Abstract
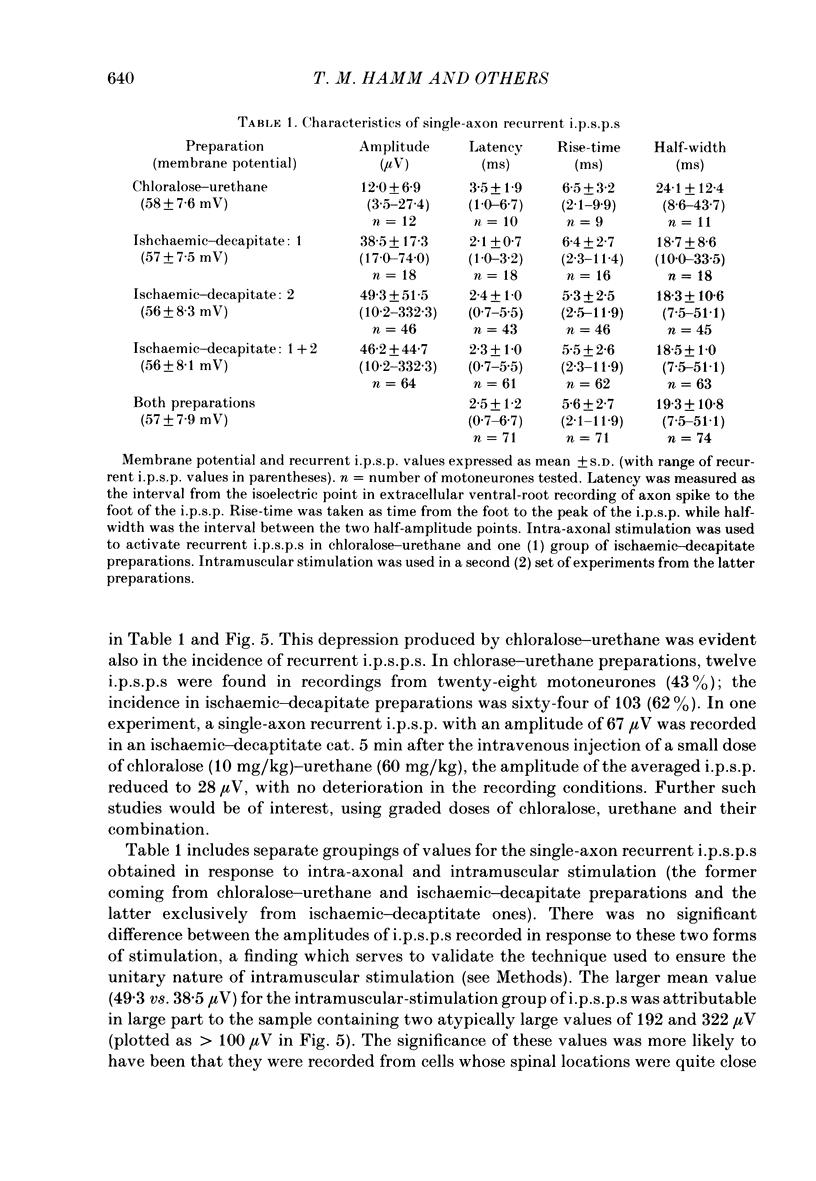
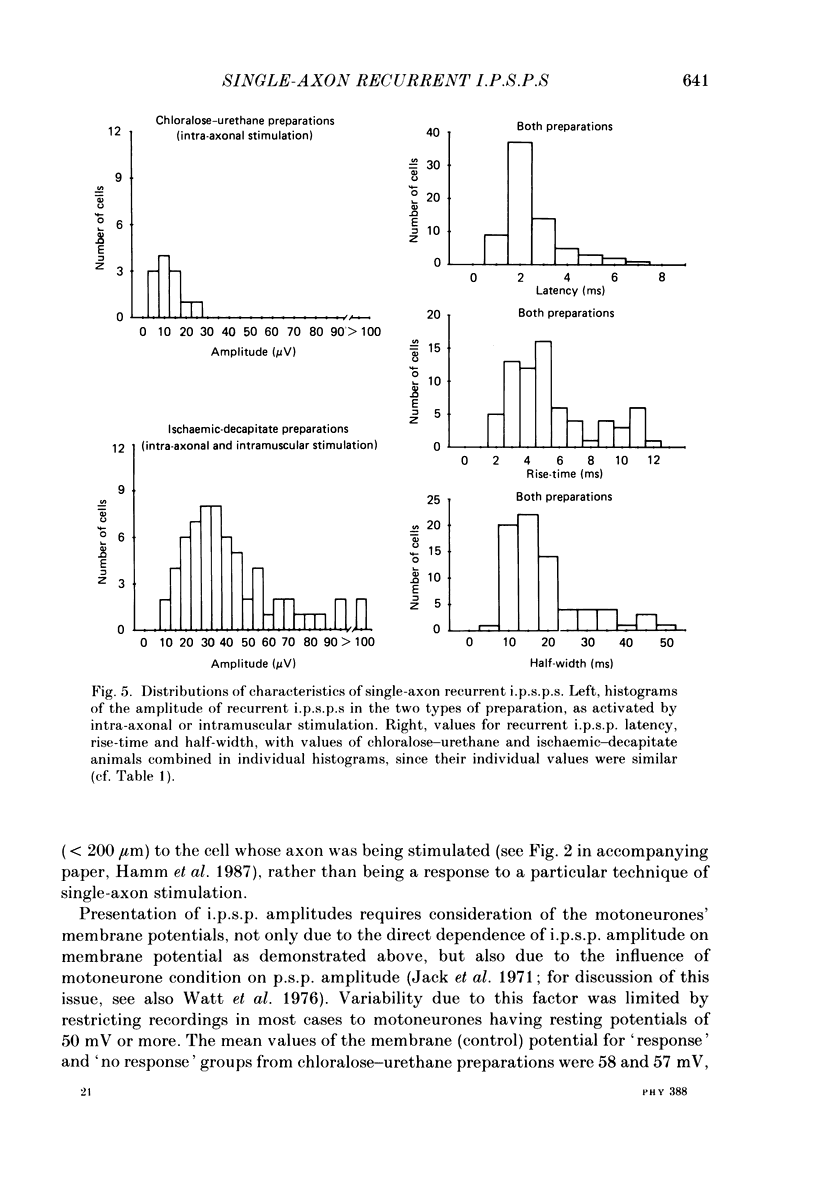
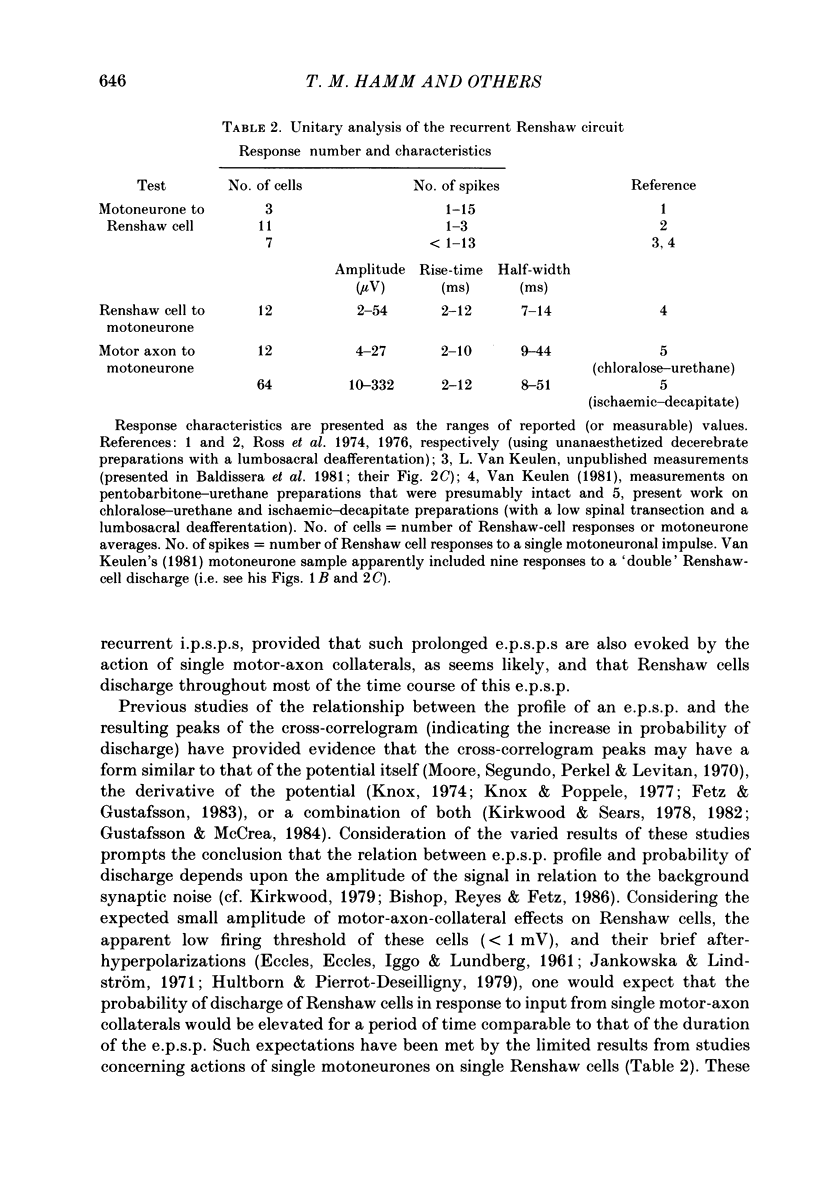
1. Signal averaging was used in forty experiments on low-spinal cats to measure and characterize the oligosynaptic responses of seventy-six motoneurons supplying the medial gastrocnemius muscle to the single impulses of antidromically stimulated single motor axons supplying the same muscle. 2. In thirteen experiments on chloralose-urethane anaesthetized preparations, twelve (43%) of the tested twenty-eight motoneurones exhibited a single-axon recurrent inhibitory post-synaptic potential (recurrent i.p.s.p.), as compared to sixty-four (62%) of the 103 motoneurones tested in twenty-seven animals in the absence of anaesthetic after ischaemic decapitation. 3. Single-axon recurrent i.p.s.p.s most often consisted of a single, long-lasting hyperpolarization. Ten of the recurrent i.p.s.p.s contained a second late peak of hyperpolarization. In another eight of the i.p.s.p.s, a small late depolarization was evident. 4. The distinct profiles of the recurrent i.p.s.p.s were readily distinguished from the relatively flat profiles with low noise levels in the averages of the fifty-five 'no-response' cells. The transmembrane and post-synaptic nature of the i.p.s.p.s was confirmed by extracellular control recordings taken immediately outside seven of the cells with positive responses. In addition, ten cells with positive responses were subjected to current passage during the averaging procedure. In all cases, depolarization increased and hyperpolarization reduced the amplitude of their single-axon recurrent i.p.s.p.s. 5. The mean amplitude of the responses was 12.0 microV in chloralose-urethane preparations as compared to a peak-to-peak noise level less than 6.0 microV in the no-response averages. Corresponding values in ischaemic-decapitate preparations were 46.2 microV and less than 7.5 microV, respectively. 6. Latency, rise-time and half-width (i.e. duration at half-amplitude) values of the i.p.s.p.s were similar for chloralose-urethane and ischaemic-decapitate preparations. The average values in both preparations were 2.5, 5.6 and 19.3 ms, respectively. The latency values indicated both disynaptic and, perhaps, longer components in the recurrent i.p.s.p.s. The rise-time and half-width values were relatively similar to those reported or measured from published records for analogous composite recurrent i.p.s.p.s (i.e. responses to antidromic stimulation of the whole muscle nerve rather than single motor axons). A weak, but significant, correlation between rise-time and half-width was observed for the sixty-six single-axon recurrent i.p.s.p.s with a single negative-going peak.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


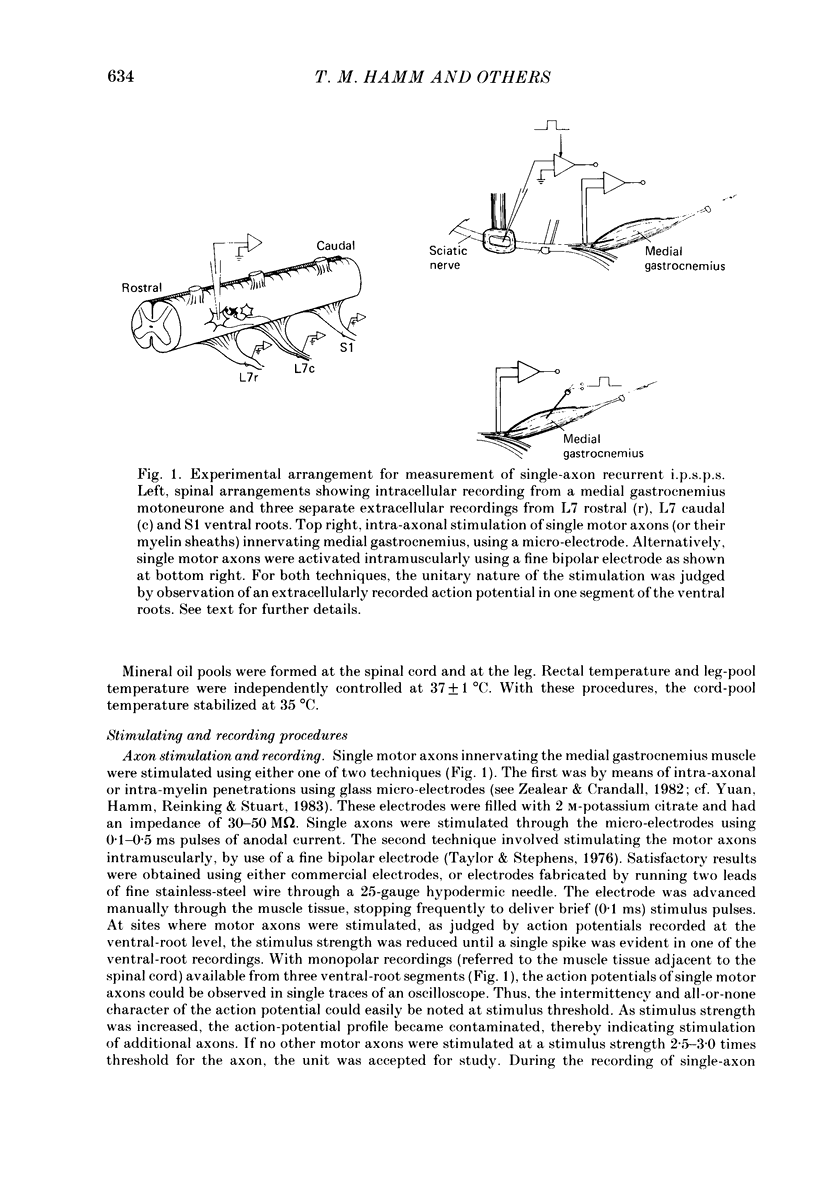


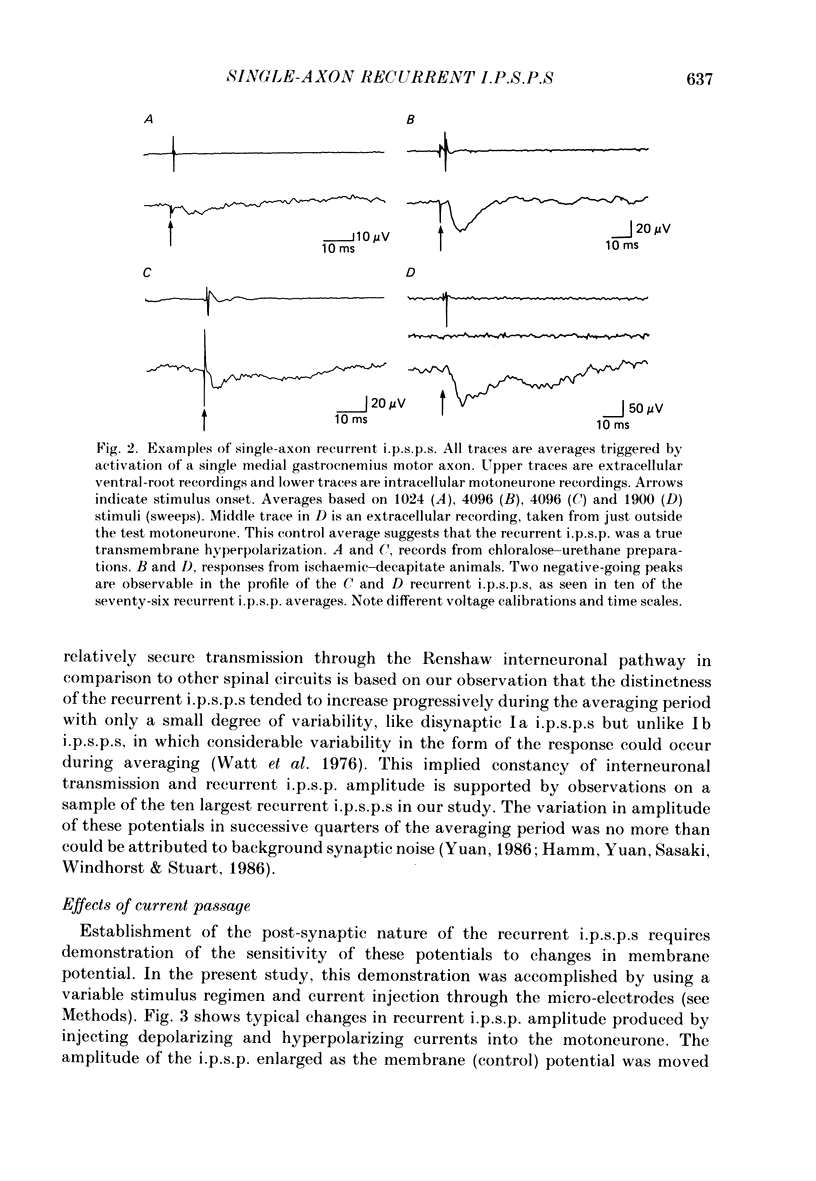
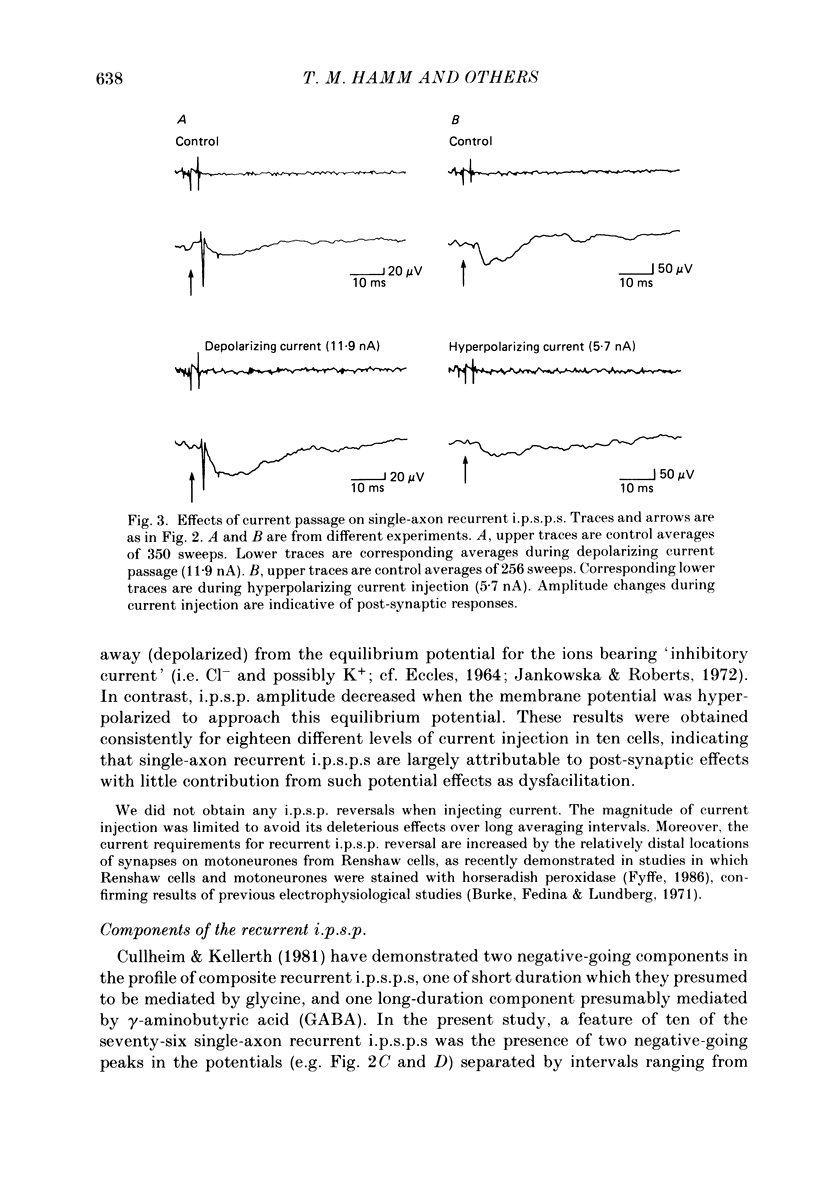
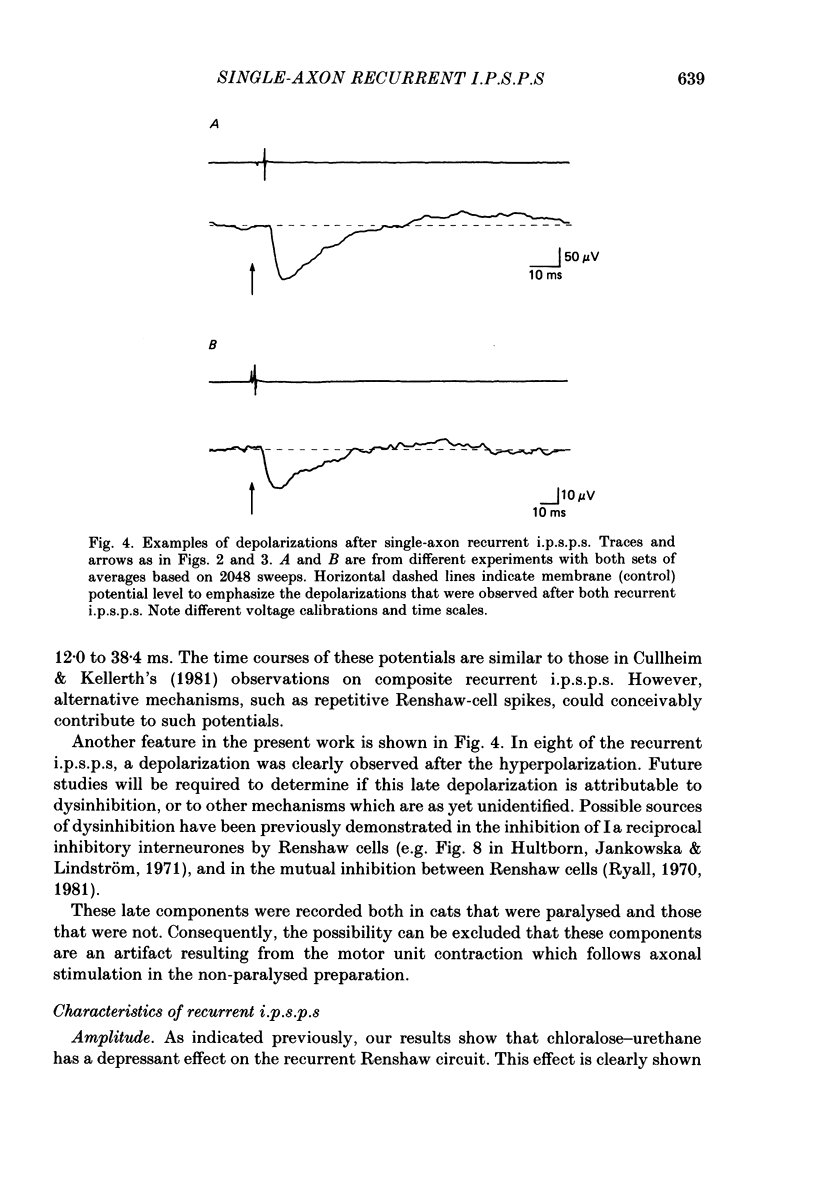









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger A. J., Averill D. B. Projection of single pulmonary stretch receptors to solitary tract region. J Neurophysiol. 1983 Mar;49(3):819–830. doi: 10.1152/jn.1983.49.3.819. [DOI] [PubMed] [Google Scholar]
- Botterman B. R., Hamm T. M., Reinking R. M., Stuart D. G. Localization of monosynaptic Ia excitatory post-synaptic potentials in the motor nucleus of the cat biceps femoris muscle. J Physiol. 1983 May;338:355–377. doi: 10.1113/jphysiol.1983.sp014677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink E., Harrison P. J., Jankowska E., McCrea D. A., Skoog B. Post-synaptic potentials in a population of motoneurones following activity of single interneurones in the cat. J Physiol. 1983 Oct;343:341–359. doi: 10.1113/jphysiol.1983.sp014896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Fedina L., Lundberg A. Spatial synaptic distribution of recurrent and group Ia inhibitory systems in cat spinal motoneurones. J Physiol. 1971 Apr;214(2):305–326. doi: 10.1113/jphysiol.1971.sp009434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Tsairis P., Zajac F. E., 3rd Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973 Nov;234(3):723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullheim S., Kellerth J. O., Conradi S. Evidence for direct synaptic interconnections between cat spinal alpha-motoneurons via the recurrent axon collaterals: a morphological study using intracellular injection of horseradish peroxidase. Brain Res. 1977 Aug 19;132(1):1–10. doi: 10.1016/0006-8993(77)90702-8. [DOI] [PubMed] [Google Scholar]
- Cullheim S., Kellerth J. O. Two kinds of recurrent inhibition of cat spinal alpha-motoneurones as differentiated pharmacologically. J Physiol. 1981 Mar;312:209–224. doi: 10.1113/jphysiol.1981.sp013624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., IGGO A., LUNDBERG A. Electrophysiological investigations on Renshaw cells. J Physiol. 1961 Dec;159:461–478. doi: 10.1113/jphysiol.1961.sp006821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., FATT P., KOKETSU K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. J Physiol. 1954 Dec 10;126(3):524–562. doi: 10.1113/jphysiol.1954.sp005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedina L., Hultborn H. Facilitation from ipsilateral primary afferents of interneuronal transmission in the Ia inhibitory pathway to motoneurones. Acta Physiol Scand. 1972 Sep;86(1):59–81. doi: 10.1111/j.1748-1716.1972.tb00225.x. [DOI] [PubMed] [Google Scholar]
- Fedina L., Hultborn H., Illert M. Facilitation from contralateral primary afferents of interneuronal transmission in the Ia inhibitory pathway to motoneurones. Acta Physiol Scand. 1975 Jun;94(2):198–221. doi: 10.1111/j.1748-1716.1975.tb05880.x. [DOI] [PubMed] [Google Scholar]
- Fetz E. E., Cheney P. D., German D. C. Corticomotoneuronal connections of precentral cells detected by postspike averages of EMG activity in behaving monkeys. Brain Res. 1976 Sep 24;114(3):505–510. doi: 10.1016/0006-8993(76)90973-2. [DOI] [PubMed] [Google Scholar]
- Fetz E. E., Gustafsson B. Relation between shapes of post-synaptic potentials and changes in firing probability of cat motoneurones. J Physiol. 1983 Aug;341:387–410. doi: 10.1113/jphysiol.1983.sp014812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel A. S., Redman S. J. The synaptic current evoked in cat spinal motoneurones by impulses in single group 1a axons. J Physiol. 1983 Sep;342:615–632. doi: 10.1113/jphysiol.1983.sp014872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B., McCrea D. Influence of stretch-evoked synaptic potentials on firing probability of cat spinal motoneurones. J Physiol. 1984 Feb;347:431–451. doi: 10.1113/jphysiol.1984.sp015074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAASE J., van der MEULEN J. [The specific effect of chloralose on recurrent inhibition of tonic motor neurons]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1961;274:272–280. doi: 10.1007/BF00362318. [DOI] [PubMed] [Google Scholar]
- Hamm T. M., Sasaki S., Stuart D. G., Windhorst U., Yuan C. S. Distribution of single-axon recurrent inhibitory post-synaptic potentials in a single spinal motor nucleus in the cat. J Physiol. 1987 Jul;388:653–664. doi: 10.1113/jphysiol.1987.sp016636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H., Jankowska E., Lindström S. Relative contribution from different nerves to recurrent depression of Ia IPSPs in motoneurones. J Physiol. 1971 Jul;215(3):637–664. doi: 10.1113/jphysiol.1971.sp009489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H., Jankowska E., Lindström S., Roberts W. Neuronal pathway of the recurrent facilitation of motoneurones. J Physiol. 1971 Oct;218(2):495–514. doi: 10.1113/jphysiol.1971.sp009630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H., Pierrot-Deseilligny E. Input-output relations in the pathway of recurrent inhibition to motoneurones in the cat. J Physiol. 1979 Dec;297(0):267–287. doi: 10.1113/jphysiol.1979.sp013039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack J. J., Miller S., Porter R., Redman S. J. The time course of minimal excitory post-synaptic potentials evoked in spinal motoneurones by group Ia afferent fibres. J Physiol. 1971 Jun;215(2):353–380. doi: 10.1113/jphysiol.1971.sp009474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E., Lindström S. Morphological identification of Renshaw cells. Acta Physiol Scand. 1971 Mar;81(3):428–430. doi: 10.1111/j.1748-1716.1971.tb04918.x. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Lundberg A., Rudomin P., Sykova E. Effects of 4-aminopyridine on transmission in excitatory and inhibitory synapses in the spinal cord. Brain Res. 1977 Nov 11;136(2):387–392. doi: 10.1016/0006-8993(77)90816-2. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Roberts W. J. Synaptic actions of single interneurones mediating reciprocal Ia inhibition of motoneurones. J Physiol. 1972 May;222(3):623–642. doi: 10.1113/jphysiol.1972.sp009818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasser R. J., Cheney P. D. Characteristics of corticomotoneuronal postspike facilitation and reciprocal suppression of EMG activity in the monkey. J Neurophysiol. 1985 Apr;53(4):959–978. doi: 10.1152/jn.1985.53.4.959. [DOI] [PubMed] [Google Scholar]
- Khatib M., Hilaire G., Monteau R. Excitatory interactions between phrenic motoneurons in the cat. Exp Brain Res. 1986;62(2):273–280. doi: 10.1007/BF00238846. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A. On the use and interpretation of cross-correlations measurements in the mammalian central nervous system. J Neurosci Methods. 1979 Aug;1(2):107–132. doi: 10.1016/0165-0270(79)90009-8. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. Excitatory post-synaptic potentials from single muscle spindle afferents in external intercostal motoneurones of the cat. J Physiol. 1982 Jan;322:287–314. doi: 10.1113/jphysiol.1982.sp014038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. The synaptic connexions to intercostal motoneurones as revealed by the average common excitation potential. J Physiol. 1978 Feb;275:103–134. doi: 10.1113/jphysiol.1978.sp012180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox C. K. Cross-correlation functions for a neuronal model. Biophys J. 1974 Aug;14(8):567–582. doi: 10.1016/S0006-3495(74)85936-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox C. K., Poppele R. E. Correlation analysis of stimulus-evoked changes in excitability of spontaneously firing neurons. J Neurophysiol. 1977 May;40(3):616–625. doi: 10.1152/jn.1977.40.3.616. [DOI] [PubMed] [Google Scholar]
- Lucas S. M., Cope T. C., Binder M. D. Analysis of individual Ia-afferent EPSPs in a homonymous motoneuron pool with respect to muscle topography. J Neurophysiol. 1984 Jan;51(1):64–74. doi: 10.1152/jn.1984.51.1.64. [DOI] [PubMed] [Google Scholar]
- Lüscher H. R., Ruenzel P., Fetz E., Henneman E. Postsynatpic population potentials recorded from ventral roots perfused with isotonic sucrose: connections of groups Ia and II spindle afferent fibers with large populations of motoneurons. J Neurophysiol. 1979 Jul;42(4):1146–1164. doi: 10.1152/jn.1979.42.4.1146. [DOI] [PubMed] [Google Scholar]
- Mendell L. M., Henneman E. Terminals of single Ia fibers: distribution within a pool of 300 homonymous motor neurons. Science. 1968 Apr 5;160(3823):96–98. doi: 10.1126/science.160.3823.96. [DOI] [PubMed] [Google Scholar]
- Moore G. P., Segundo J. P., Perkel D. H., Levitan H. Statistical signs of synaptic interaction in neurons. Biophys J. 1970 Sep;10(9):876–900. doi: 10.1016/S0006-3495(70)86341-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson J. B., Fleshman J. W., Sypert G. W. Properties of single-fiber spindle group II EPSPs in triceps surae motoneurons. J Neurophysiol. 1980 Oct;44(4):713–725. doi: 10.1152/jn.1980.44.4.713. [DOI] [PubMed] [Google Scholar]
- Rall W. Distinguishing theoretical synaptic potentials computed for different soma-dendritic distributions of synaptic input. J Neurophysiol. 1967 Sep;30(5):1138–1168. doi: 10.1152/jn.1967.30.5.1138. [DOI] [PubMed] [Google Scholar]
- Ryall R. W. Patterns of recurrent excitation and mutual inhibition of cat Renshaw cells. J Physiol. 1981 Jul;316:439–452. doi: 10.1113/jphysiol.1981.sp013799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryall R. W. Renshaw cell mediated inhibition of Renshaw cells: patterns of excitation and inhibition from impulses in motor axon collaterals. J Neurophysiol. 1970 Mar;33(2):257–270. doi: 10.1152/jn.1970.33.2.257. [DOI] [PubMed] [Google Scholar]
- Rymer W. Z., Houk J. C., Crago P. E. Mechanisms of the clasp-knife reflex studied in an animal model. Exp Brain Res. 1979 Sep;37(1):93–113. doi: 10.1007/BF01474257. [DOI] [PubMed] [Google Scholar]
- Smith T. G., Wuerker R. B., Frank K. Membrane impedance changes during synaptic transmission in cat spinal motoneurons. J Neurophysiol. 1967 Sep;30(5):1072–1096. doi: 10.1152/jn.1967.30.5.1072. [DOI] [PubMed] [Google Scholar]
- Stauffer E. K., Watt D. G., Taylor A., Reinking R. M., Stuart D. G. Analysis of muscle receptor connections by spike-triggered averaging. 2. Spindle group II afferents. J Neurophysiol. 1976 Nov;39(6):1393–1402. doi: 10.1152/jn.1976.39.6.1393. [DOI] [PubMed] [Google Scholar]
- Taylor A., Stephens J. A. Study of human motor unit contractions by controlled intramuscular microstimulation. Brain Res. 1976 Nov 26;117(2):331–335. doi: 10.1016/0006-8993(76)90742-3. [DOI] [PubMed] [Google Scholar]
- Van Keulen L. Autogenetic recurrent inhibition of individual spinal motoneurones of the cat. Neurosci Lett. 1981 Feb 6;21(3):297–300. doi: 10.1016/0304-3940(81)90220-2. [DOI] [PubMed] [Google Scholar]
- Walmsley B., Tracey D. J. An intracellular study of Renshaw cells. Brain Res. 1981 Oct 26;223(1):170–175. doi: 10.1016/0006-8993(81)90818-0. [DOI] [PubMed] [Google Scholar]
- Watt D. G., Stauffer E. K., Taylor A., Reinking R. M., Stuart D. G. Analysis of muscle receptor connections by spike-triggered averaging. 1. Spindle primary and tendon organ afferents. J Neurophysiol. 1976 Nov;39(6):1375–1392. doi: 10.1152/jn.1976.39.6.1375. [DOI] [PubMed] [Google Scholar]
- Winters W. D., Spooner C. E. A neurophysiological comparison of alpha-chloralose with gamma-hydroxybutyrate in cats. Electroencephalogr Clin Neurophysiol. 1966 Jan;20(1):83–90. doi: 10.1016/0013-4694(66)90144-1. [DOI] [PubMed] [Google Scholar]
- Zealear D. L., Crandall W. F. Stimulating and recording from axons within their myelin sheaths: a stable and nondamaging method for studying single motor units. J Neurosci Methods. 1982 Jan;5(1-2):47–54. doi: 10.1016/0165-0270(82)90050-4. [DOI] [PubMed] [Google Scholar]
- Zengel J. E., Reid S. A., Sypert G. W., Munson J. B. Membrane electrical properties and prediction of motor-unit type of medial gastrocnemius motoneurons in the cat. J Neurophysiol. 1985 May;53(5):1323–1344. doi: 10.1152/jn.1985.53.5.1323. [DOI] [PubMed] [Google Scholar]
- Zieglgänsberger W., Reiter C. A cholinergic mechanism in the spinal cord of cats. Neuropharmacology. 1974 Jun;13(6):519–527. doi: 10.1016/0028-3908(74)90141-5. [DOI] [PubMed] [Google Scholar]


