Abstract
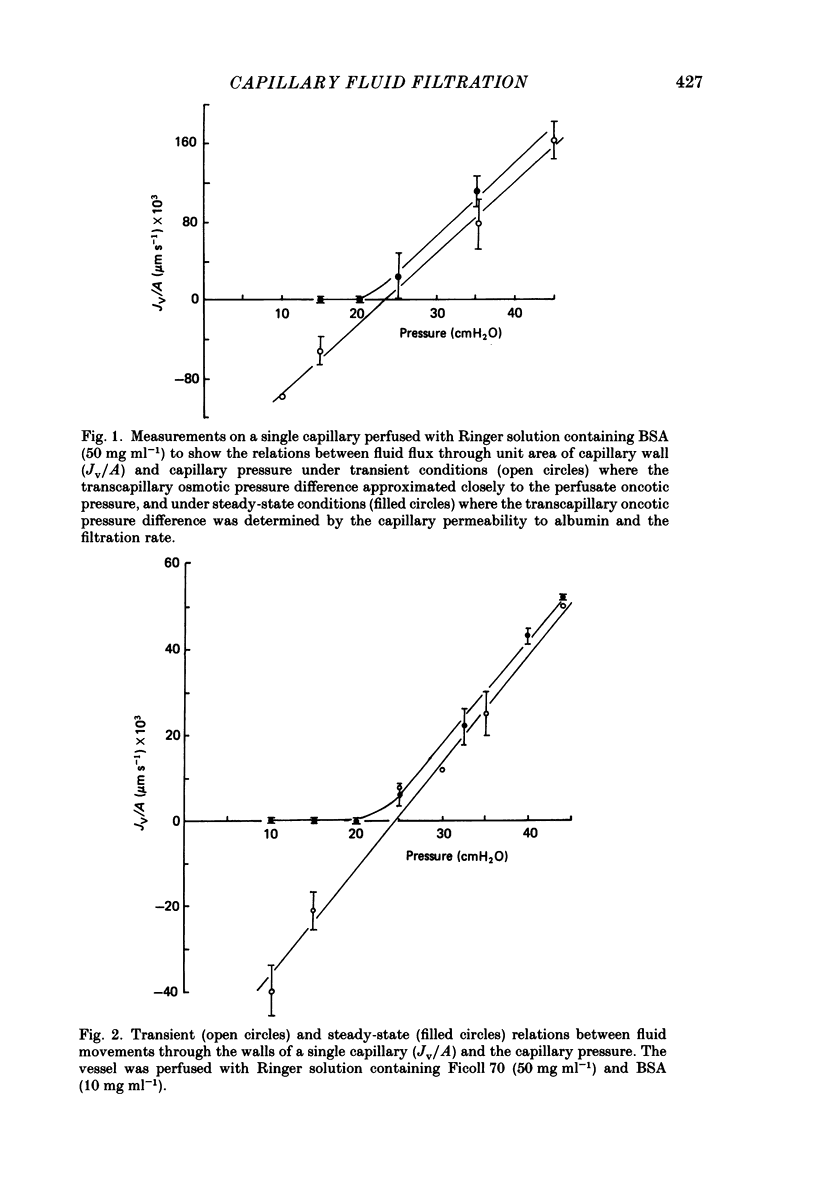
1. The theory of steady-state filtration through capillary walls (Michel, 1984) has been developed and investigated in experiments on single capillaries of the frog mesentery perfused with Ringer solutions containing bovine serum albumin (BSA) and Ficoll 70. 2. In each experiment, the micro-occlusion technique of Michel, Mason, Curry, Tooke & Hunter (1974) has been used to investigate the relation between fluid movements per unit area of capillary wall (Jv/A) and capillary pressure (Pc) under two sets of conditions in the same vessel. First, the relation has been determined following brief perfusions, where the difference in oncotic pressure across the capillary wall approximated to the perfusate oncotic pressure at all values of Pc. These results are referred to as the transient data. Secondly, the relation was investigated by estimating Jv/A at values of Pc which had been maintained constant during at least 2 min of perfusion prior to the measurement. Under these conditions the concentration of macromolecules in the pericapillary fluid was determined by the steady-state composition of the filtrate passing through the capillary wall, and these results are referred to as steady-state data. 3. In all fifteen capillaries investigated, the relationship between Jv/A and Pc was linear for the transient data and conspicuously non-linear in the steady state. When Pc exceeded the oncotic pressure of the perfusate, steady-state values for Jv/A lay slightly above but parallel to the transient values for the same vessel. At values of Pc less than the perfusate oncotic pressure, the transient data showed reabsorption of fluid from the tissues, but in the steady state either fluid movements were so small as to be undetected or slight filtration was observed. The steady-state data followed the pattern predicted by theory. 4. The transient data were used to estimate the reflection coefficient of the capillary wall (sigma) to the macromolecular solute. In seven vessels, the mean sigma to BSA was 0.76 (S.E. of mean +/- 0.04) and in eight different vessels mean sigma to Ficoll 70 in the presence of BSA (10 mg ml-1) was 0.98 (S.E. of mean +/- 0.05). The steady-state data were consistent with the prediction that the oncotic pressure opposing high filtration rates approximates to sigma 2 pi c in the steady state, where pi c is the perfusate oncotic pressure.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





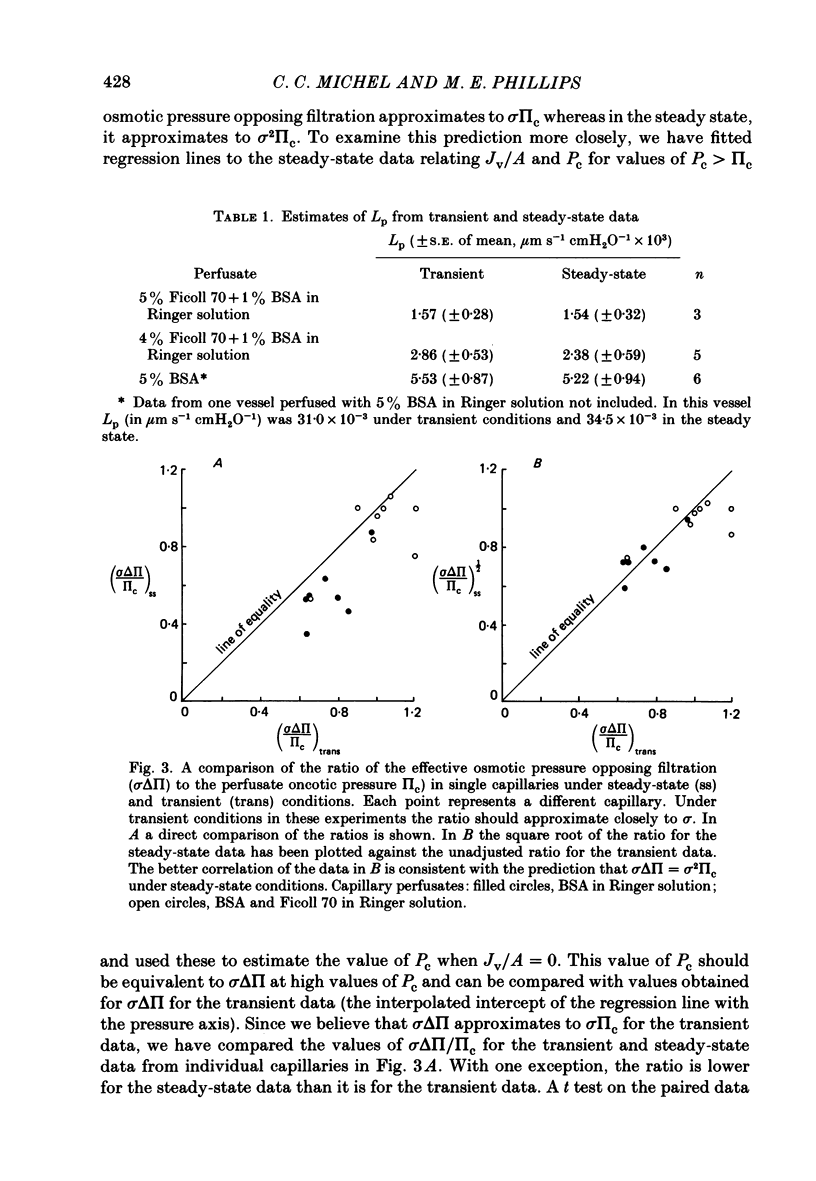

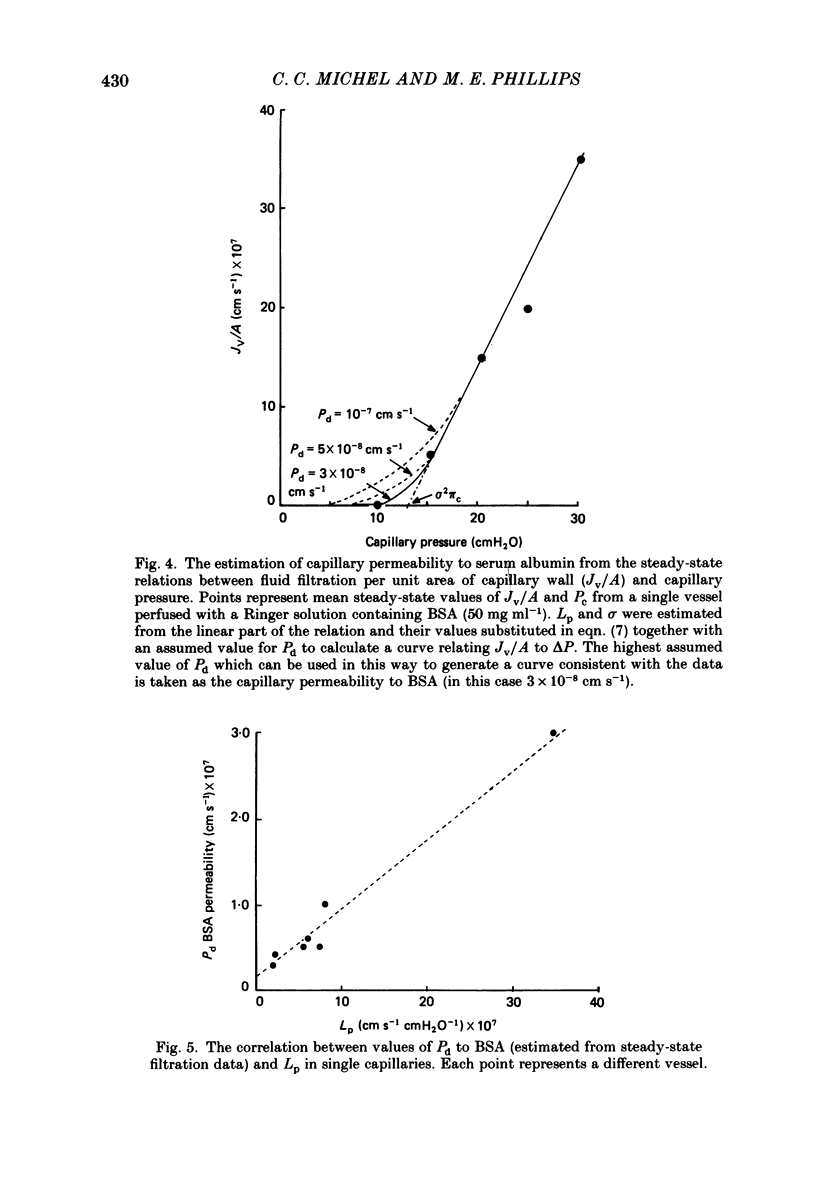


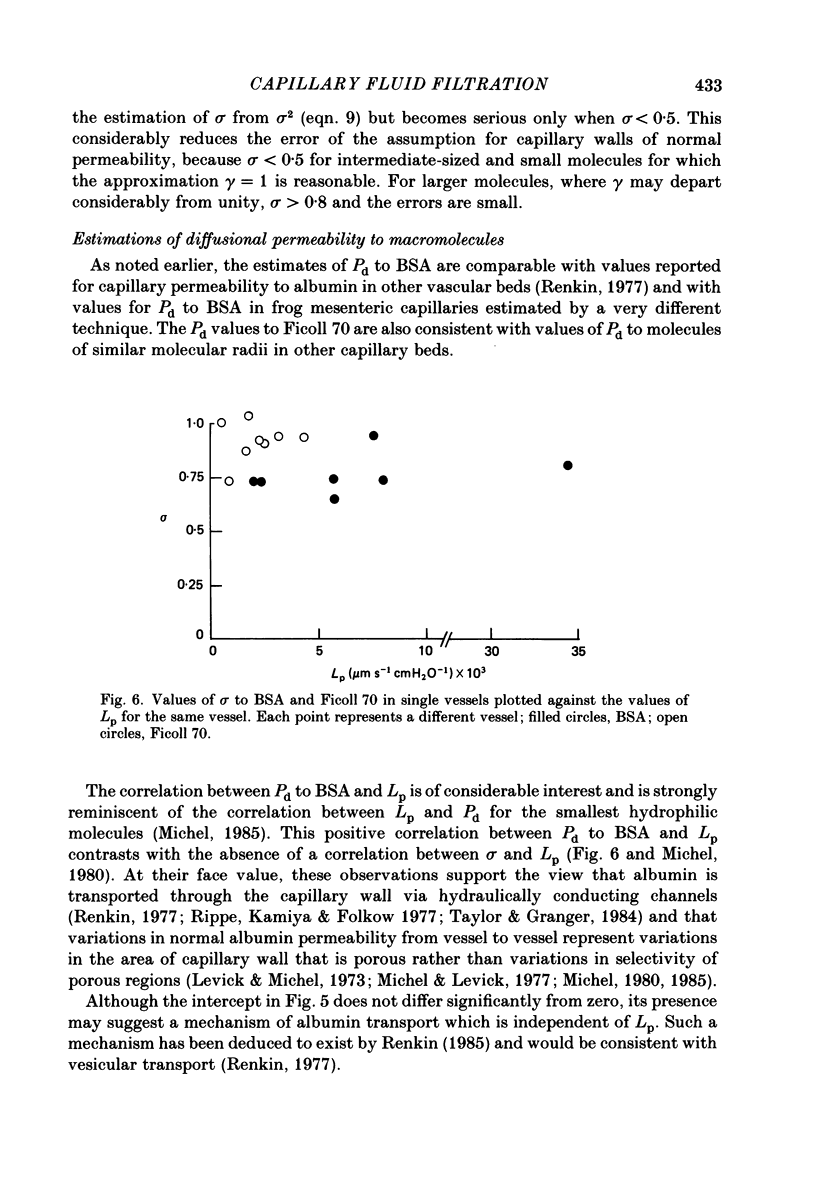


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bert J. L., Pinder K. L. An analog computer simulation showing the effect of volume exclusion on capillary fluid exchange. Microvasc Res. 1982 Jul;24(1):94–103. doi: 10.1016/0026-2862(82)90046-2. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Harding T. H. Response to the velocity of moving visual stimuli of the brisk classes of ganglion cells in the cat retina. J Physiol. 1983 Dec;345:47–63. doi: 10.1113/jphysiol.1983.sp014964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry F. E., Frøkjaer-Jensen J. Water flow across the walls of single muscle capillaries in the frog, Rana pipiens. J Physiol. 1984 May;350:293–307. doi: 10.1113/jphysiol.1984.sp015202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry F. E., Michel C. C., Mason J. C. Osmotic reflextion coefficients of capillary walls to low molecular weight hydrophilic solutes measured in single perfused capillaries of the frog mesentery. J Physiol. 1976 Oct;261(2):319–336. doi: 10.1113/jphysiol.1976.sp011561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUYTON A. C. A concept of negative interstitial pressure based on pressures in implanted perforated capsules. Circ Res. 1963 Apr;12:399–414. doi: 10.1161/01.res.12.4.399. [DOI] [PubMed] [Google Scholar]
- Granger H. J. Role of the interstitial matrix and lymphatic pump in regulation of transcapillary fluid balance. Microvasc Res. 1979 Sep;18(2):209–216. doi: 10.1016/0026-2862(79)90029-3. [DOI] [PubMed] [Google Scholar]
- HUGHES R., MAY A. J., WIDDICOMBE J. G. Mechanical factors in the formation of oedema in perfused rabbits' lungs. J Physiol. 1958 Jul 14;142(2):292–305. doi: 10.1113/jphysiol.1958.sp006016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight A. D., Levick J. R. Effect of fluid pressure on the hydraulic conductance of interstitium and fenestrated endothelium in the rabbit knee. J Physiol. 1985 Mar;360:311–332. doi: 10.1113/jphysiol.1985.sp015619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh A., Landis E. M., Turner A. H. THE MOVEMENT OF FLUID THROUGH THE HUMAN CAPILLARY WALL IN RELATION TO VENOUS PRESSURE AND TO THE COLLOID OSMOTIC PRESSURE OF THE BLOOD. J Clin Invest. 1932 Jan;11(1):63–95. doi: 10.1172/JCI100408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick J. R., Michel C. C. The permeability of individually perfused frog mesenteric capillaries to T1824 and T1824-albumin as evidence for a large pore system. Q J Exp Physiol Cogn Med Sci. 1973 Jan;58(1):67–85. doi: 10.1113/expphysiol.1973.sp002192. [DOI] [PubMed] [Google Scholar]
- Lunde P. K., Waaler B. A. Transvascular fluid balance in the lung. J Physiol. 1969 Nov;205(1):1–18. doi: 10.1113/jphysiol.1969.sp008947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason J. C., Curry F. E., Michel C. C. The effects of proteins upon the filtration coefficient of individually perfused frog mesenteric capillaries. Microvasc Res. 1977 Mar;13(2):185–202. doi: 10.1016/0026-2862(77)90084-x. [DOI] [PubMed] [Google Scholar]
- Michel C. C. Filtration coefficients and osmotic reflexion coefficients of the walls of single frog mesenteric capillaries. J Physiol. 1980 Dec;309:341–355. doi: 10.1113/jphysiol.1980.sp013512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel C. C., Levick J. R. Variations in permeability along individually perfused capillaries of the frog mesentery. Q J Exp Physiol Cogn Med Sci. 1977 Jan;62(1):1–10. doi: 10.1113/expphysiol.1977.sp002369. [DOI] [PubMed] [Google Scholar]
- Michel C. C., Mason J. C., Curry F. E., Tooke J. E., Hunter P. J. A development of the Landis technique for measuring the filtration coefficient of individual capillaries in the frog mesentery. Q J Exp Physiol Cogn Med Sci. 1974 Oct;59(4):283–309. doi: 10.1113/expphysiol.1974.sp002275. [DOI] [PubMed] [Google Scholar]
- Michel C. C., Phillips M. E. The effects of bovine serum albumin and a form of cationised ferritin upon the molecular selectivity of the walls of single frog capillaries. Microvasc Res. 1985 Mar;29(2):190–203. doi: 10.1016/0026-2862(85)90016-0. [DOI] [PubMed] [Google Scholar]
- Michel C. C., Phillips M. E., Turner M. R. The effects of native and modified bovine serum albumin on the permeability of frog mesenteric capillaries. J Physiol. 1985 Mar;360:333–346. doi: 10.1113/jphysiol.1985.sp015620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel C. C. The Malpighi lecture. Vascular permeability--the consequence of Malpighi's hypothesis. Int J Microcirc Clin Exp. 1985;4(3):265–284. [PubMed] [Google Scholar]
- Patlak C. S., Goldstein D. A., Hoffman J. F. The flow of solute and solvent across a two-membrane system. J Theor Biol. 1963 Nov;5(3):426–442. doi: 10.1016/0022-5193(63)90088-2. [DOI] [PubMed] [Google Scholar]
- Renkin E. M. Capillary transport of macromolecules: pores and other endothelial pathways. J Appl Physiol (1985) 1985 Feb;58(2):315–325. doi: 10.1152/jappl.1985.58.2.315. [DOI] [PubMed] [Google Scholar]
- Renkin E. M. Multiple pathways of capillary permeability. Circ Res. 1977 Dec;41(6):735–743. doi: 10.1161/01.res.41.6.735. [DOI] [PubMed] [Google Scholar]
- Rippe B., Kamiya A., Folkow B. Is capillary micropinocytosis of any significance for the transcapillary transfer of plasma proteins? Acta Physiol Scand. 1977 Jun;100(2):258–260. doi: 10.1111/j.1748-1716.1977.tb05945.x. [DOI] [PubMed] [Google Scholar]
- Starling E. H. On the Absorption of Fluids from the Connective Tissue Spaces. J Physiol. 1896 May 5;19(4):312–326. doi: 10.1113/jphysiol.1896.sp000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. E. Capillary fluid filtration. Starling forces and lymph flow. Circ Res. 1981 Sep;49(3):557–575. doi: 10.1161/01.res.49.3.557. [DOI] [PubMed] [Google Scholar]
- Wiederhielm C. A. Dynamics of capillary fluid exchange: a nonlinear computer simulation. Microvasc Res. 1979 Jul;18(1):48–82. doi: 10.1016/0026-2862(79)90017-7. [DOI] [PubMed] [Google Scholar]


