Abstract
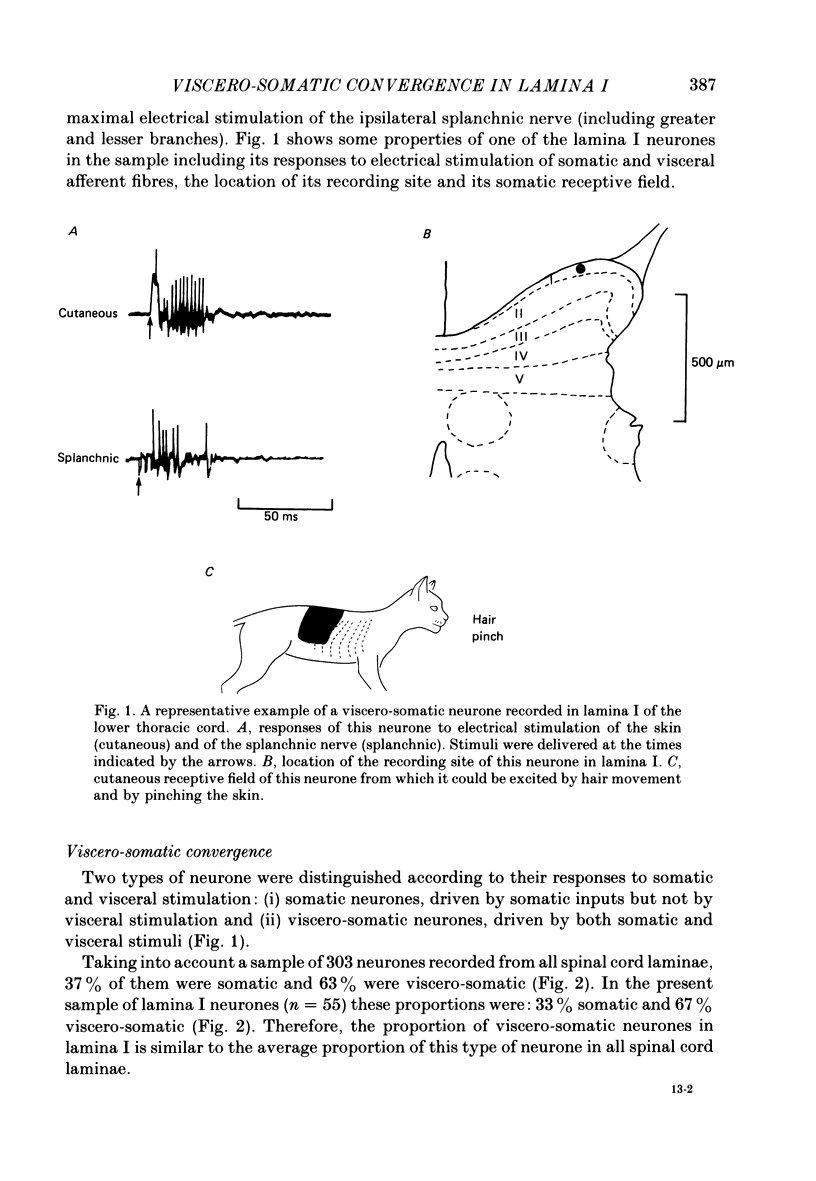
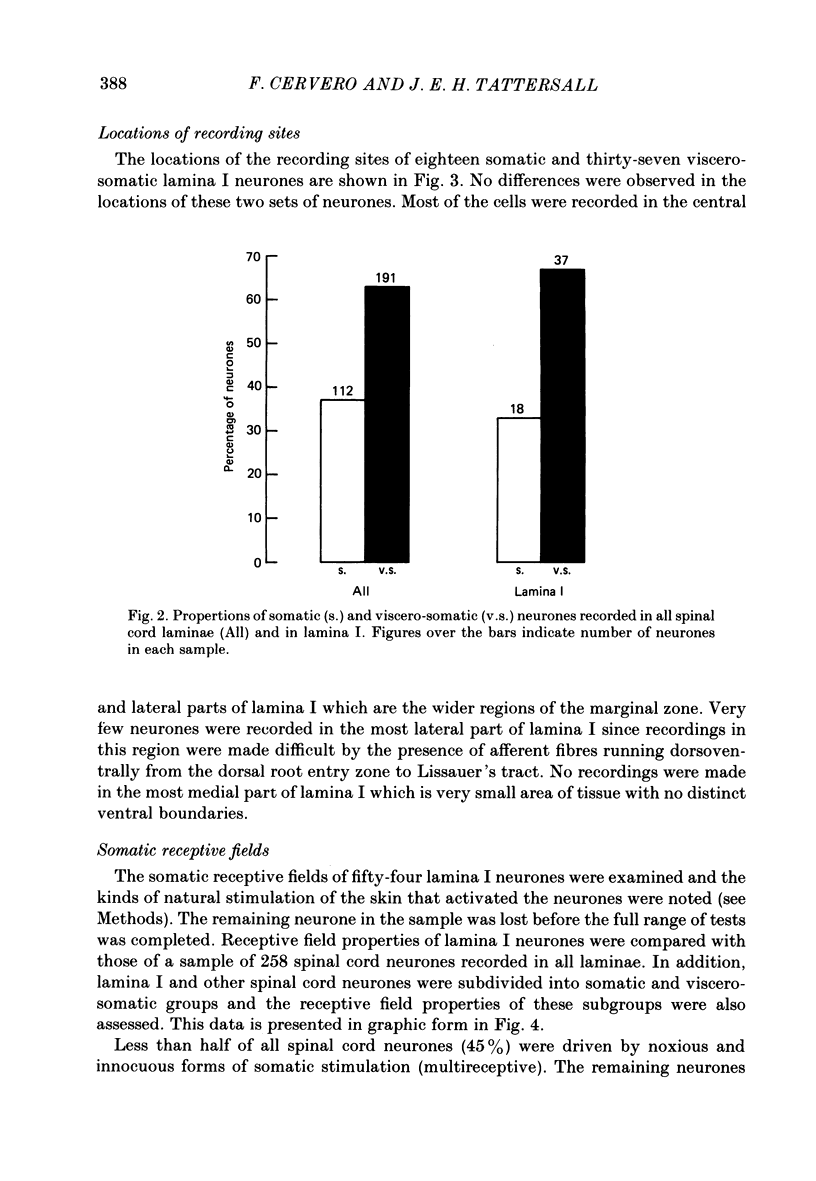
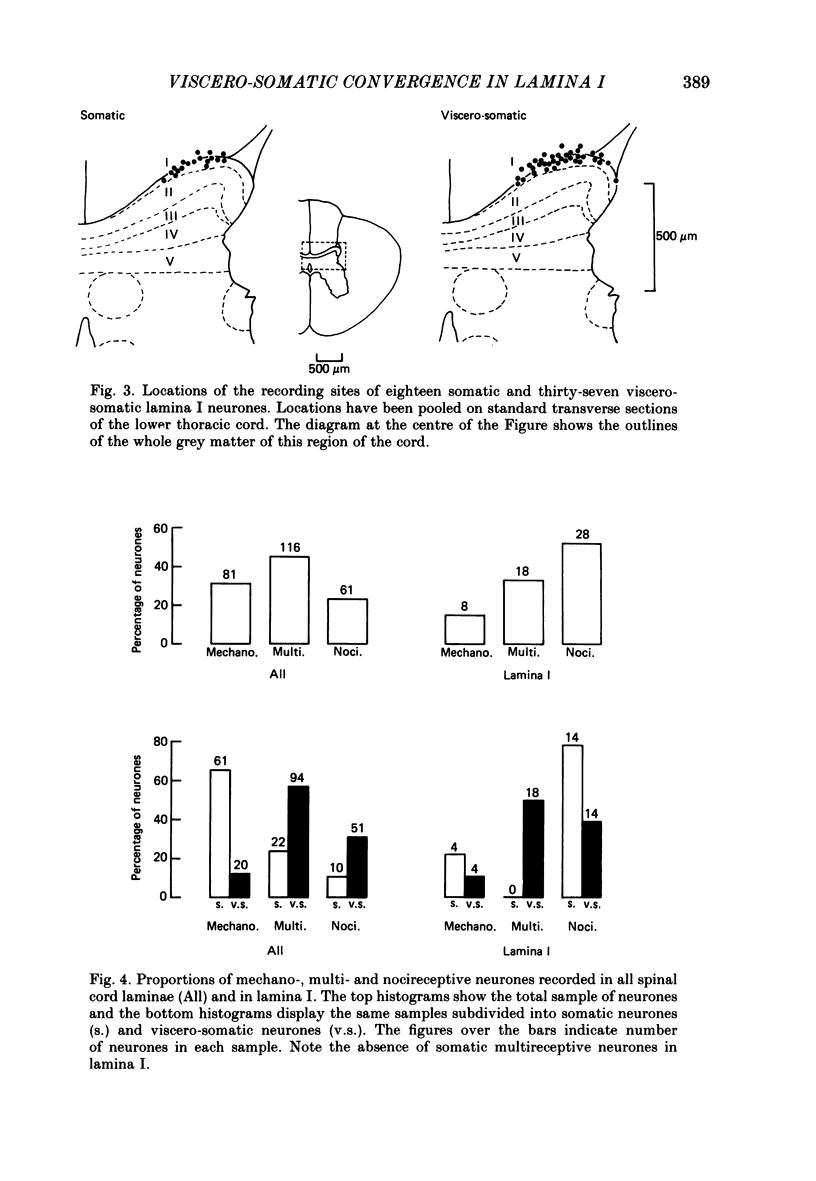
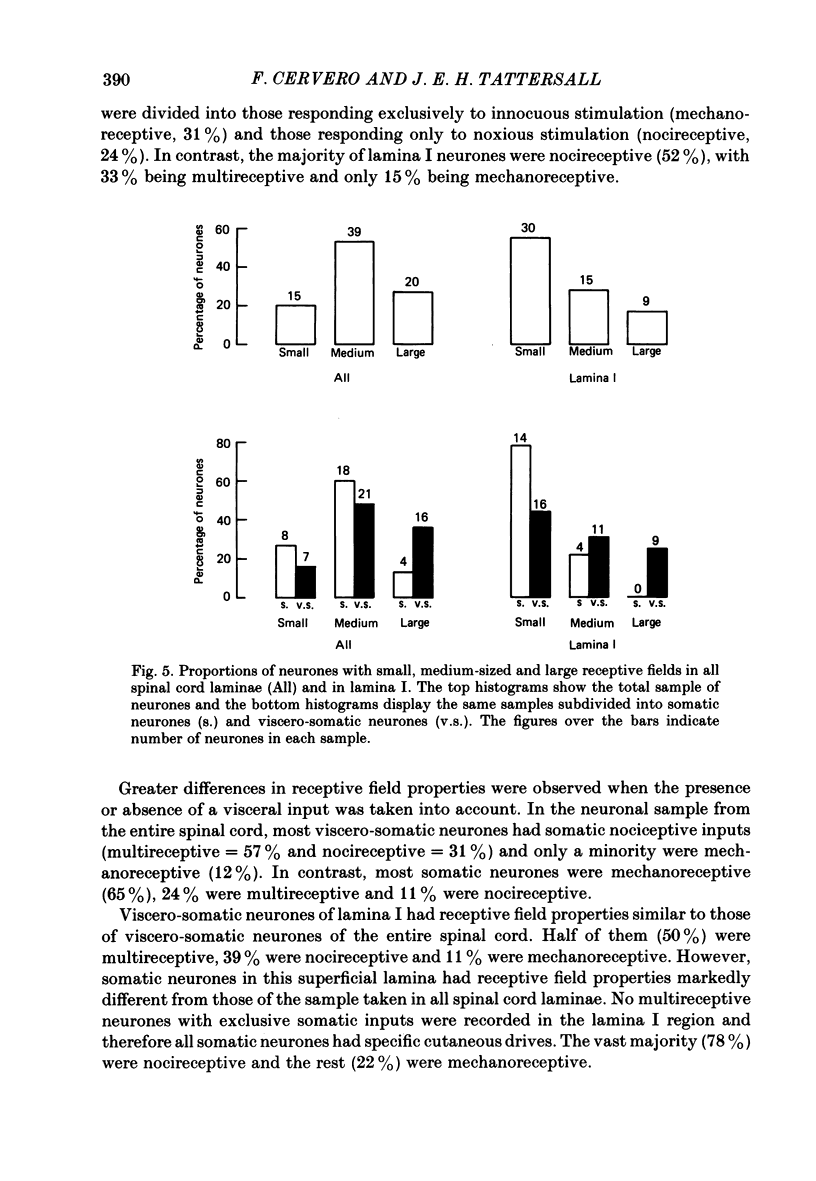
1. Single-unit electrical activity has been recorded from fifty-five neurones whose recording sites were located in or immediately adjacent to the marginal zone (lamina I) of the lower thoracic spinal cord (T8-T12) of anaesthetized or decerebrate cats. Their responses to stimulation of somatic and visceral afferent fibres and the sizes of their cutaneous receptive fields have been analysed and compared with the responses and receptive fields of neurones recorded throughout the spinal grey matter. 2. Neurones were classified according to their responses to innocuous stimulation of their somatic receptive fields (i.e. brushing and stroking) or to noxious stimulation (i.e. pinching, squeezing and/or heating above 45 degrees C). 52% of all the neurones recorded in lamina I were driven exclusively by noxious stimulation of the skin (nocireceptive); 33% were driven by both noxious and innocuous stimulation of the skin (multireceptive) and 15% were driven exclusively by innocuous stimulation of the skin (mechanoreceptive). 3. Visceral afferent inputs to these neurones were tested by supramaximal electrical stimulation of the ipsilateral splanchnic nerve (15 V, 0.2 ms, 0.3 Hz). Two types of neurone were distinguished according to their responses to visceral stimulation: (i) somatic neurones, driven only by stimulation of somatic afferent fibres and (ii) viscero-somatic neurones, driven by stimulation of somatic and visceral afferent fibres. Of the neurones recorded in lamina I, 33% were somatic and 67% were viscero-somatic. This proportion was very similar to the percentages of somatic and viscero-somatic neurones recorded throughout the grey matter (37 and 63%, respectively). 4. Viscero-somatic neurones in lamina I had somatic receptive field properties similar to those of viscero-somatic neurones of the entire spinal cord. Half of them were multireceptive, 39% were nocireceptive and 11% were mechanoreceptive. However, somatic neurones in lamina I had receptive field properties different from those of somatic neurones from other laminae: no multireceptive somatic neurones were recorded in lamina I; the vast majority (78%) were nocireceptive and 22% were mechanoreceptive. 5. The majority of somatic and viscero-somatic neurones in lamina I had small somatic receptive fields but, even in this group of cells, viscero-somatic neurones had larger receptive fields than somatic cells. 6. Ascending axonal projections in both dorsolateral funiculi and in the contralateral ventrolateral quadrant were tested in eighteen lamina I neurones. Only one neurone was found to project to the cervical cord.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahams V. C., Richmond F. J., Keane J. Projections from C2 and C3 nerves supplying muscles and skin of the cat neck: a study using transganglionic transport of horseradish peroxidase. J Comp Neurol. 1984 Nov 20;230(1):142–154. doi: 10.1002/cne.902300113. [DOI] [PubMed] [Google Scholar]
- Abrahams V. C., Swett J. E. The pattern of spinal and medullary projections from a cutaneous nerve and a muscle nerve of the forelimb of the cat: a study using the transganglionic transport of HRP. J Comp Neurol. 1986 Apr 1;246(1):70–84. doi: 10.1002/cne.902460105. [DOI] [PubMed] [Google Scholar]
- Bennett G. J., Abdelmoumene M., Hayashi H., Dubner R. Physiology and morphology of substantia gelatinosa neurons intracellularly stained with horseradish peroxidase. J Comp Neurol. 1980 Dec 15;194(4):809–827. doi: 10.1002/cne.901940407. [DOI] [PubMed] [Google Scholar]
- Cervero F., Connell L. A. Distribution of somatic and visceral primary afferent fibres within the thoracic spinal cord of the cat. J Comp Neurol. 1984 Nov 20;230(1):88–98. doi: 10.1002/cne.902300108. [DOI] [PubMed] [Google Scholar]
- Cervero F., Iggo A., Molony V. An electrophysiological study of neurones in the Substantia Gelatinosa Rolandi of the cat's spinal cord. Q J Exp Physiol Cogn Med Sci. 1979 Oct;64(4):297–314. doi: 10.1113/expphysiol.1979.sp002484. [DOI] [PubMed] [Google Scholar]
- Cervero F., Iggo A., Molony V. Ascending projections of nociceptor-driven Lamina I neurones in the cat. Exp Brain Res. 1979 Mar 9;35(1):135–149. doi: 10.1007/BF00236790. [DOI] [PubMed] [Google Scholar]
- Cervero F., Iggo A., Ogawa H. Nociceptor-driven dorsal horn neurones in the lumbar spinal cord of the cat. Pain. 1976 Mar;2(1):5–24. doi: 10.1016/0304-3959(76)90042-7. [DOI] [PubMed] [Google Scholar]
- Cervero F., Iggo A. The substantia gelatinosa of the spinal cord: a critical review. Brain. 1980 Dec;103(4):717–772. doi: 10.1093/brain/103.4.717. [DOI] [PubMed] [Google Scholar]
- Cervero F. Somatic and visceral inputs to the thoracic spinal cord of the cat: effects of noxious stimulation of the biliary system. J Physiol. 1983 Apr;337:51–67. doi: 10.1113/jphysiol.1983.sp014611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervero F. Supraspinal connections of neurones in the thoracic spinal cord of the cat: ascending projections and effects of descending impulses. Brain Res. 1983 Sep 26;275(2):251–261. doi: 10.1016/0006-8993(83)90986-1. [DOI] [PubMed] [Google Scholar]
- Cervero F., Tattersall J. E. Cutaneous receptive fields of somatic and viscerosomatic neurones in the thoracic spinal cord of the cat. J Comp Neurol. 1985 Jul 15;237(3):325–332. doi: 10.1002/cne.902370304. [DOI] [PubMed] [Google Scholar]
- Cervero F., Tattersall J. E. Somatic and visceral sensory integration in the thoracic spinal cord. Prog Brain Res. 1986;67:189–205. doi: 10.1016/s0079-6123(08)62763-6. [DOI] [PubMed] [Google Scholar]
- Christensen B. N., Perl E. R. Spinal neurons specifically excited by noxious or thermal stimuli: marginal zone of the dorsal horn. J Neurophysiol. 1970 Mar;33(2):293–307. doi: 10.1152/jn.1970.33.2.293. [DOI] [PubMed] [Google Scholar]
- Craig A. D., Kniffki K. D. Spinothalamic lumbosacral lamina I cells responsive to skin and muscle stimulation in the cat. J Physiol. 1985 Aug;365:197–221. doi: 10.1113/jphysiol.1985.sp015767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groat W. C. Spinal cord projections and neuropeptides in visceral afferent neurons. Prog Brain Res. 1986;67:165–187. doi: 10.1016/s0079-6123(08)62762-4. [DOI] [PubMed] [Google Scholar]
- Dubner R., Bennett G. J. Spinal and trigeminal mechanisms of nociception. Annu Rev Neurosci. 1983;6:381–418. doi: 10.1146/annurev.ne.06.030183.002121. [DOI] [PubMed] [Google Scholar]
- Light A. R., Perl E. R. Spinal termination of functionally identified primary afferent neurons with slowly conducting myelinated fibers. J Comp Neurol. 1979 Jul 15;186(2):133–150. doi: 10.1002/cne.901860203. [DOI] [PubMed] [Google Scholar]
- Light A. R., Trevino D. L., Perl E. R. Morphological features of functionally defined neurons in the marginal zone and substantia gelatinosa of the spinal dorsal horn. J Comp Neurol. 1979 Jul 15;186(2):151–171. doi: 10.1002/cne.901860204. [DOI] [PubMed] [Google Scholar]
- Lima D., Coimbra A. A Golgi study of the neuronal population of the marginal zone (lamina I) of the rat spinal cord. J Comp Neurol. 1986 Feb 1;244(1):53–71. doi: 10.1002/cne.902440105. [DOI] [PubMed] [Google Scholar]
- Lima D., Coimbra A. The neuronal population of the marginal zone (lamina I) of the rat spinal cord. A study based on reconstructions of serially sectioned cells. Anat Embryol (Berl) 1983;167(2):273–288. doi: 10.1007/BF00298516. [DOI] [PubMed] [Google Scholar]
- Molony V. Fine glass micro-electrodes for recording from small neurones in the spinal cord of the cat [proceedings]. J Physiol. 1978 Nov;284:27P–28P. [PubMed] [Google Scholar]
- Molony V., Steedman W. M., Cervero F., Iggo A. Intracellular marking of identified neurones in the superficial dorsal horn of the cat spinal cord. Q J Exp Physiol. 1981 Jul;66(3):211–223. doi: 10.1113/expphysiol.1981.sp002551. [DOI] [PubMed] [Google Scholar]
- Nyberg G., Blomqvist A. The central projection of muscle afferent fibres to the lower medulla and upper spinal cord: an anatomical study in the cat with the transganglionic transport method. J Comp Neurol. 1984 Nov 20;230(1):99–109. doi: 10.1002/cne.902300109. [DOI] [PubMed] [Google Scholar]
- Pierau F. K., Fellmer G., Taylor D. C. Somato-visceral convergence in cat dorsal root ganglion neurones demonstrated by double-labelling with fluorescent tracers. Brain Res. 1984 Oct 29;321(1):63–70. doi: 10.1016/0006-8993(84)90681-4. [DOI] [PubMed] [Google Scholar]
- Tattersall J. E., Cervero F., Lumb B. M. Effects of reversible spinalization on the visceral input to viscerosomatic neurons in the lower thoracic spinal cord of the cat. J Neurophysiol. 1986 Sep;56(3):785–796. doi: 10.1152/jn.1986.56.3.785. [DOI] [PubMed] [Google Scholar]


