Abstract
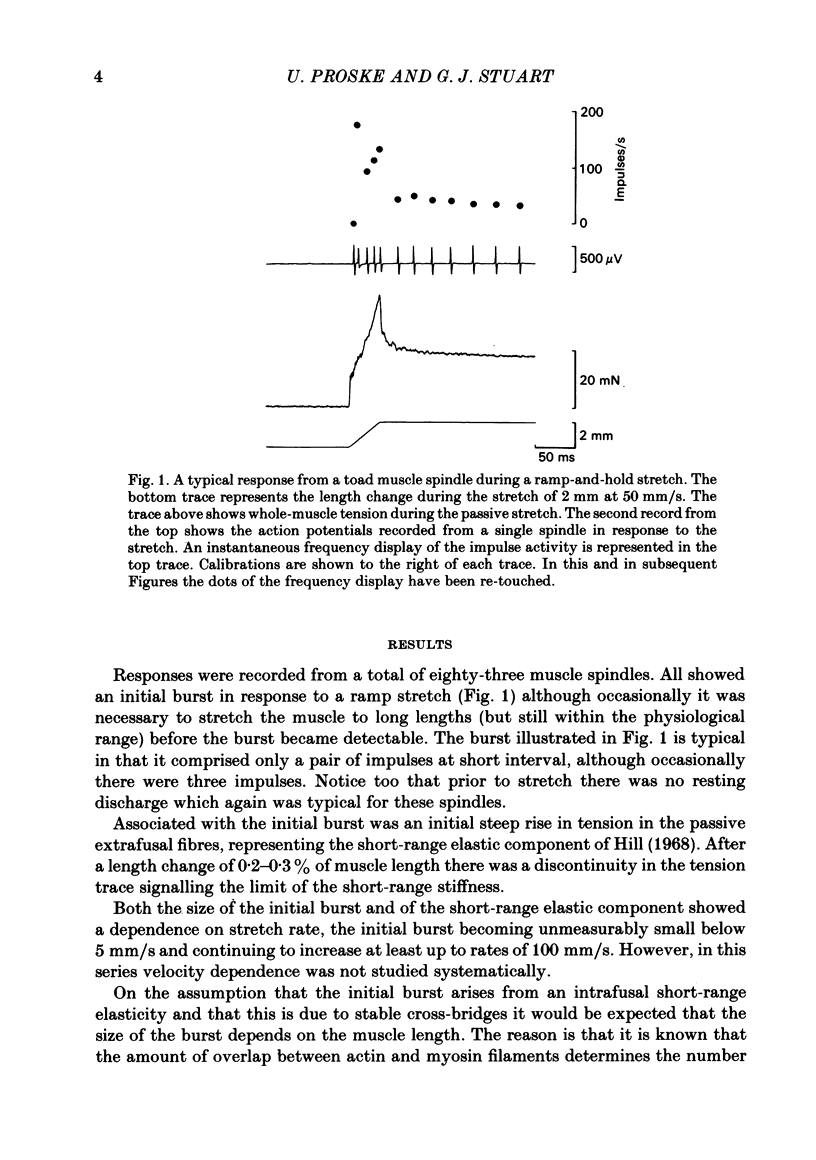
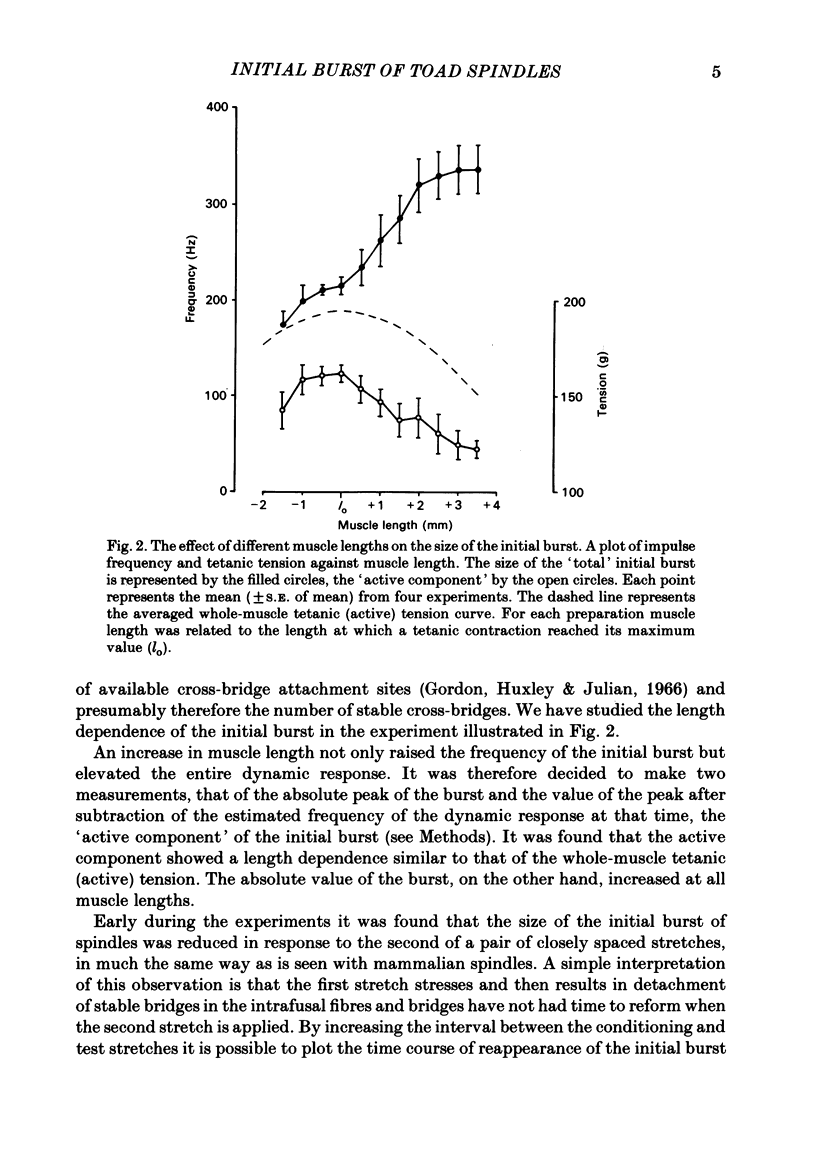
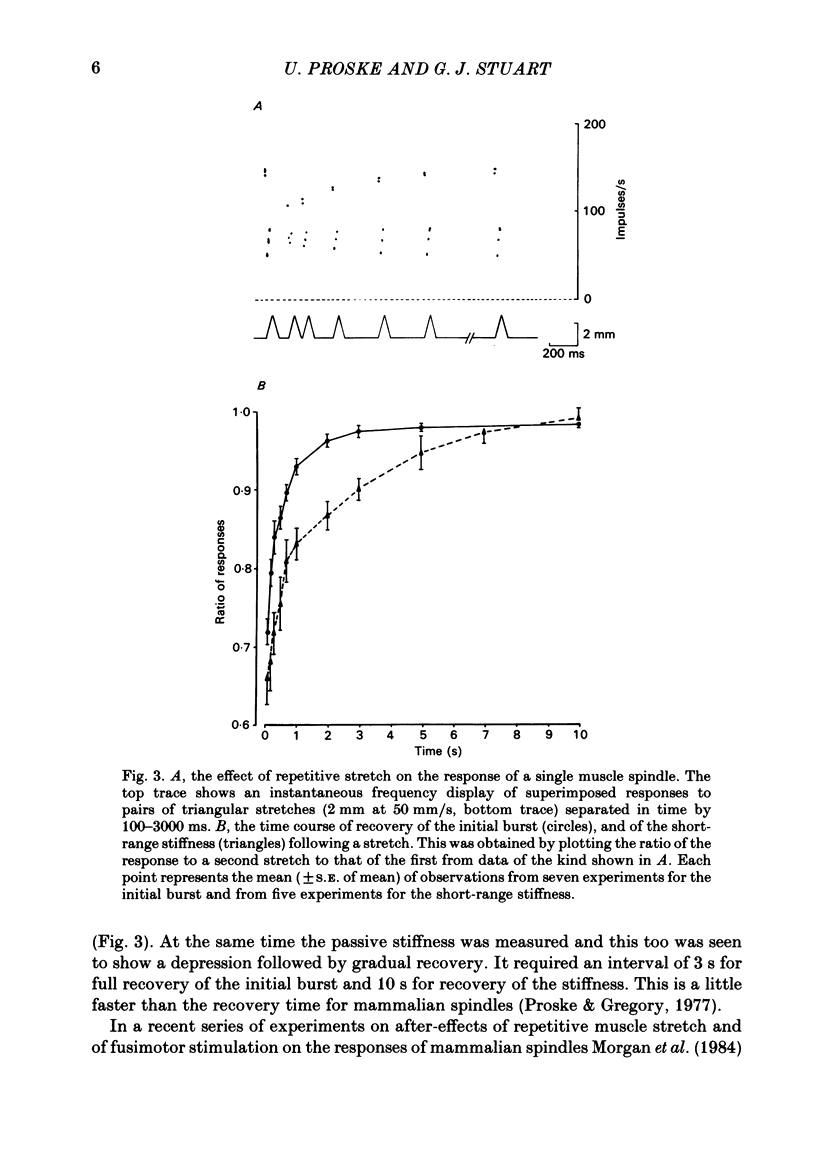
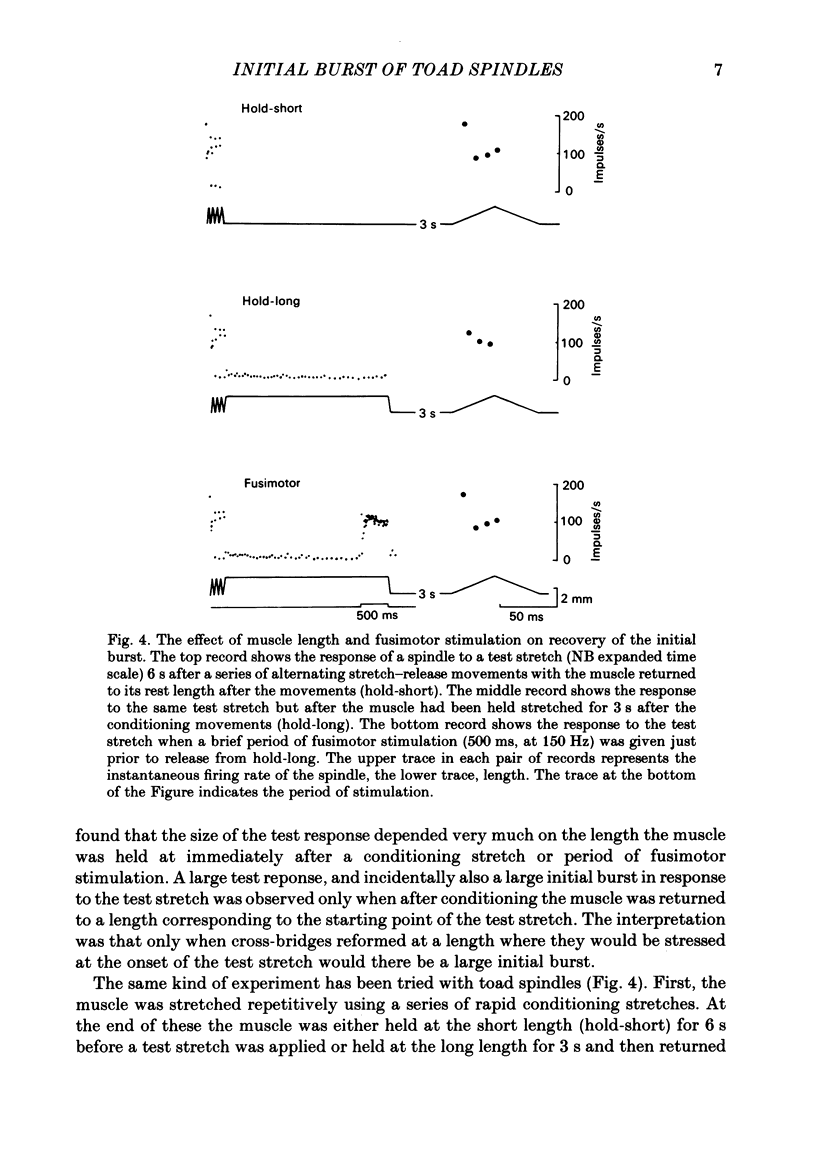
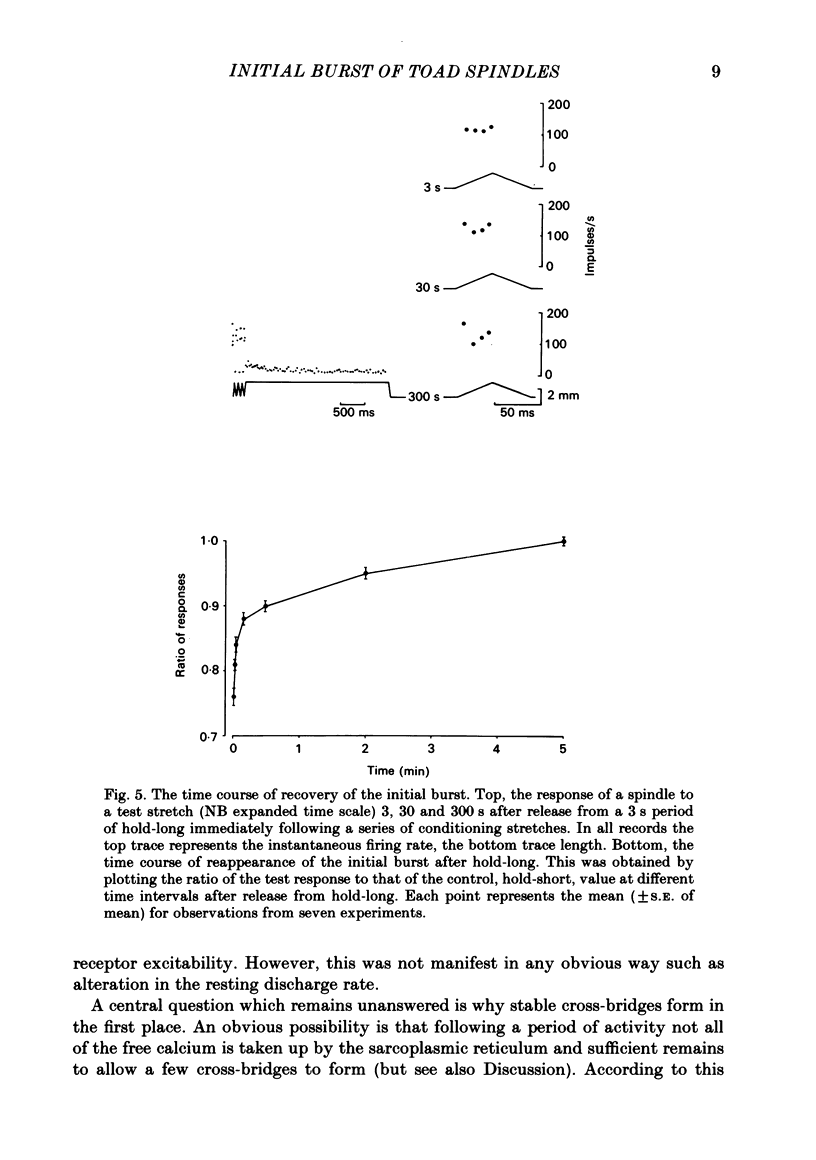
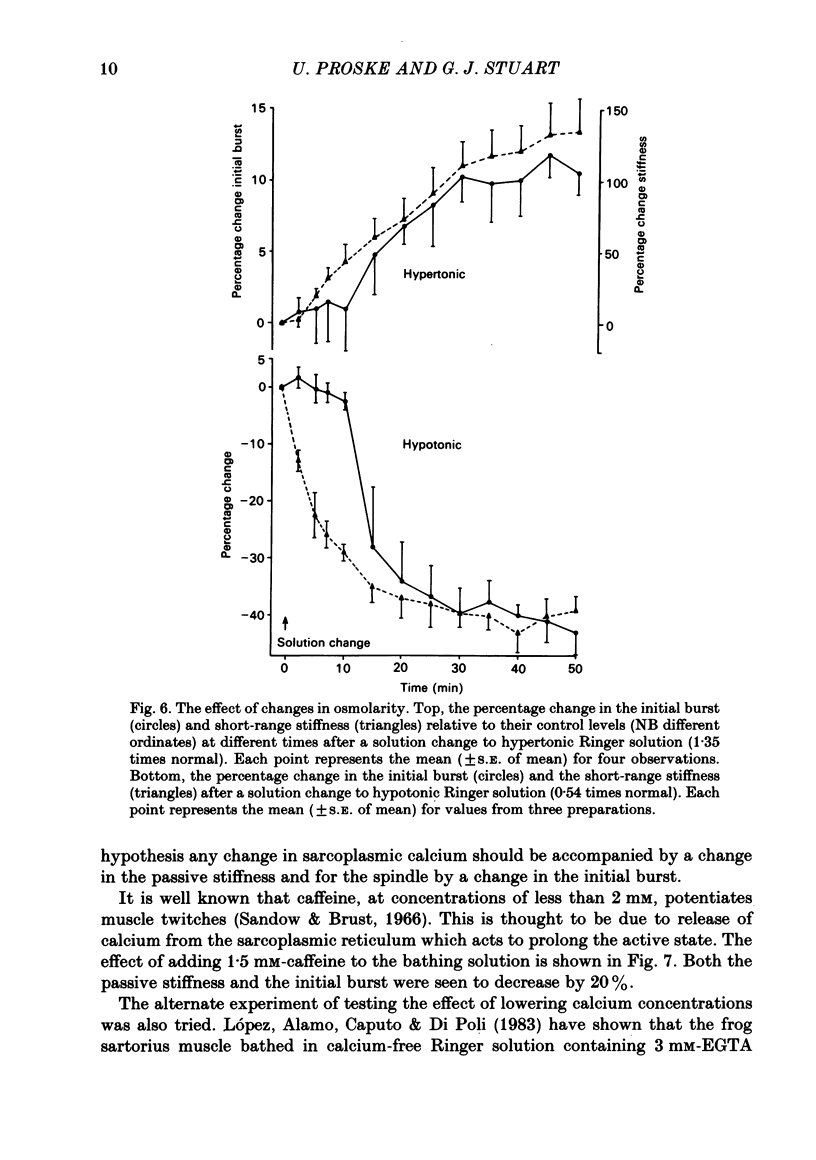
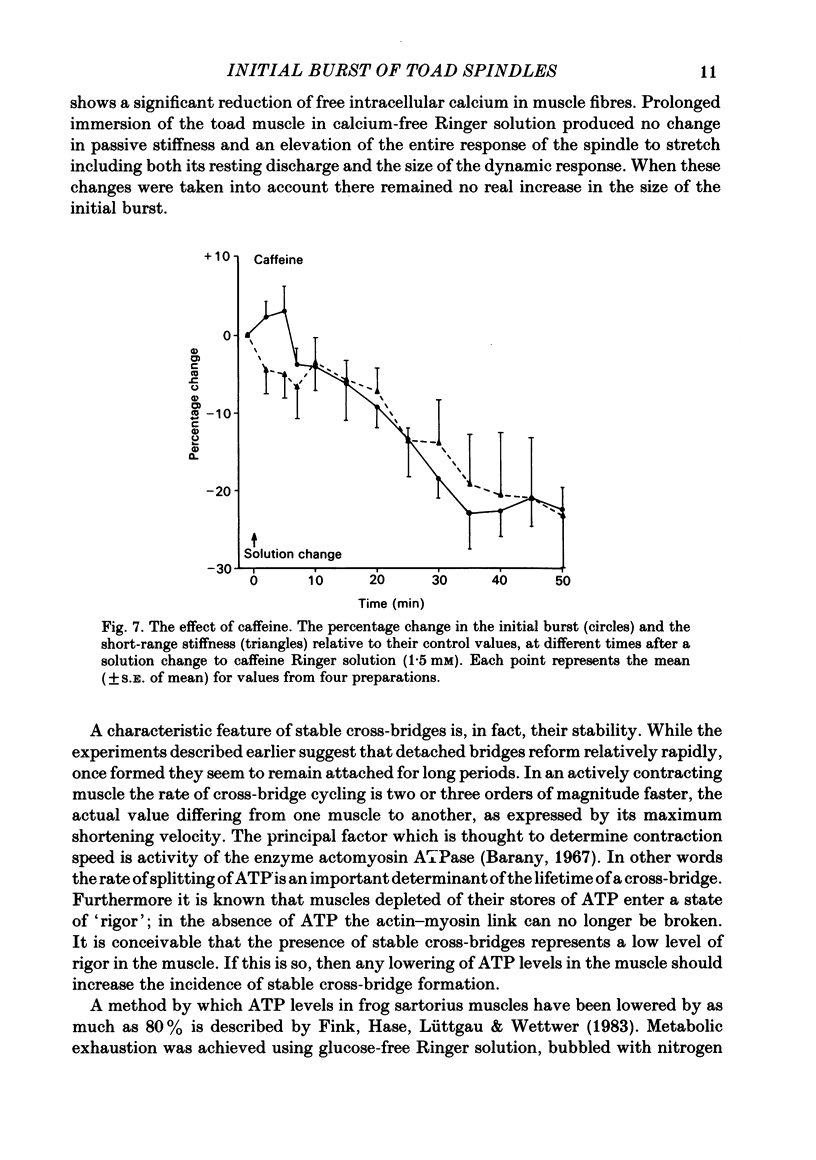
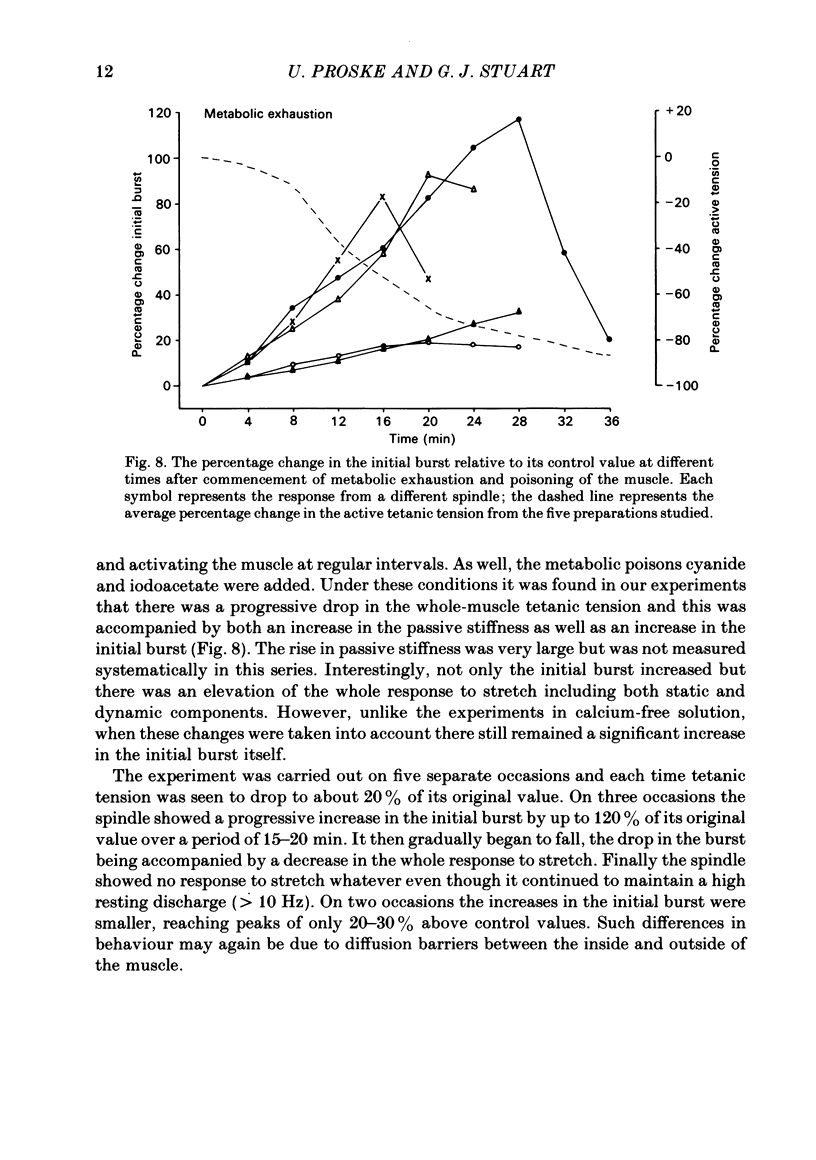
The responses of muscle spindles in the iliofibularis muscle of the cane toad Bufo marinus were examined during constant velocity stretch of the passive muscle. Spindles were found to show an 'initial burst' of high frequency impulses at the onset of stretch. Associated with the initial burst was a steep passive tension rise in the whole muscle, the short-range elastic component (Hill, 1968), called here the passive stiffness. The size of the initial burst was found to depend on muscle length in a similar way as whole-muscle tetanic tension. Repetitive stretch was found to reduce both the initial burst and passive stiffness. The time taken for both to return to their control values was 3 and 10 s respectively. If immediately following repetitive stretch the muscle, and hence the spindle, was held stretched for 3 s, the initial burst in response to a subsequent stretch from a shorter length remained reduced in size for 300 s. The depression could be reversed by a brief period of fusimotor stimulation. Hypertonic Ringer solutions were found to increase the initial burst and passive stiffness, while both were reduced in hypotonic solutions. Low concentrations of caffeine (1.5 mM) produced a similar decrease in both the initial burst and the passive stiffness. Calcium-free Ringer solution left the stiffness unchanged, and increased the whole dynamic response of the spindle. Metabolic exhaustion and poisoning of the muscle caused the initial burst to increase while decreasing the active tension. It is concluded that the initial burst is an intrafusal manifestation of the passive short-range stiffness of extrafusal muscle which is thought to be due to the formation of stable cross-bridges between the actin and myosin filaments of myofibrils.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenner B., Schoenberg M., Chalovich J. M., Greene L. E., Eisenberg E. Evidence for cross-bridge attachment in relaxed muscle at low ionic strength. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7288–7291. doi: 10.1073/pnas.79.23.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B., Yu L. C., Podolsky R. J. X-ray diffraction evidence for cross-bridge formation in relaxed muscle fibers at various ionic strengths. Biophys J. 1984 Sep;46(3):299–306. doi: 10.1016/S0006-3495(84)84026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Goodwin G. M., Matthews P. B. After-effects of fusimotor stimulation on the response of muscle spindle primary afferent endings. J Physiol. 1969 Dec;205(3):677–694. doi: 10.1113/jphysiol.1969.sp008990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bárány M. ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol. 1967 Jul;50(6 Suppl):197–218. doi: 10.1085/jgp.50.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalovich J. M., Eisenberg E. Inhibition of actomyosin ATPase activity by troponin-tropomyosin without blocking the binding of myosin to actin. J Biol Chem. 1982 Mar 10;257(5):2432–2437. [PMC free article] [PubMed] [Google Scholar]
- Fink R., Hase S., Lüttgau H. C., Wettwer E. The effect of cellular energy reserves and internal calcium ions on the potassium conductance in skeletal muscle of the frog. J Physiol. 1983 Mar;336:211–228. doi: 10.1113/jphysiol.1983.sp014577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAY E. G. The spindle and extrafusal innervation of a frog muscle. Proc R Soc Lond B Biol Sci. 1957 May 7;146(924):416–430. doi: 10.1098/rspb.1957.0021. [DOI] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966 May;184(1):170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen P., Sten-Knudsen O. The dependence of the short-range elasticity on sarcomere length in resting isolated frog muscle fibres. Acta Physiol Scand. 1981 Jun;112(2):113–120. doi: 10.1111/j.1748-1716.1981.tb06793.x. [DOI] [PubMed] [Google Scholar]
- Hill D. K. Tension due to interaction between the sliding filaments in resting striated muscle. The effect of stimulation. J Physiol. 1968 Dec;199(3):637–684. doi: 10.1113/jphysiol.1968.sp008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt C. C., Ottoson D. Initial burst of primary endings of isolated mammalian muscle spindles. J Neurophysiol. 1976 Mar;39(2):324–330. doi: 10.1152/jn.1976.39.2.324. [DOI] [PubMed] [Google Scholar]
- Ito F., Komatsu Y. Calcium-dependent regenerative responses in the afferent nerve terminal of the frog muscle spindle. Brain Res. 1979 Oct 12;175(1):160–164. doi: 10.1016/0006-8993(79)90525-0. [DOI] [PubMed] [Google Scholar]
- JANSEN J. K., MATTHEWS P. B. The central control of the dynamic response of muscle spindle receptors. J Physiol. 1962 May;161:357–378. doi: 10.1113/jphysiol.1962.sp006892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennerstrand G., Thoden U. Dynamic analysis of muscle spindle endings in the cat using length changes of different length-time relations. Acta Physiol Scand. 1968 May-Jun;73(1):234–250. doi: 10.1111/j.1748-1716.1968.tb04100.x. [DOI] [PubMed] [Google Scholar]
- Lewis D. M., Proske U. The effect of muscle length and rate of fusimotor stimulation on the frequency of discharge in primary endings from muscle spindles in the cat. J Physiol. 1972 May;222(3):511–535. doi: 10.1113/jphysiol.1972.sp009812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J., Noth J. The effect of bathing solution tonicity on resting tension in frog muscle fibers. J Gen Physiol. 1973 Dec;62(6):737–755. doi: 10.1085/jgp.62.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J. The effect of low-level activation on the mechanical properties of isolated frog muscle fibers. J Gen Physiol. 1971 Aug;58(2):145–162. doi: 10.1085/jgp.58.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. L., Prochazka A., Proske U. The after-effects of stretch and fusimotor stimulation on the responses of primary endings of cat muscle spindles. J Physiol. 1984 Nov;356:465–477. doi: 10.1113/jphysiol.1984.sp015477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R. L., Sollins M. R., Julian F. J. Calcium activation produces a characteristic response to stretch in both skeletal and cardiac muscle. Nature. 1976 Apr 15;260(5552):619–621. doi: 10.1038/260619a0. [DOI] [PubMed] [Google Scholar]
- OTTOSON D. THE EFFECT OF OSMOTIC PRESSURE CHANGES ON THE ISOLATED MUSCLE SPINDLE. Acta Physiol Scand. 1965 May-Jun;64:93–105. doi: 10.1111/j.1748-1716.1965.tb04157.x. [DOI] [PubMed] [Google Scholar]
- Proske U., Gregory J. E. The time-course of recovery of the initial burst of primary endings of muscle spindles. Brain Res. 1977 Feb;121(2):358–361. doi: 10.1016/0006-8993(77)90159-7. [DOI] [PubMed] [Google Scholar]
- Schäfer S. S. The acceleration response of a primary muscle-spindle ending to ramp stretch of the extrafusal muscle. Experientia. 1967 Dec 15;23(12):1026–1027. doi: 10.1007/BF02136428. [DOI] [PubMed] [Google Scholar]


