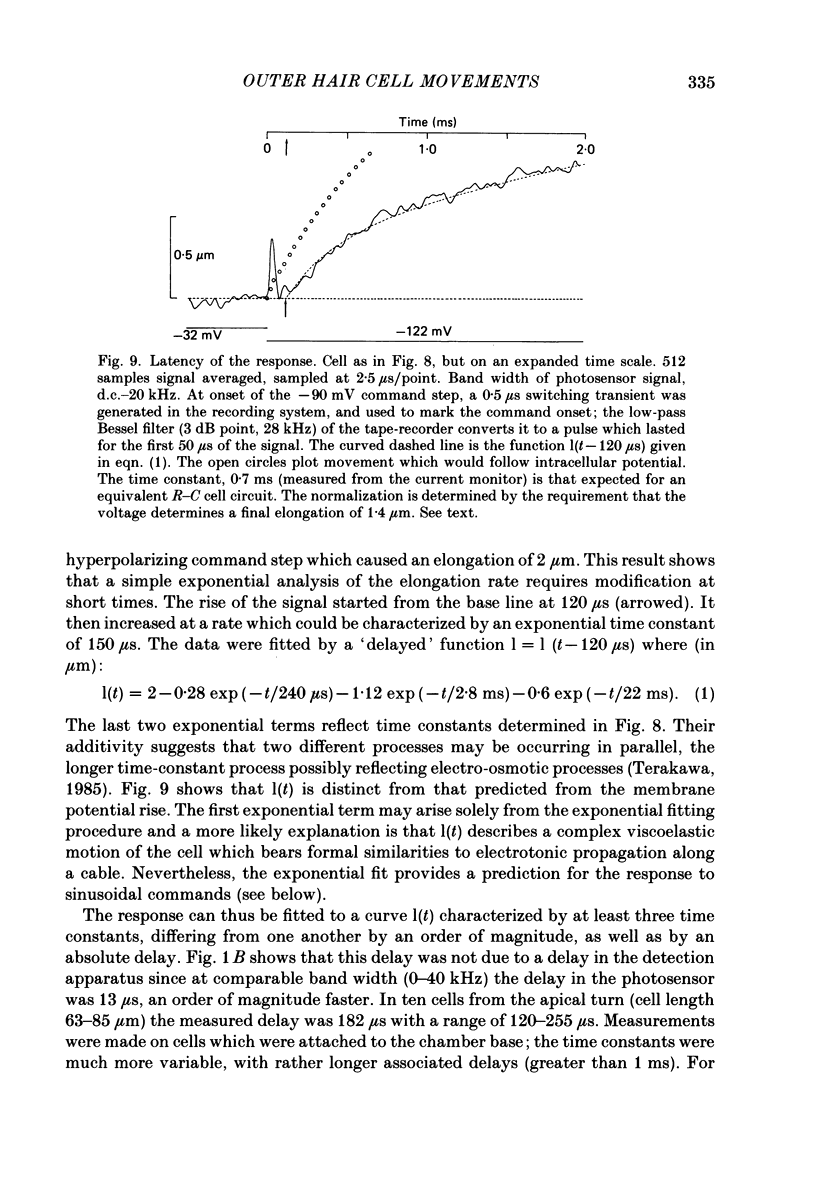
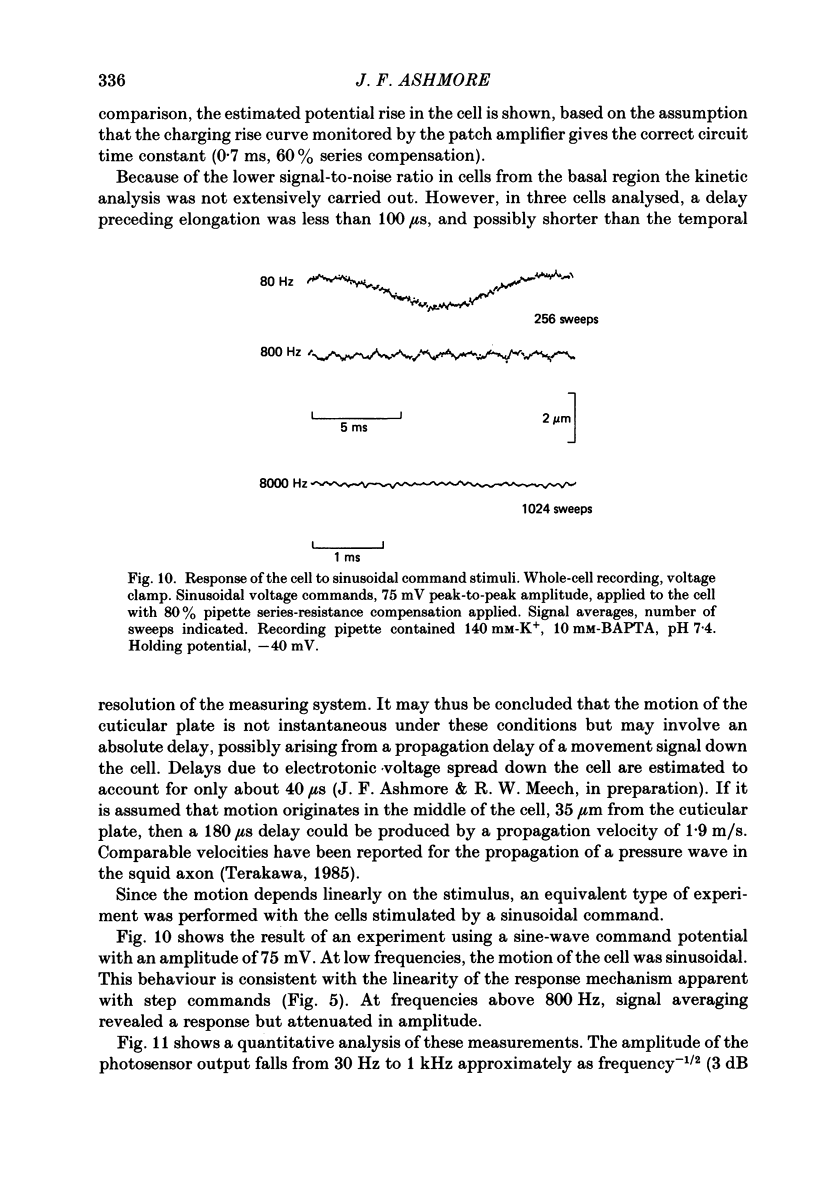
1. Outer hair cells from the cochlea of the guinea-pig were isolated and their motile properties studied in short-term culture by the whole-cell variant of the patch recording technique. 2. Cells elongated and shortened when subjected to voltage steps. Cells from both high- and low-frequency regions of the cochlea responded with an elongation when hyperpolarized and a shortening when depolarized. The longitudinal motion of the cell was measured by a differential photosensor capable of responding to motion frequencies 0-40 kHz. 3. Under voltage clamp the length change of the cell was graded with command voltage over a range +/- 2 microns (approximately 4% of the length) for cells from the apical turns of the cochlea. The mean sensitivity of the movement was 2.11 nm/pA injected current, or 19.8 nm/mV membrane polarization. 4. The kinetics of the cell length change during a voltage step were measured. Stimulated at their basal end, cells from the apical (low-frequency) cochlear turns responded with a latency of between 120 and 255 microseconds. The cells thereafter elongated exponentially by a process which could be characterized by three time constants, one with value 240 microseconds, and a second in the range 1.3-2.8 ms. A third time constant with a value 20-40 ms characterized a slower component which may represent osmotic changes. 5. Consistent with the linearity shown to voltage steps, sinusoidal stimulation of the cell generated movements which could be measured at frequencies above 1 kHz. The phase of the movement relative to the stimulus continued to grow with frequency, suggesting the presence of an absolute delay in the response of about 200 microseconds. 6. The electrically stimulated movements were insensitive to the ionic composition of the cell, manipulated by dialysis from the patch pipette. The responses occurred when the major cation was K+ or Na+ in the pipette. Loading the cell with ATP-free solutions or calcium buffers did not inhibit the response. 7. It is concluded that interaction between actin and myosin, although present in the cell, is unlikely to account for the cell motility. Instead, it is proposed that outer hair cell motility is associated with structures in the cell cortex. The implications for cochlear mechanics of such force generation in outer hair cells are discussed.
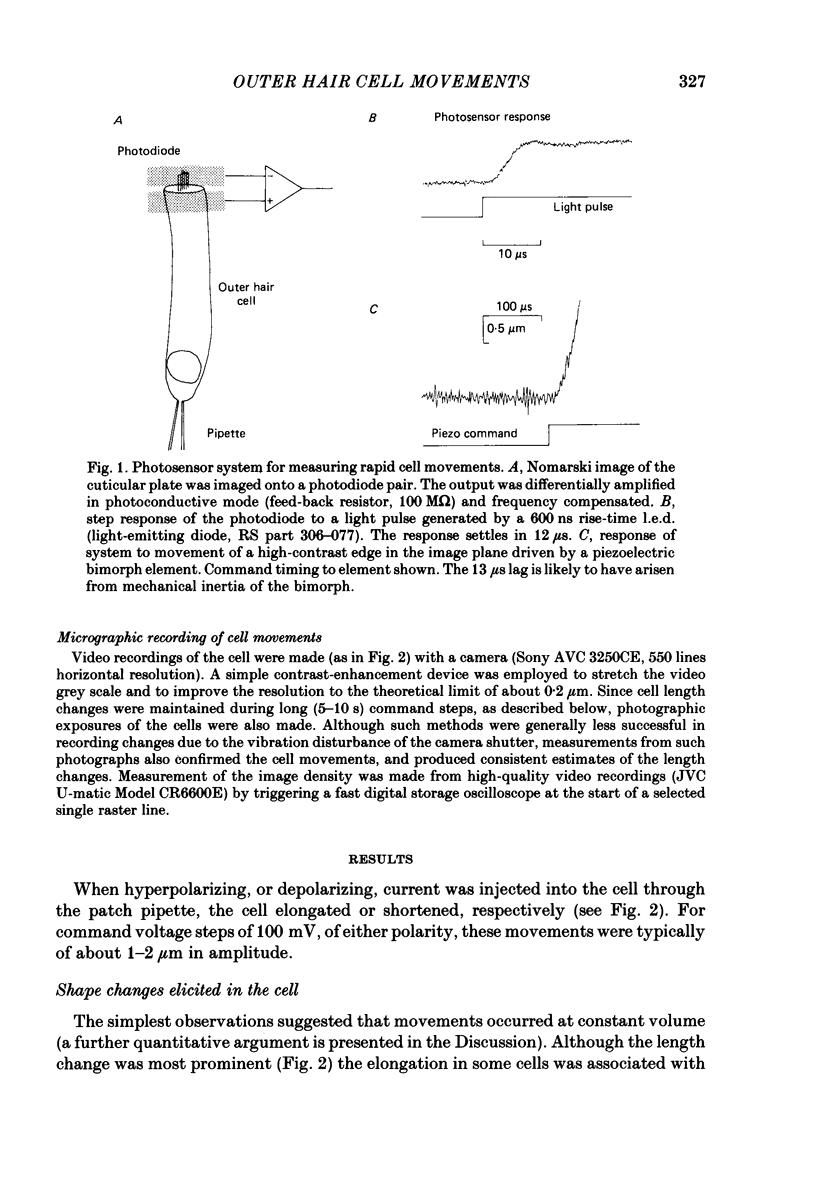
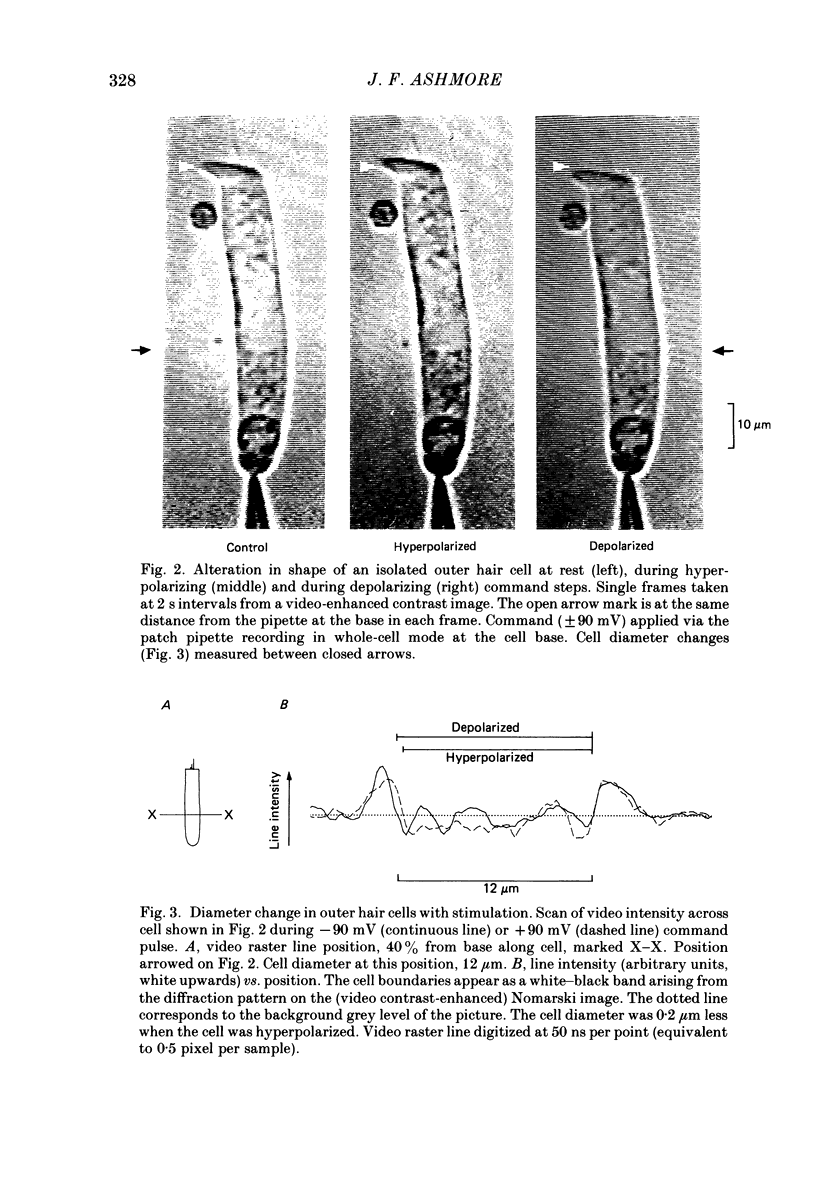
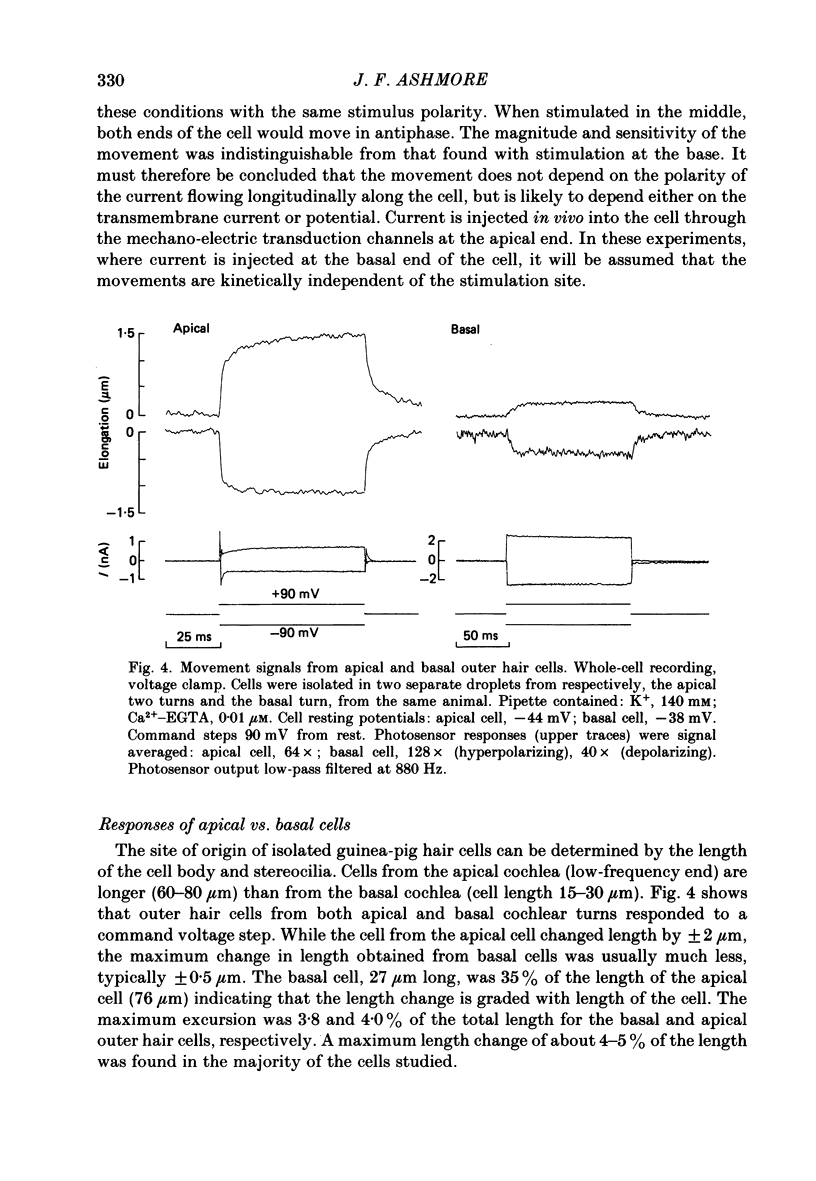
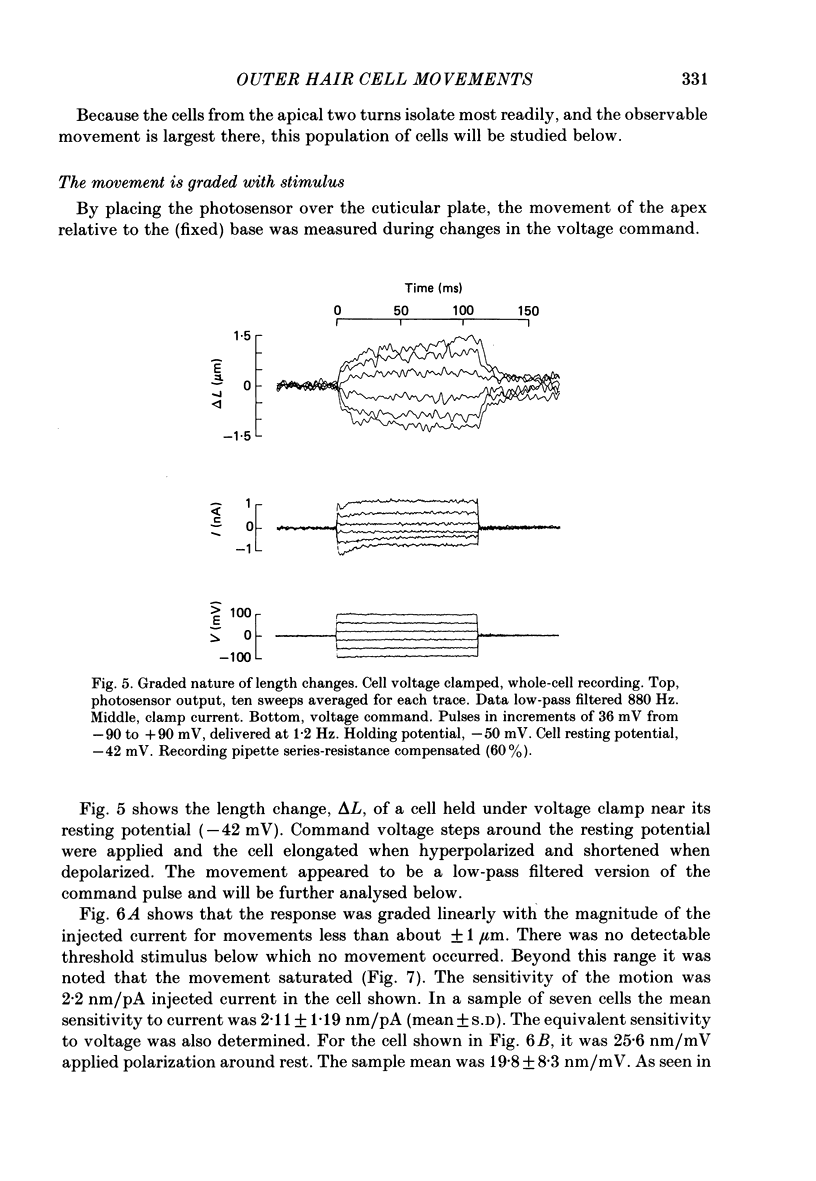
Full text
PDF





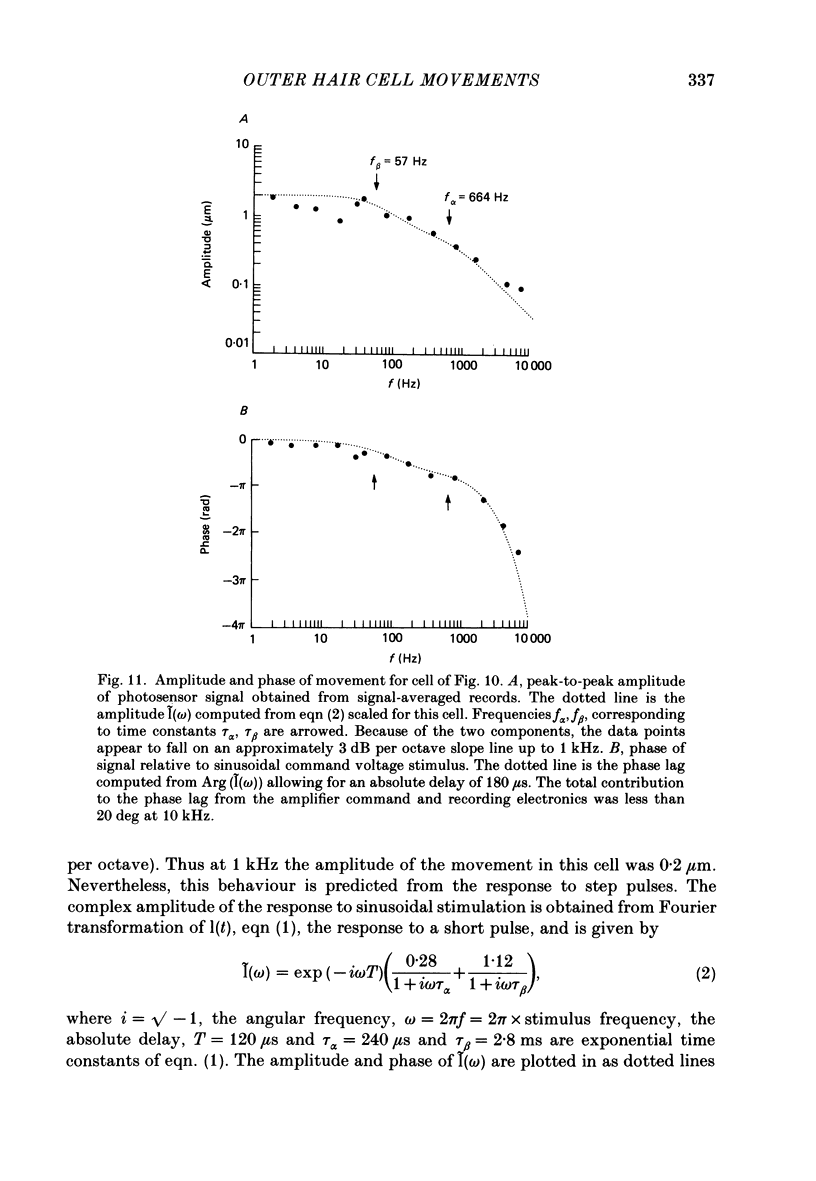

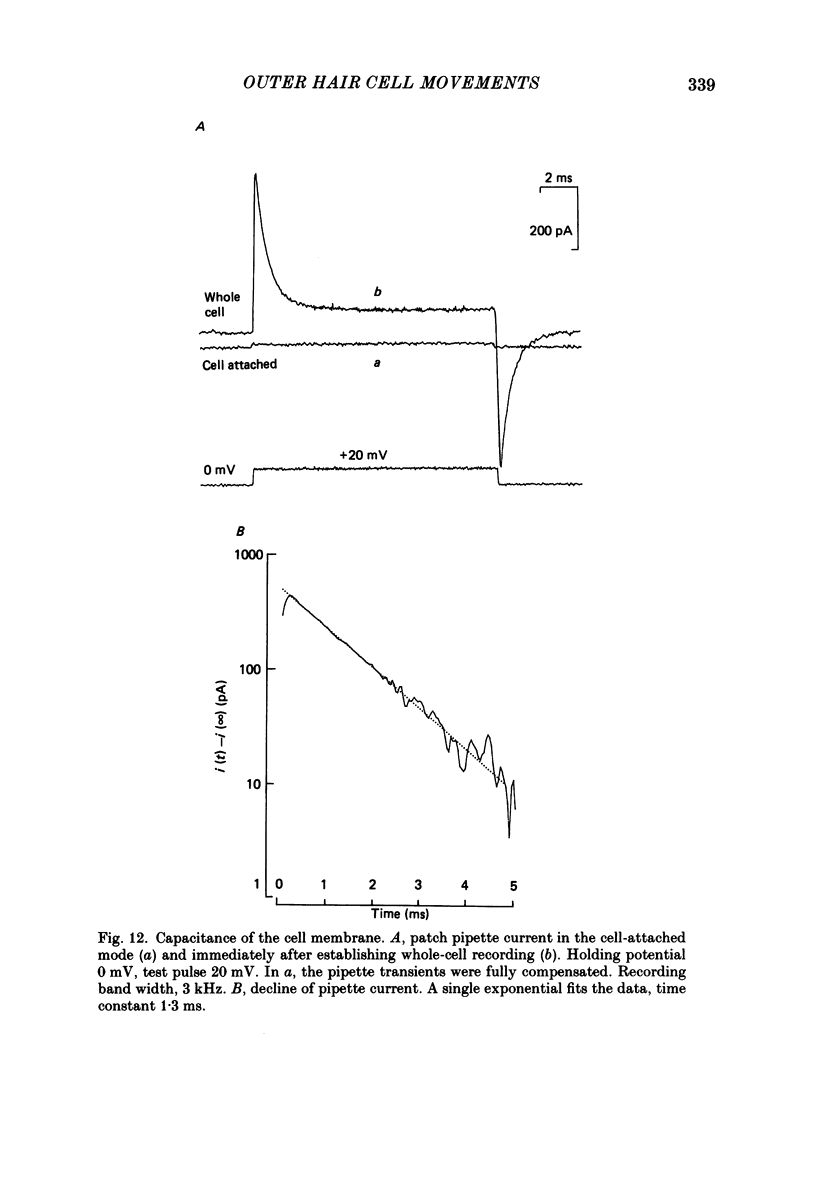

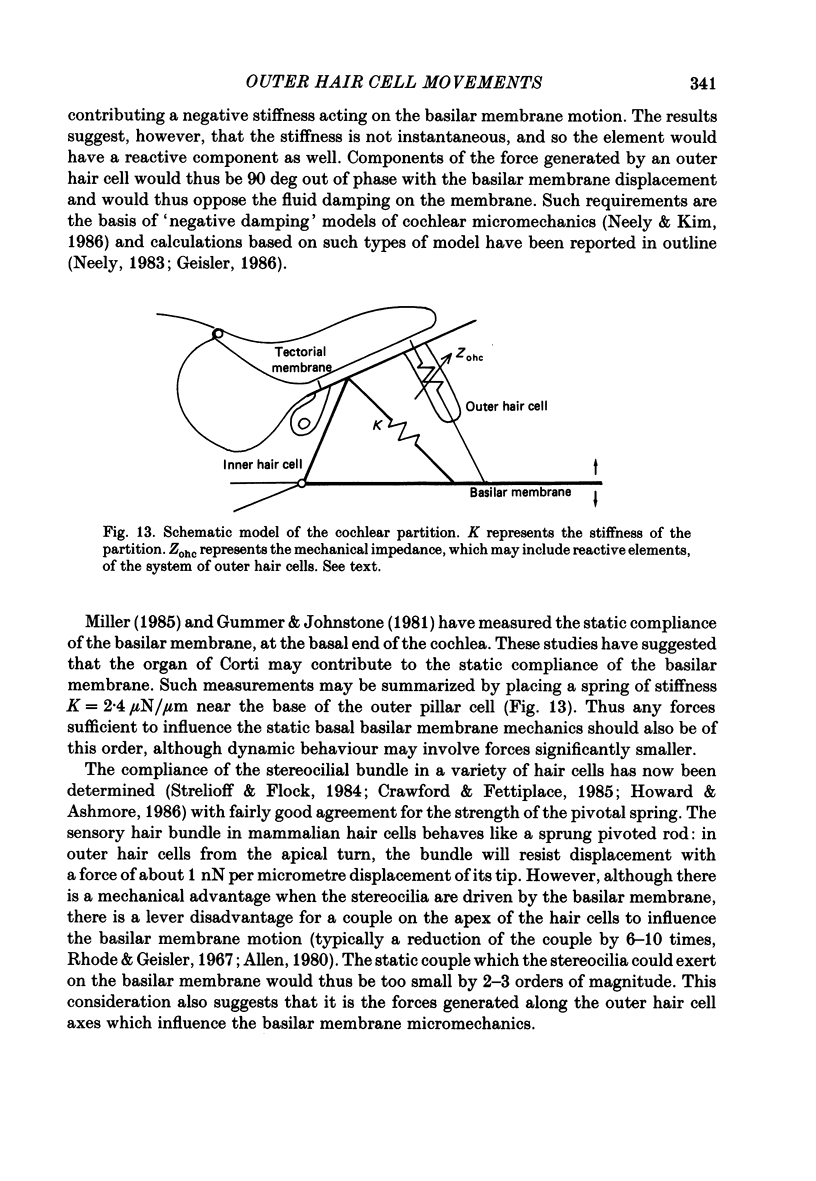
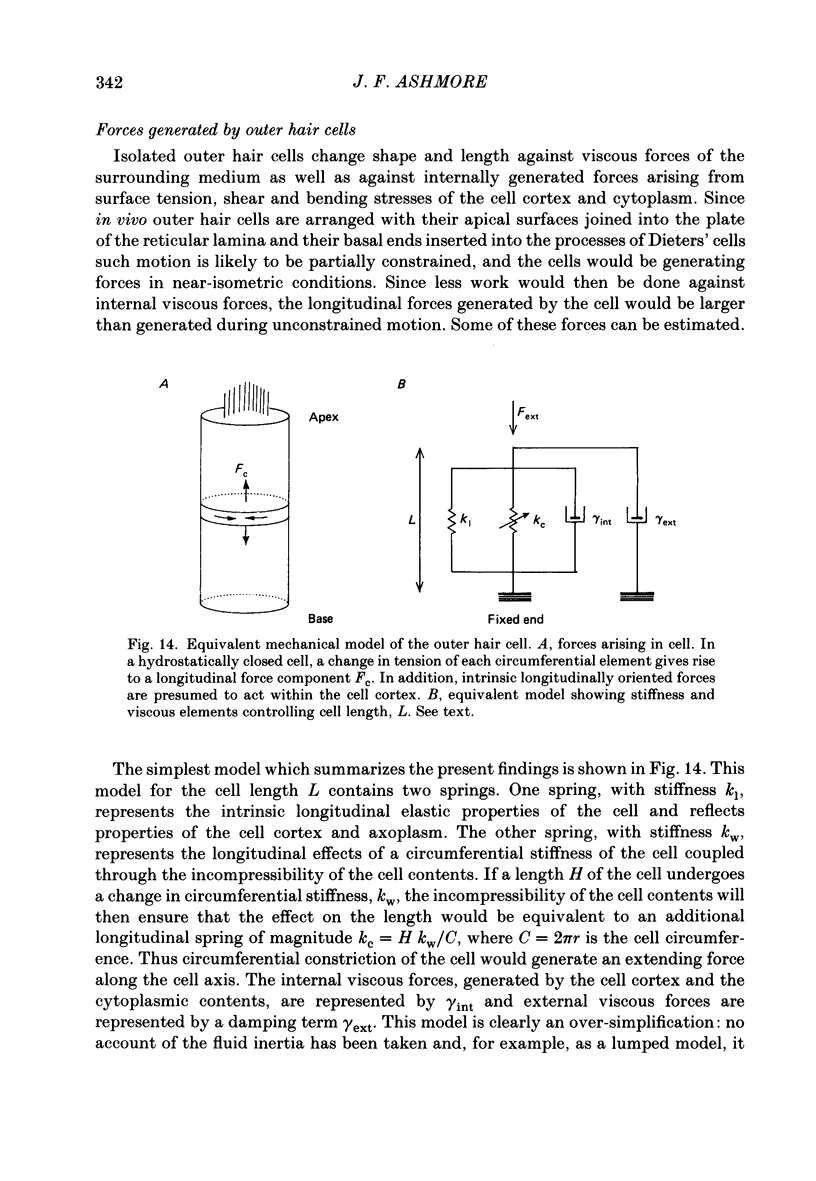





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. B. Cochlear micromechanics--a physical model of transduction. J Acoust Soc Am. 1980 Dec;68(6):1660–1670. doi: 10.1121/1.385198. [DOI] [PubMed] [Google Scholar]
- Ashmore J. F., Meech R. W. Ionic basis of membrane potential in outer hair cells of guinea pig cochlea. Nature. 1986 Jul 24;322(6077):368–371. doi: 10.1038/322368a0. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Nuttall A. L. Efferent control of cochlear inner hair cell responses in the guinea-pig. J Physiol. 1984 Sep;354:625–646. doi: 10.1113/jphysiol.1984.sp015396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Smith D. I., Nuttall A. L. The temperature dependency of neural and hair cell responses evoked by high frequencies. J Acoust Soc Am. 1983 May;73(5):1662–1670. doi: 10.1121/1.389387. [DOI] [PubMed] [Google Scholar]
- Brownell W. E., Bader C. R., Bertrand D., de Ribaupierre Y. Evoked mechanical responses of isolated cochlear outer hair cells. Science. 1985 Jan 11;227(4683):194–196. doi: 10.1126/science.3966153. [DOI] [PubMed] [Google Scholar]
- Cody A. R., Russell I. J. Outer hair cells in the mammalian cochlea and noise-induced hearing loss. Nature. 1985 Jun 20;315(6021):662–665. doi: 10.1038/315662a0. [DOI] [PubMed] [Google Scholar]
- Crawford A. C., Fettiplace R. The mechanical properties of ciliary bundles of turtle cochlear hair cells. J Physiol. 1985 Jul;364:359–379. doi: 10.1113/jphysiol.1985.sp015750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P. Response characteristics of mammalian cochlear hair cells. J Neurosci. 1985 Jun;5(6):1591–1608. doi: 10.1523/JNEUROSCI.05-06-01591.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P., Santos-Sacchi J., Flock A. Intracellular recordings from cochlear outer hair cells. Science. 1982 Nov 5;218(4572):582–584. doi: 10.1126/science.7123260. [DOI] [PubMed] [Google Scholar]
- Davis H. A model for transducer action in the cochlea. Cold Spring Harb Symp Quant Biol. 1965;30:181–190. doi: 10.1101/sqb.1965.030.01.020. [DOI] [PubMed] [Google Scholar]
- Davis H. An active process in cochlear mechanics. Hear Res. 1983 Jan;9(1):79–90. doi: 10.1016/0378-5955(83)90136-3. [DOI] [PubMed] [Google Scholar]
- Evans E. F., Harrison R. V. Proceedings: Correlation between cochlear outer hair cell damage and deterioration of cochlear nerve tuning properties in the guinea-pig. J Physiol. 1976 Mar;256(1):43P–44P. [PubMed] [Google Scholar]
- Flock A., Flock B., Ulfendahl M. Mechanisms of movement in outer hair cells and a possible structural basis. Arch Otorhinolaryngol. 1986;243(2):83–90. doi: 10.1007/BF00453755. [DOI] [PubMed] [Google Scholar]
- Geisler C. D. A model of the effect of outer hair cell motility on cochlear vibrations. Hear Res. 1986;24(2):125–131. doi: 10.1016/0378-5955(86)90056-0. [DOI] [PubMed] [Google Scholar]
- Goldman Y. E., Hibberd M. G., McCray J. A., Trentham D. R. Relaxation of muscle fibres by photolysis of caged ATP. Nature. 1982 Dec 23;300(5894):701–705. doi: 10.1038/300701a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Howard J., Ashmore J. F. Stiffness of sensory hair bundles in the sacculus of the frog. Hear Res. 1986;23(1):93–104. doi: 10.1016/0378-5955(86)90178-4. [DOI] [PubMed] [Google Scholar]
- Khanna S. M., Leonard D. G. Basilar membrane tuning in the cat cochlea. Science. 1982 Jan 15;215(4530):305–306. doi: 10.1126/science.7053580. [DOI] [PubMed] [Google Scholar]
- Lamb T. D., Matthews H. R., Torre V. Incorporation of calcium buffers into salamander retinal rods: a rejection of the calcium hypothesis of phototransduction. J Physiol. 1986 Mar;372:315–349. doi: 10.1113/jphysiol.1986.sp016011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman M. C., Dodds L. W. Single-neuron labeling and chronic cochlear pathology. II. Stereocilia damage and alterations of spontaneous discharge rates. Hear Res. 1984 Oct;16(1):43–53. doi: 10.1016/0378-5955(84)90024-8. [DOI] [PubMed] [Google Scholar]
- Lim D. J. Cochlear anatomy related to cochlear micromechanics. A review. J Acoust Soc Am. 1980 May;67(5):1686–1695. doi: 10.1121/1.384295. [DOI] [PubMed] [Google Scholar]
- Lim D. J., Flock A. Ultrastructural morphology of enzyme-dissociated cochlear sensory cells. Acta Otolaryngol. 1985 Mar-Apr;99(3-4):478–492. doi: 10.3109/00016488509108941. [DOI] [PubMed] [Google Scholar]
- Luff A. R. Dynamic properties of the inferior rectus, extensor digitorum longus, diaphragm and soleus muscles of the mouse. J Physiol. 1981;313:161–171. doi: 10.1113/jphysiol.1981.sp013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Pallotta B. S. Calcium dependence of open and shut interval distributions from calcium-activated potassium channels in cultured rat muscle. J Physiol. 1983 Nov;344:585–604. doi: 10.1113/jphysiol.1983.sp014957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. E. Structural implications of basilar membrane compliance measurements. J Acoust Soc Am. 1985 Apr;77(4):1465–1474. doi: 10.1121/1.392041. [DOI] [PubMed] [Google Scholar]
- Neely S. T., Kim D. O. A model for active elements in cochlear biomechanics. J Acoust Soc Am. 1986 May;79(5):1472–1480. doi: 10.1121/1.393674. [DOI] [PubMed] [Google Scholar]
- Pringle J. W. The Croonian Lecture, 1977. Stretch activation of muscle: function and mechanism. Proc R Soc Lond B Biol Sci. 1978 May 5;201(1143):107–130. doi: 10.1098/rspb.1978.0035. [DOI] [PubMed] [Google Scholar]
- Rhode W. S., Geisler C. D. Model of the displacement between opposing points on the tectorial membrane and reticular lamina. J Acoust Soc Am. 1967 Jul;42(1):185–190. doi: 10.1121/1.1910547. [DOI] [PubMed] [Google Scholar]
- Russell I. J., Cody A. R., Richardson G. P. The responses of inner and outer hair cells in the basal turn of the guinea-pig cochlea and in the mouse cochlea grown in vitro. Hear Res. 1986;22:199–216. doi: 10.1016/0378-5955(86)90096-1. [DOI] [PubMed] [Google Scholar]
- Russell I. J., Sellick P. M. Low-frequency characteristics of intracellularly recorded receptor potentials in guinea-pig cochlear hair cells. J Physiol. 1983 May;338:179–206. doi: 10.1113/jphysiol.1983.sp014668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K. Fine structure of the sensory epithelium of guinea-pig organ of Corti: subsurface cisternae and lamellar bodies in the outer hair cells. Cell Tissue Res. 1983;229(3):467–481. doi: 10.1007/BF00207692. [DOI] [PubMed] [Google Scholar]
- Sellick P. M., Patuzzi R., Johnstone B. M. Measurement of basilar membrane motion in the guinea pig using the Mössbauer technique. J Acoust Soc Am. 1982 Jul;72(1):131–141. doi: 10.1121/1.387996. [DOI] [PubMed] [Google Scholar]
- Strelioff D., Flock A. Stiffness of sensory-cell hair bundles in the isolated guinea pig cochlea. Hear Res. 1984 Jul;15(1):19–28. doi: 10.1016/0378-5955(84)90221-1. [DOI] [PubMed] [Google Scholar]
- Tasaki I., Iwasa K. Rapid pressure changes and surface displacements in the squid giant axon associated with production of action potentials. Jpn J Physiol. 1982;32(1):69–81. doi: 10.2170/jjphysiol.32.69. [DOI] [PubMed] [Google Scholar]
- Terakawa S. Potential-dependent variations of the intracellular pressure in the intracellularly perfused squid giant axon. J Physiol. 1985 Dec;369:229–248. doi: 10.1113/jphysiol.1985.sp015898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., Inoué S. Acrosomal reaction of Thyone sperm. II. The kinetics and possible mechanism of acrosomal process elongation. J Cell Biol. 1982 Jun;93(3):820–827. doi: 10.1083/jcb.93.3.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenner H. P., Zimmermann U., Schmitt U. Reversible contraction of isolated mammalian cochlear hair cells. Hear Res. 1985 May;18(2):127–133. doi: 10.1016/0378-5955(85)90004-8. [DOI] [PubMed] [Google Scholar]
- Zwislocki J. J. Five decades of research on cochlear mechanics. J Acoust Soc Am. 1980 May;67(5):1679–1685. doi: 10.1121/1.384294. [DOI] [PubMed] [Google Scholar]
- de Boer E. No sharpening? a challenge for cochlear mechanics. J Acoust Soc Am. 1983 Feb;73(2):567–573. doi: 10.1121/1.389002. [DOI] [PubMed] [Google Scholar]



