Abstract
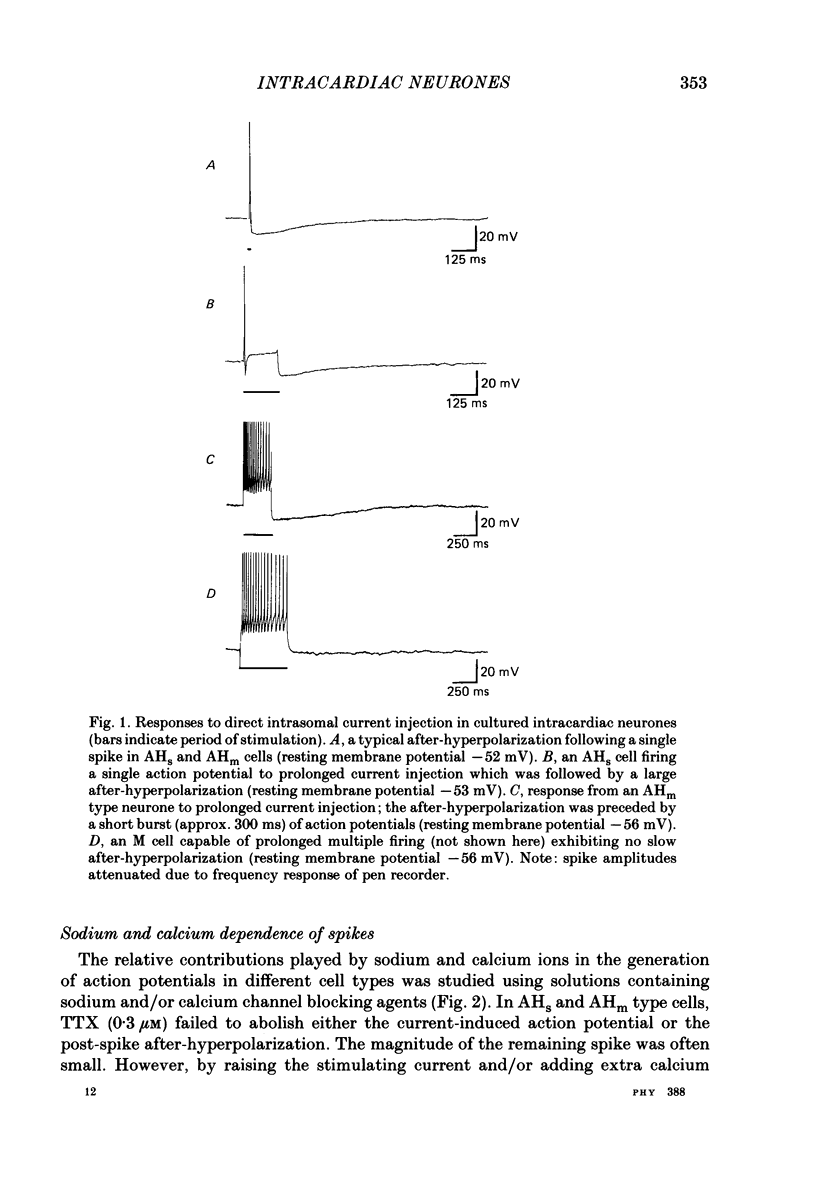
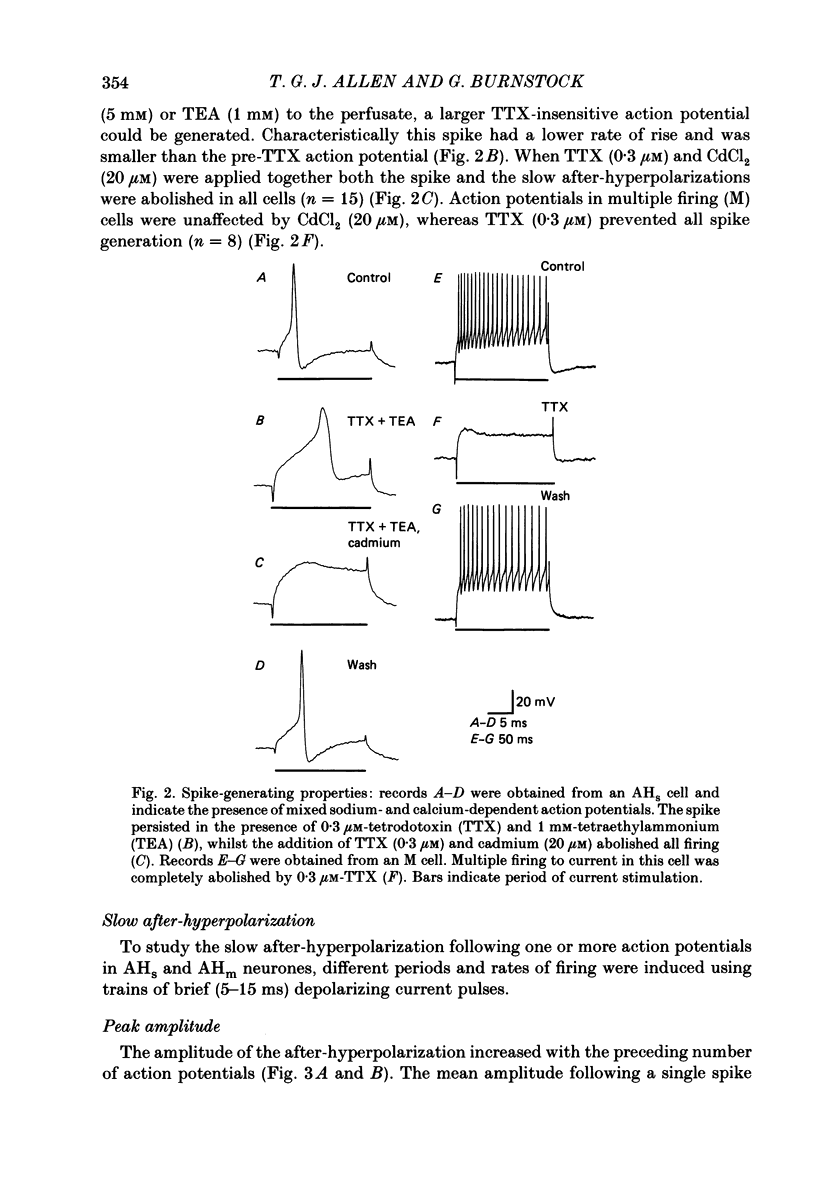
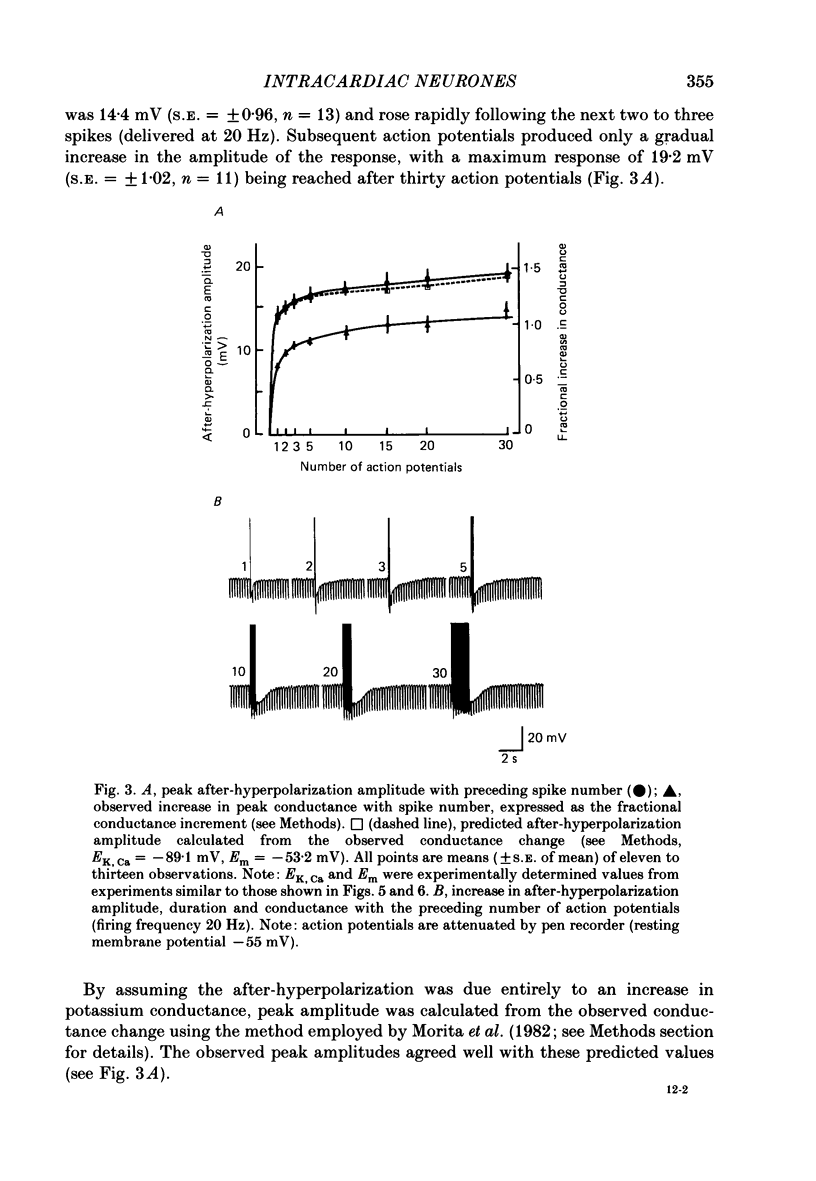
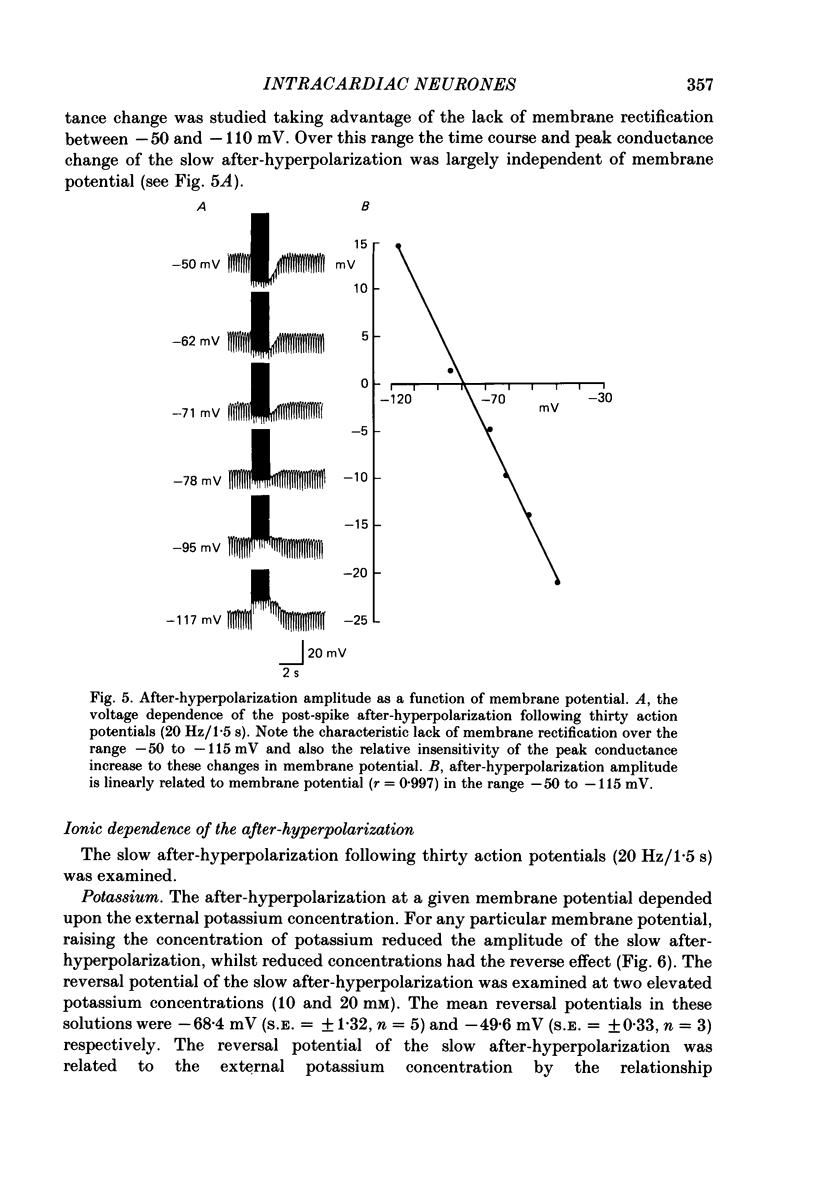
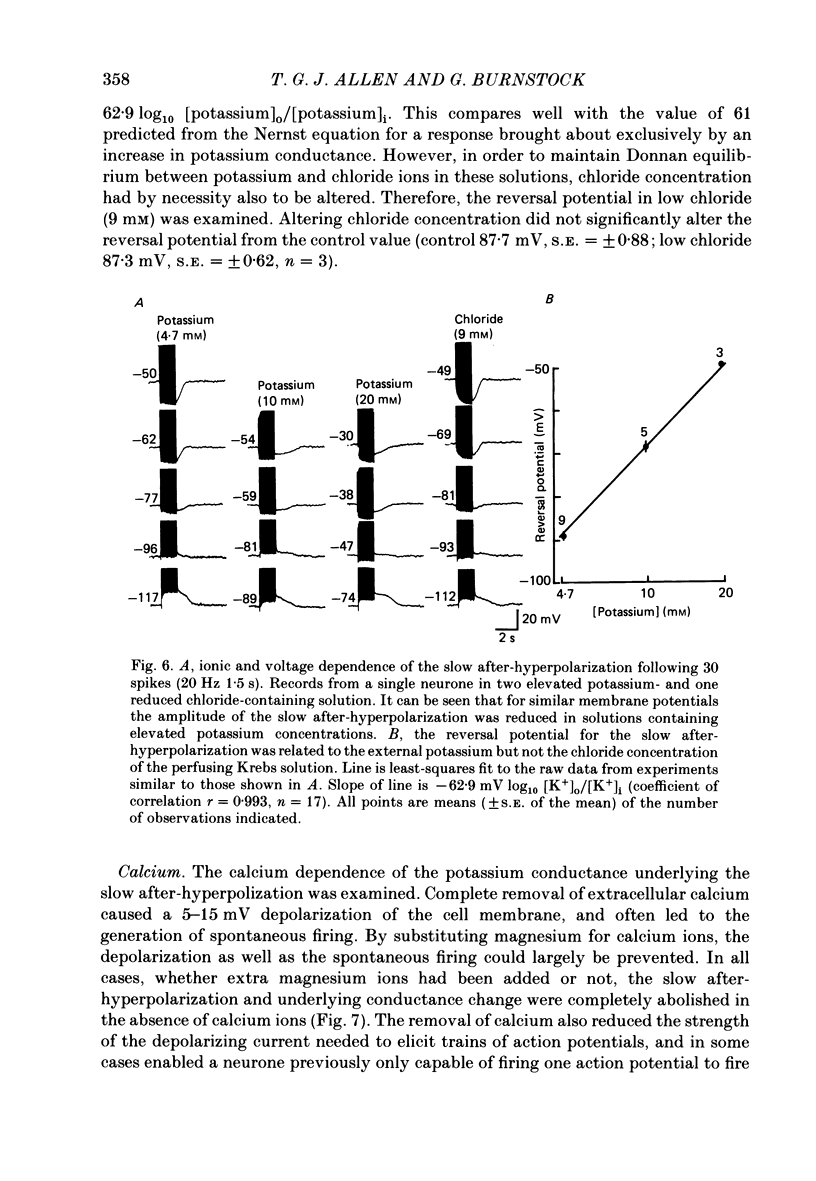
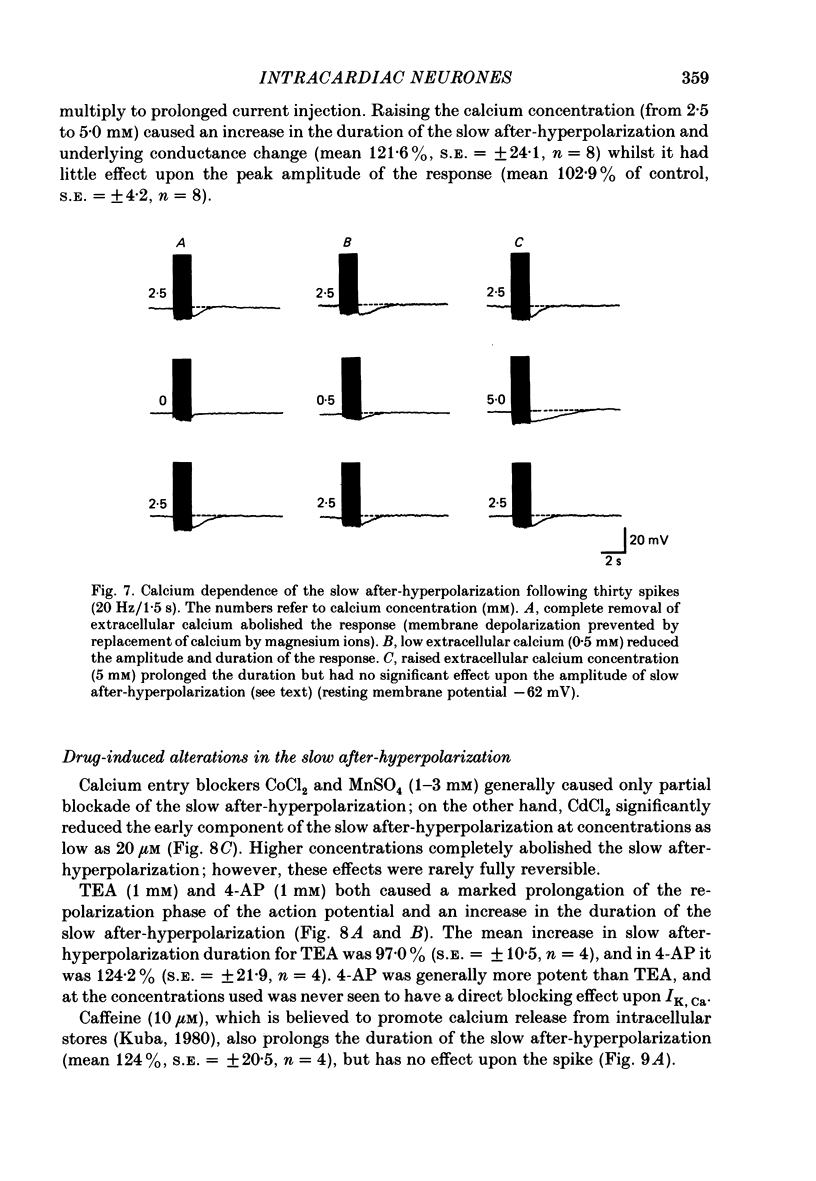
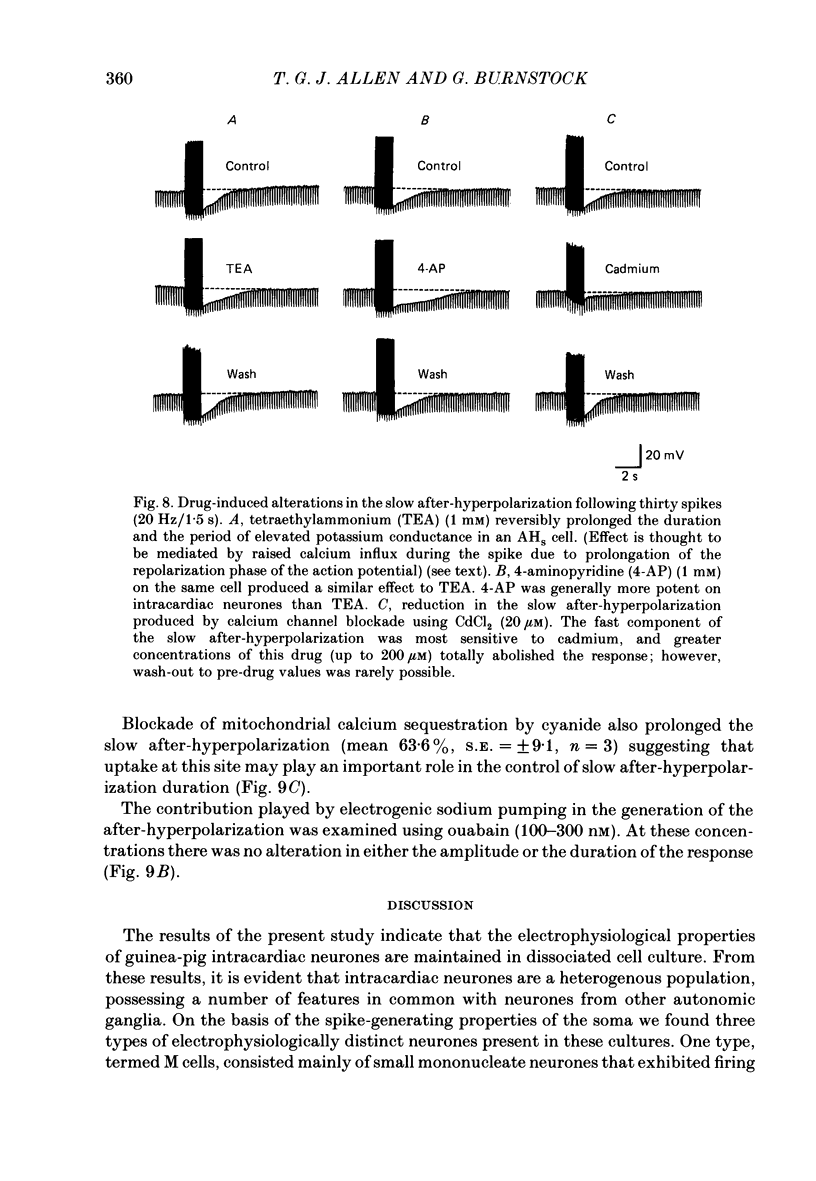
1. The electrophysiological properties of intracardiac neurones cultured from ganglia within the atria and interatrial septum of the new-born guinea-pig heart were studied using intracellular micro-electrodes. 2. Three types of neurones with resting membrane potentials in the range -45 to -76 mV were detected. The first type, AHs cells, had high (15-28 mV) firing thresholds, pronounced slow post-spike after-hyperpolarizations and fired only once to prolonged intrasomal current injection. The second type, AHm cells, were similar to AHs cells, except that they could fire short bursts of spikes (100-400 ms) at the onset of current injection. The third type, M cells, had low firing thresholds (10-15 mV), no slow after-hyperpolarizations and produced non-adapting trains of action potentials in response to depolarizing current injection. 3. The generation of action potentials in M cells was prevented by tetrodotoxin (TTX; 0.3 microM), whereas in AHs and AHm cells action potentials displayed a channel blockade using solutions containing the divalent cations cadmium, cobalt or manganese (0.02-1 mM). 4. The post-spike after-hyperpolarization in AHs and AHm cells was abolished by the removal of extracellular calcium, shortened in solutions containing the calcium entry blockers CdCl2, MnSO4 and CoCl2 (0.02-1 mM) and prolonged by the addition of calcium (5.0 mM), tetraethylammonium (1-3 mM), 4-aminopyridine (1-3 mM), cyanide (10 microM) or caffeine (100 microM) to the perfusate. 5. The reversal potential of the post-spike after-hyperpolarization was -89.1 mV. This value changed by 62.9 mV for a 10-fold increase in extracellular potassium concentration. 6. The peak conductance change during the post-spike after-hyperpolarization (gK,Ca), was largely independent of membrane potential between -50 and -110 mV. The peak increase in gK,Ca and the duration of the after-hyperpolarization increased with the number of spikes preceding it. 7. It is concluded that calcium entry during the action potential is responsible for the activation of an outward potassium current in the two types of AH cells; the roles played by intracellular calcium extrusion as well as sequestration mechanisms in the generation of the response are discussed.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed Z., Connor J. A. Measurement of calcium influx under voltage clamp in molluscan neurones using the metallochromic dye arsenazo III. J Physiol. 1979 Jan;286:61–82. doi: 10.1113/jphysiol.1979.sp012607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. N., Magleby K. L., Pallotta B. S. Properties of single calcium-activated potassium channels in cultured rat muscle. J Physiol. 1982 Oct;331:211–230. doi: 10.1113/jphysiol.1982.sp014370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman J. G., Purves R. D. Intracellular recordings from ganglia of the thoracic sympathetic chain of the guinea-pig. J Physiol. 1969 Jul;203(1):173–198. doi: 10.1113/jphysiol.1969.sp008858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Adams P. R. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980 Feb 14;283(5748):673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Constanti A., Adams P. R. Ca-activated potassium current in vertebrate sympathetic neurons. Cell Calcium. 1983 Dec;4(5-6):407–420. doi: 10.1016/0143-4160(83)90017-9. [DOI] [PubMed] [Google Scholar]
- Dalsgaard C. J., Franco-Cereceda A., Saria A., Lundberg J. M., Theodorsson-Norheim E., Hökfelt T. Distribution and origin of substance P- and neuropeptide Y-immunoreactive nerves in the guinea-pig heart. Cell Tissue Res. 1986;243(3):477–485. doi: 10.1007/BF00218054. [DOI] [PubMed] [Google Scholar]
- Ellison J. P., Hibbs R. G. An ultrastructural study of mammalian cardiac ganglia. J Mol Cell Cardiol. 1976 Feb;8(2):89–101. doi: 10.1016/0022-2828(76)90023-7. [DOI] [PubMed] [Google Scholar]
- Fowler J. C., Greene R., Weinreich D. Two calcium-sensitive spike after-hyperpolarizations in visceral sensory neurones of the rabbit. J Physiol. 1985 Aug;365:59–75. doi: 10.1113/jphysiol.1985.sp015759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto S., Yamamoto K., Kuba K., Morita K., Kato E. Calcium localization in the sympathetic ganglion of the bullfrog and effects of caffeine. Brain Res. 1980 Nov 24;202(1):21–32. [PubMed] [Google Scholar]
- Gallego R., Eyzaguirre C. Membrane and action potential characteristics of A and C nodose ganglion cells studied in whole ganglia and in tissue slices. J Neurophysiol. 1978 Sep;41(5):1217–1232. doi: 10.1152/jn.1978.41.5.1217. [DOI] [PubMed] [Google Scholar]
- Gibbins I. L., Furness J. B., Costa M., MacIntyre I., Hillyard C. J., Girgis S. Co-localization of calcitonin gene-related peptide-like immunoreactivity with substance P in cutaneous, vascular and visceral sensory neurons of guinea pigs. Neurosci Lett. 1985 Jun 12;57(2):125–130. doi: 10.1016/0304-3940(85)90050-3. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Stickgold R., Yoshikami D. Synaptic excitation and inhibition resulting from direct action of acetylcholine on two types of chemoreceptors on individual amphibian parasympathetic neurones. J Physiol. 1977 Oct;271(3):817–846. doi: 10.1113/jphysiol.1977.sp012027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassall C. J., Burnstock G. Intrinsic neurones and associated cells of the guinea-pig heart in culture. Brain Res. 1986 Jan 29;364(1):102–113. doi: 10.1016/0006-8993(86)90991-1. [DOI] [PubMed] [Google Scholar]
- Hassall C. J., Burnstock G. Neuropeptide Y-like immunoreactivity in cultured intrinsic neurones of the heart. Neurosci Lett. 1984 Nov 23;52(1-2):111–115. doi: 10.1016/0304-3940(84)90359-8. [DOI] [PubMed] [Google Scholar]
- Higashi H., Morita K., North R. A. Calcium-dependent after-potentials in visceral afferent neurones of the rabbit. J Physiol. 1984 Oct;355:479–492. doi: 10.1113/jphysiol.1984.sp015433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Holman M. E., Spence I. Two types of neurones in the myenteric plexus of duodenum in the guinea-pig. J Physiol. 1974 Jan;236(2):303–326. doi: 10.1113/jphysiol.1974.sp010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Johnson S. M., van Helden D. F. The slow calcium-dependent potassium current in a myenteric neurone of the guinea-pig ileum. J Physiol. 1985 Apr;361:315–337. doi: 10.1113/jphysiol.1985.sp015648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Spence I. Calcium action potentials in mammalian peripheral neurones. Nat New Biol. 1973 May 9;243(123):54–56. [PubMed] [Google Scholar]
- Ito H. Evidence for initiation of calcium spikes in C-cells of the rabbit nodose ganglion. Pflugers Arch. 1982 Aug;394(2):106–112. doi: 10.1007/BF00582910. [DOI] [PubMed] [Google Scholar]
- Jansen J. K., Nicholls J. G. Conductance changes, an electrogenic pump and the hyperpolarization of leech neurones following impulses. J Physiol. 1973 Mar;229(3):635–655. doi: 10.1113/jphysiol.1973.sp010158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING T. S., COAKLEY J. B. The intrinsic nerve cells of the cardiac atria of mammals and man. J Anat. 1958 Jul;92(3):353–376. [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y., Hassall C. J., Burnstock G. Culture of intramural cardiac ganglia of the newborn guinea-pig. I. Neuronal elements. Cell Tissue Res. 1986;244(3):595–604. doi: 10.1007/BF00212539. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Hassall C. J., Burnstock G. Culture of intramural cardiac ganglia of the newborn guinea-pig. II. Non-neuronal elements. Cell Tissue Res. 1986;244(3):605–612. doi: 10.1007/BF00212540. [DOI] [PubMed] [Google Scholar]
- Kuba K. Release of calcium ions linked to the activation of potassium conductance in a caffeine-treated sympathetic neurone. J Physiol. 1980 Jan;298:251–269. doi: 10.1113/jphysiol.1980.sp013079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A. Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature. 1981 Jun 11;291(5815):497–500. doi: 10.1038/291497a0. [DOI] [PubMed] [Google Scholar]
- McAfee D. A., Yarowsky P. J. Calcium-dependent potentials in the mammalian sympathetic neurone. J Physiol. 1979 May;290(2):507–523. doi: 10.1113/jphysiol.1979.sp012787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan U. J., Purves D. Visual identification of two kinds of nerve cells and their synaptic contacts in a living autonomic ganglion of the mudpuppy (Necturus maculosus). J Physiol. 1976 Jan;254(2):405–425. doi: 10.1113/jphysiol.1976.sp011238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W. Calcium-dependent potassium activation in nervous tissues. Annu Rev Biophys Bioeng. 1978;7:1–18. doi: 10.1146/annurev.bb.07.060178.000245. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K., North R. A., Tokimasa T. The calcium-activated potassium conductance in guinea-pig myenteric neurones. J Physiol. 1982 Aug;329:341–354. doi: 10.1113/jphysiol.1982.sp014306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi S., North R. A. Intracellular recording from the myenteric plexus of the guinea-pig ileum. J Physiol. 1973 Jun;231(3):471–491. doi: 10.1113/jphysiol.1973.sp010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozdrachev A. D., Pogorelov A. G. Extracellular recording of neuronal activity of the cat heart ganglia. J Auton Nerv Syst. 1982 Jul;6(1):73–81. doi: 10.1016/0165-1838(82)90024-8. [DOI] [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. On the electrogenic sodium pump in mammalian non-myelinated nerve fibres and its activation by various external cations. J Physiol. 1968 May;196(1):183–221. doi: 10.1113/jphysiol.1968.sp008502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper S. The acetylcholine sensitivity of the surface membrane of multiply-innervated parasympathetic ganglion cells in the mudpuppy before and after partial denervation. J Physiol. 1976 Jan;254(2):455–473. doi: 10.1113/jphysiol.1976.sp011240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihe E., McKnight A. T., Corbett A. D., Hartschuh W., Reinecke M., Kosterlitz H. W. Characterization of opioid peptides in guinea-pig heart and skin. Life Sci. 1983;33 (Suppl 1):711–714. doi: 10.1016/0024-3205(83)90601-x. [DOI] [PubMed] [Google Scholar]
- Weihe E., Reinecke M., Forssmann W. G. Distribution of vasoactive intestinal polypeptide-like immunoreactivity in the mammalian heart. Interrelation with neurotensin- and substance P-like immunoreactive nerves. Cell Tissue Res. 1984;236(3):527–540. doi: 10.1007/BF00217219. [DOI] [PubMed] [Google Scholar]
- Wood J. D. Enteric neurophysiology. Am J Physiol. 1984 Dec;247(6 Pt 1):G585–G598. doi: 10.1152/ajpgi.1984.247.6.G585. [DOI] [PubMed] [Google Scholar]
- Yeh J. Z., Oxford G. S., Wu C. H., Narahashi T. Dynamics of aminopyridine block of potassium channels in squid axon membrane. J Gen Physiol. 1976 Nov;68(5):519–535. doi: 10.1085/jgp.68.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J. Z., Oxford G. S., Wu C. H., Narahashi T. Interactions of aminopyridines with potassium channels of squid axon membranes. Biophys J. 1976 Jan;16(1):77–81. doi: 10.1016/S0006-3495(76)85663-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


