Abstract
Objective
To analyze the quantity and distribution of intramuscular nerves within the striated urogenital sphincter and test the hypothesis that decreased nerve density is associated with decreased striated sphincter muscle and cadaver age.
Methods
Thirteen cadaveric urethras (mean age 47 years, range 15–78 years) were selected for study. A sagittal histologic section was stained with S100 stain to identify intramuscular nerves. The number of times that a nerve was seen within the striated urogenital sphincter (nerve number) was counted. The number of axons within each nerve fascicle was also counted. Regression analysis of nerve density against muscle cell number and age was performed.
Results
Remarkable variation was found in the quantity of intramuscular nerves in the striated urogenital sphincter of the 13 urethras studied. The number of nerves ranged from 72 to 543, a sevenfold variation (mean 247.1 ± standard deviation 123.2), and the range of number of axons was 431 to 3523 (2201 ± 1152.6). The larger nerve fascicles were seen predominantly in the distal (13.1 ± 5.7 axons per nerve) compared with the proximal part of the striated urogenital sphincter (1.2 ± 2). Reduced nerve density throughout the striated urogenital sphincter correlated with fewer muscle cells (P = .02). Nerve density also decreased with advancing age (P = .004).
Conclusion
Remarkable variation in the quantity of intramuscular nerves was found. Women with sparse intramuscular nerves had fewer striated muscle cells. Intramuscular nerve density declined with age.
Stress urinary incontinence is a remarkably common condition yet the pathophysiology of this distressing problem is still unknown. A neurogenic hypothesis that stress urinary incontinence is related to pudendal nerve injury is based on abnormal conduction times,1 but a 2-millisecond conduction delay does not seem sufficient to explain significant dysfunction. If nerve injury were to compromise the continence mechanism, a most likely mechanism might be atrophy of sphincteric muscle secondary to denervation.
Urethral sphincteric strength is an important determinant of stress continence. The degree of sphincter weakness as evidenced by decreased maximum urethral closure pressure correlates with severity of stress incontinence.2 Poor sphincter function is associated with operative failure.3,4 It is not known whether poor sphincter function due to muscle loss within the striated urogenital sphincter correlates with reduced intramuscular nerves. Understanding the cause of sphincter weakness would further our understanding of the pathophysiology of stress urinary incontinence.
At present, however, there has been no direct histologic demonstration that there are differences among individuals in the amount of intramuscular nerves within the urethra. Furthermore it is not known whether a decrease in neural tissue within the urethra is correlated with a decrease in muscle cells.
This study of intramuscular nerves within the striated urogenital sphincter was undertaken to study the spatial distribution of intramuscular nerves along the striated urogenital sphincter; to examine interindividual variation in neural tissue; and to test the hypothesis that a decrease in nerve density within the urethra correlates with fewer striated muscle cells and with older age.
Materials and Methods
The urethra and its surrounding tissues were removed en bloc at postmortem examination from 34 female cadavers. Twenty-five samples were deemed suitable for histologic measurements and were processed. Among these samples, sufficient tissue for neural staining remained in the sagittal section block of 13 specimens. The mean age of the cadavers was 47 years (range 15–78 years, standard deviation [SD] ± 23). The specimens were harvested an average of 15.6 ± 6.1 (range 9–27) hours after death. Five samples were from nulliparous and seven were from parous women as assessed from patient charts, careful assessment of the cervix, and evidence of parturition seen in the perineal tissues (eg, episiotomy scar). In one woman, parity could not be established. None of the women had neurologic or muscular disease known to affect pelvic innervation.
The specimens were fixed by suspension in 10% buffered formalin and divided along the midsagittal plane. One half of the specimen was embedded in paraffin and used to produce a median sagittal section including the urethra and bladder base. The sections were stained with S100 stain specific to the nucleus and cytoplasm of Schwann cells of all neural tissue. This enabled identification of neural tissue within the urethra.
The striated urogenital sphincter muscle region of the ventral urethral wall was analyzed because its three component parts, the sphincter urethrae, the compressor urethrae, and the urethrovaginal sphincter, are all identifiable in this wall.5.
The region of striated urogenital sphincter was marked from the caudal margin of the detrusor loop to the caudal end of the striated muscle. This region was then divided into ten equal segments called “deciles” (Figure 1). The number of times a nerve section was seen in the striated urogenital sphincter was counted under 400× magnification. The total number of times a nerve section was seen within the striated urogenital sphincter was obtained by adding the counts obtained in every decile and is referred to as nerve count in this text. Similar counts for all of the axons (axon count) within nerve fascicles were made. All nerve fascicles greater than 3.8 μm or containing two or more axons were counted. Nerve fascicle diameter was measured on a video monitor screen and converted to actual measurements using a magnification factor of 23.3 cm on the screen = 100 μm. The minimum resolvable nerve fascicle diameter was 3.8 μm, therefore smaller fascicles were not counted, as that would have increased the counting error. Counting was done systematically from the outer edge of the striated urogenital sphincter to the lumen. The number of axons contained in each nerve fascicle, whether cut in axial, longitudinal, or oblique section was also counted.
Figure 1.
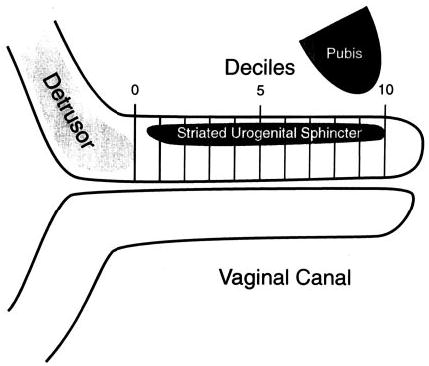
Urethral sagittal section with decile markings.
The density of axons and the density of nerves was calculated separately by dividing the nerve and axon counts by the cross-sectional area of tissue examined. To accomplish this, the length and width of each decile within the region of striated sphincter muscle were measured to the nearest 0.1 mm using the Vernier scale on the microscope stage. Their product was calculated to obtain the area of muscle in each decile. The nerve or axon count was divided by the area of the sampled region to obtain the density of intramuscular nerves.
Average nerve size, as determined by the number of axons per nerve, was derived by dividing the total nerve count by the total axon count in each decile. These data were then plotted per decile to display the average nerve size at different locations along the urethra.
Regression analyses were performed using a statistical program (Statview, Abacus Concepts, Berkeley, CA). Logarithmic regression was used to test the hypothesis of a relationship between nerve density and striated muscle cell number. These data were obtained from 11 specimens, and linear regression was used to test the hypothesis of a relationship between nerve density and cadaver age. P values less than .05 were considered statistically significant.
Results
The nerve count in the striated urogenital sphincter of all 13 urethras varied from 72 to 543 (247.1 ± 123.2), a sevenfold variation. The corresponding axon count ranged from 431 to 3523 (2201 ± 1152.6). The average nerve count and density in each decile for this group of urethral specimens is shown in Figure 2. Nerve number and nerve density show similar trends along the urethra. However, in the distal urethra fewer but more densely packed nerves were found. A similar finding was seen for axon number and density (Figure 3).
Figure 2.
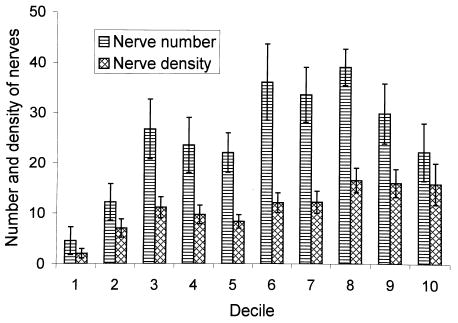
Regional variation in nerve number and nerve density with each decile of the urethral length in the striated urogenital sphincter muscle.
Figure 3.
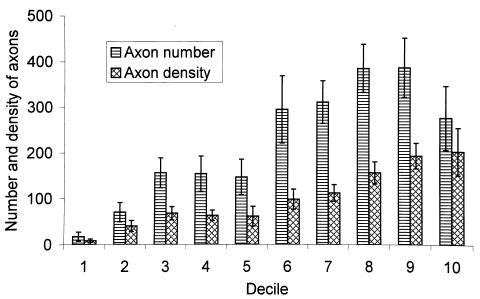
Regional variation in axon number and axon density with each decile of the urethral length in the striated urogenital sphincter muscle.
The nerves with most axons were found in the distal part of the striated urogenital sphincter (13.1 ± 5.7) with decreased nerve size proximally (1.2 ± 2) (Figure 4). The experimental hypothesis was supported in that nerve density in the striated urogenital sphincter and muscle cell numbers showed a highly significant relationship (r = .69; P = .02), (Figure 5). A significant decrease in nerve density was found with older age (r = −.73; P = .004) (Figure 6).
Figure 4.
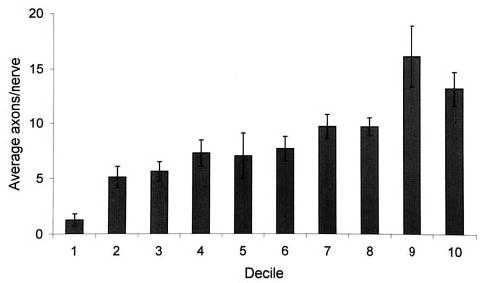
Frequency distribution showing regional variations in average nerve size based on the number of axons per nerve.
Figure 5.
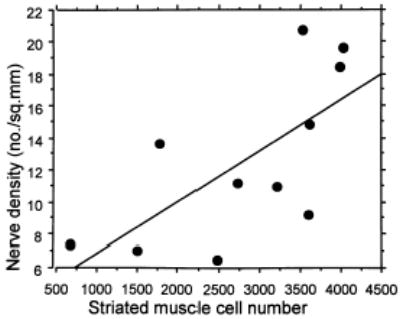
Linear regression analysis between nerve density and striated muscle cell number.
Figure 6.
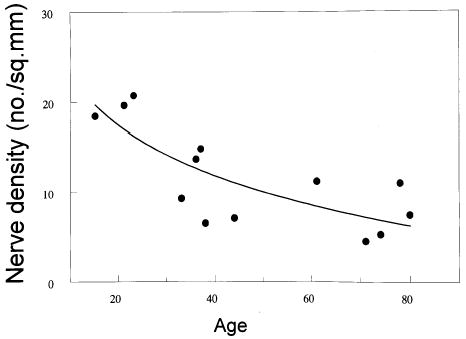
Logarithmic regression analysis between nerve density and cadaver age.
Discussion
The results of the present study support the hypothesis that decreased intramuscular nerve density in the urethra correlates with decreased muscular tissue, providing a plausible association between nerve injury and muscle loss. This finding supports the neurogenic hypothesis for stress urinary incontinence.
We found considerable variation in the amount of neural tissue present in a group of cadaver urethras as well as an age-related trend toward fewer intramuscular nerves within the striated urogenital sphincter.
There are many other factors yet to be determined that might help explain this finding. For example, we found an association between age and loss of neural tissue within the urethra. Many factors likely contribute to this age-related loss, including parity, disease, disuse, prior surgery, and genetic and environmental factors. Now that we have developed a quantitative scheme for analyzing urethral nerves and axons, further studies of urethral innervation in physiologic and pathologic states can be conducted. Furthermore, understanding the locations and patterns of nerve loss can help the clinician select an appropriate electrophysiologic technique to study the neural function of the striated urogenital sphincter in vivo.
The nature of the nerve supply to the urethra has not been resolved. There has been little information regarding the distribution of the nerves once they have reached the urethra, although the autonomic innervation of smooth muscle in the proximal urethra and bladder neck has been determined.6 A dual innervation has been proposed.7,8 Our finding that the largest nerves in the striated urogenital sphincter arrive at the distal urethra in the region of the urogenital diaphragm supports the pudendal nerve as the source of nerve supply to the striated urogenital sphincter. The present study did not identify the nerve supply to the smooth muscle in other portions and did not exclude the possibility that nerves from the cranial end of the urethra, near the bladder neck, might also be present.
More recently, a detailed gross and histologic neuroanatomic study demonstrating an intrapelvic somatic pathway derived from S2, S3, and S4 sacral roots, distinct from the peripheral pudendal nerve, supplying the levator ani and the urethra was performed.9 Our findings extend those studies by examining the nerve tissue within the urethra itself.
This study has limitations. First, the specimen sample was small; therefore, it might be premature to extrapolate the results of this study to the general population. Next, the specimens were collected by a physician who performed the urethral dissection when the opportunity arose between clinical duties, so they were not consecutive autopsies and do not belong to any particular series. Power analyses were not done for this explorative study but should be an important factor in the design of future studies of this kind. The histologic technique required special immunohistochemical staining but did not involve plastic embedding of sections, which would have increased the accuracy of the results. The axons were seen in axial, longitudinal, and oblique sections within nerve fascicles. Maintaining uniformity in counting all the axons in all the specimens minimized counting error. Despite these limitations, this study provides a logical step toward future more detailed, quantitative analyses of urethral innervation.
Footnotes
Funded by the National Institutes of Health grants RO1 DK 47516 and RO1 DK 51405
References
- 1.Snooks SJ, Badenoch DF, Tiptaft RC, Swash M. Perineal nerve damage in genuine stress urinary incontinence. An electrophysiological study. Br J Urol. 1985;57:422–6. doi: 10.1111/j.1464-410x.1985.tb06302.x. [DOI] [PubMed] [Google Scholar]
- 2.Hilton P, Stanton SL. Urethral pressure measurement by microtransducer: The results in symptom-free and in those with genuine stress incontinence. Br J Obstet Gynecol. 1983;90:919–33. doi: 10.1111/j.1471-0528.1983.tb06764.x. [DOI] [PubMed] [Google Scholar]
- 3.McGuire EJ. Urodynamic findings in patients after failure of stress incontinence operations. Prog Clin Biol Res. 1981;78:351–60. [PubMed] [Google Scholar]
- 4.Bowen LW, Sand PK, Ostergard DR, Franti CE. Unsuccessful Burch retropubic urethropexy: A case controlled study. Am J Obstet Gynecol. 1989;160:452–8. doi: 10.1016/0002-9378(89)90471-7. [DOI] [PubMed] [Google Scholar]
- 5.Oelrich TM. The striated urogenital sphincter muscle in the female. Anat Rec. 1983;205:223–32. doi: 10.1002/ar.1092050213. [DOI] [PubMed] [Google Scholar]
- 6.Gosling JA, Dixon JS, Lendon RG. The autonomic innervation of the human male and female bladder neck and proximal urethra. J Urol. 1977;118:302–5. doi: 10.1016/s0022-5347(17)57981-1. [DOI] [PubMed] [Google Scholar]
- 7.Elbadawi A, Schenk EA. A new theory of the innervation of bladder musculature. J Urol. 1974;111:613–5. doi: 10.1016/s0022-5347(17)60028-4. [DOI] [PubMed] [Google Scholar]
- 8.DeLancey JOL. Correlative study of paraurethral anatomy. Obstet Gynecol. 1986;68:91–7. [PubMed] [Google Scholar]
- 9.Borirakchanyavat S, Aboseif R, Carroll PR, Tanagho EA, Lue TF. Continence mechanism of the isolated female urethra: An anatomic study of the intrapelvic somatic nerves. J Urol. 1997;158:822–6. doi: 10.1097/00005392-199709000-00035. [DOI] [PubMed] [Google Scholar]


