Abstract
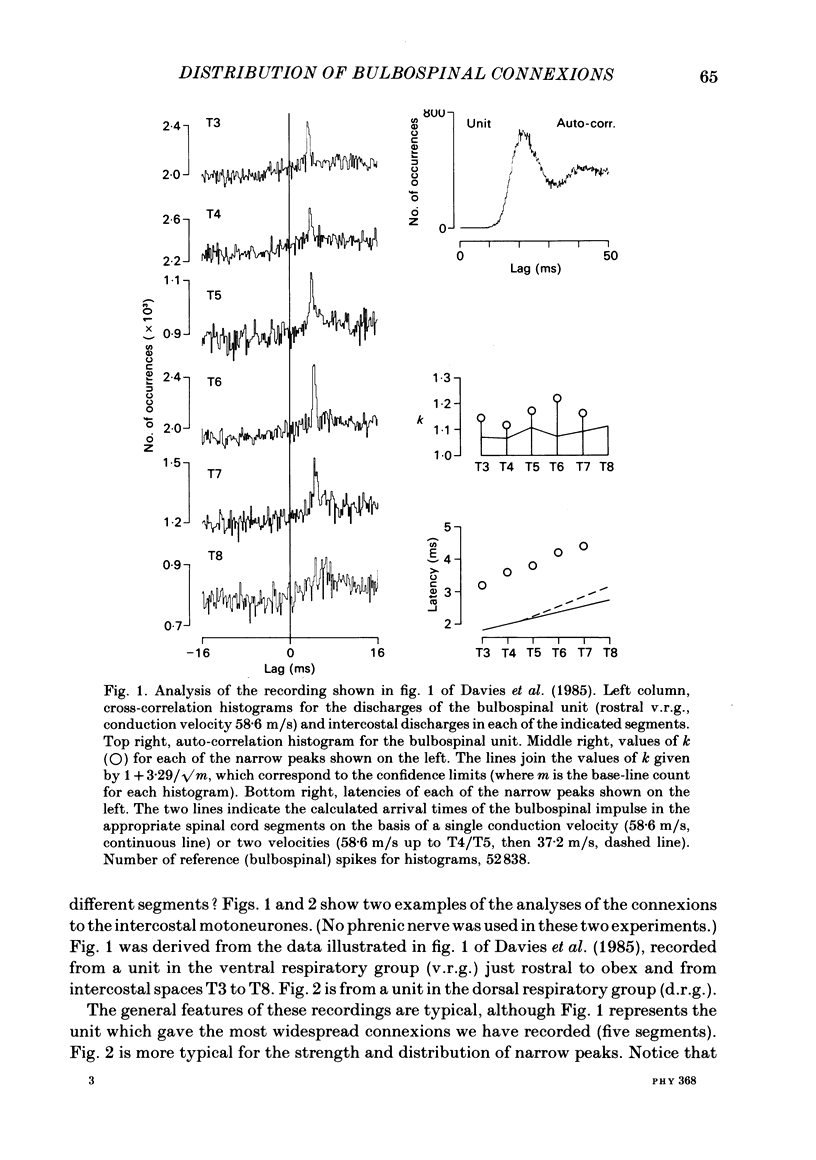
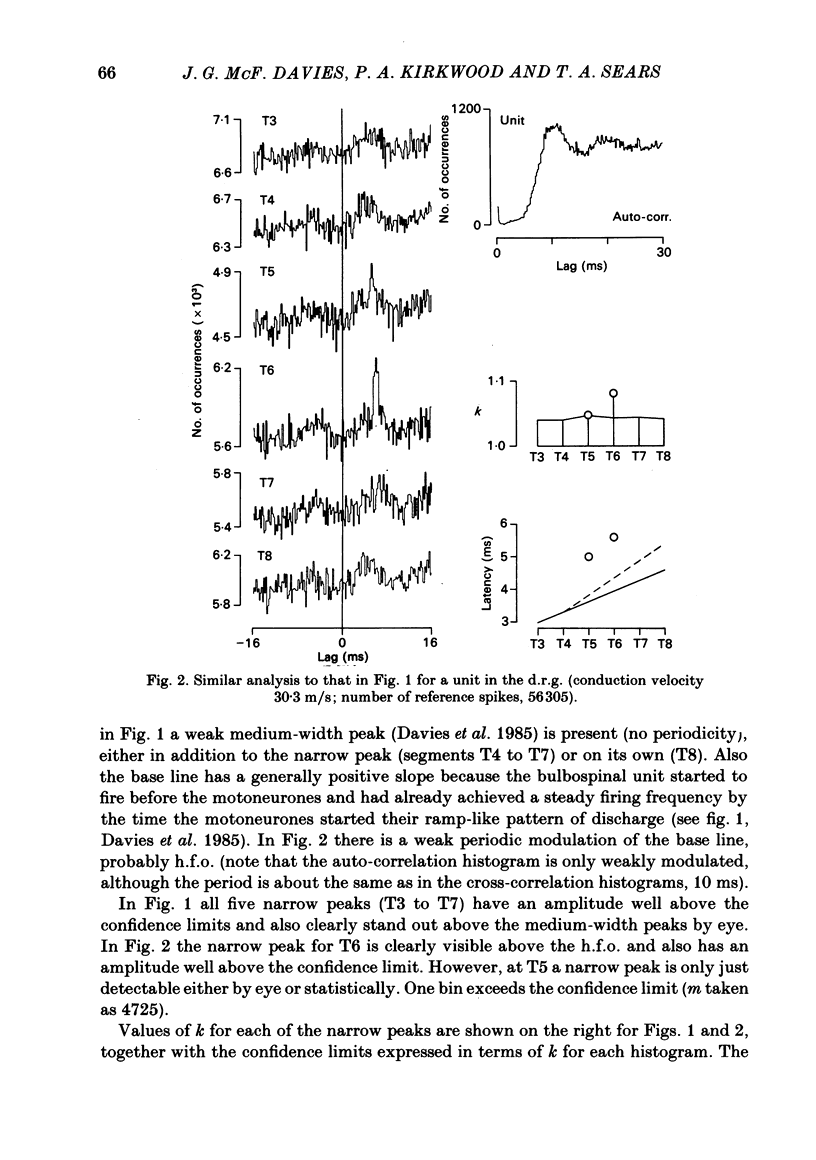
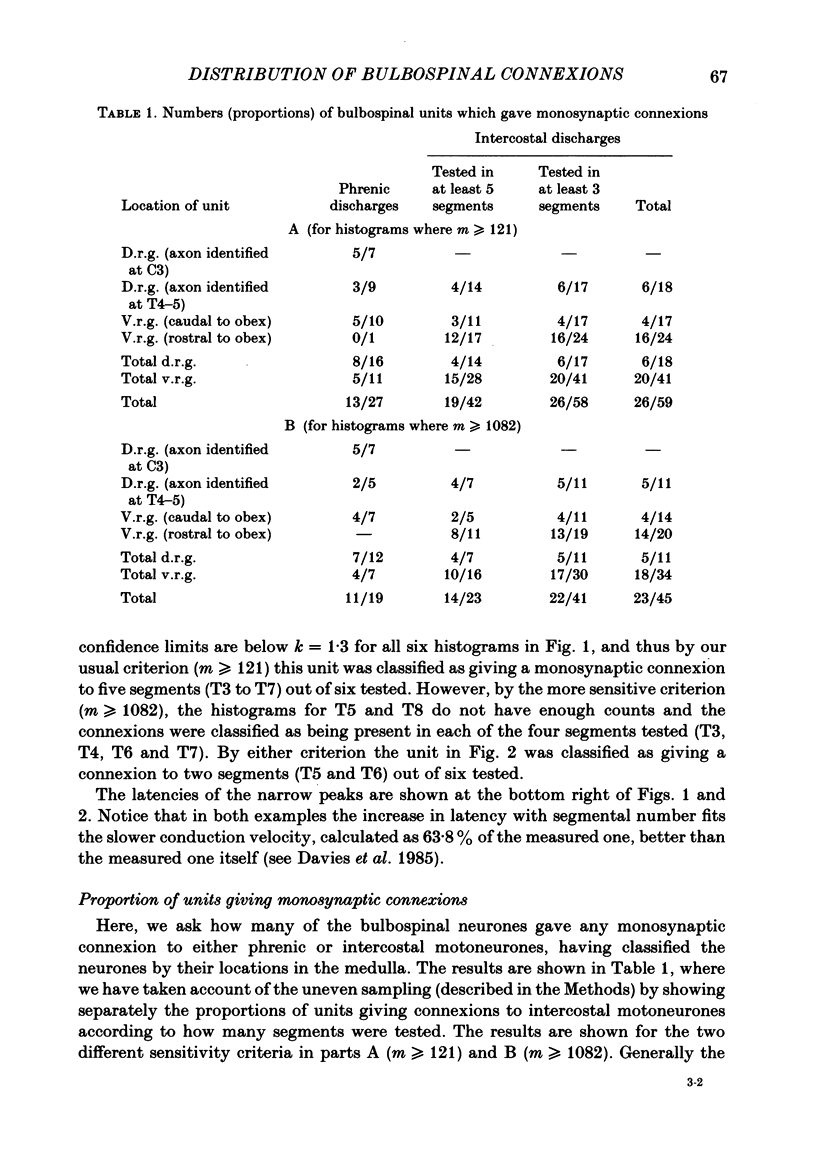
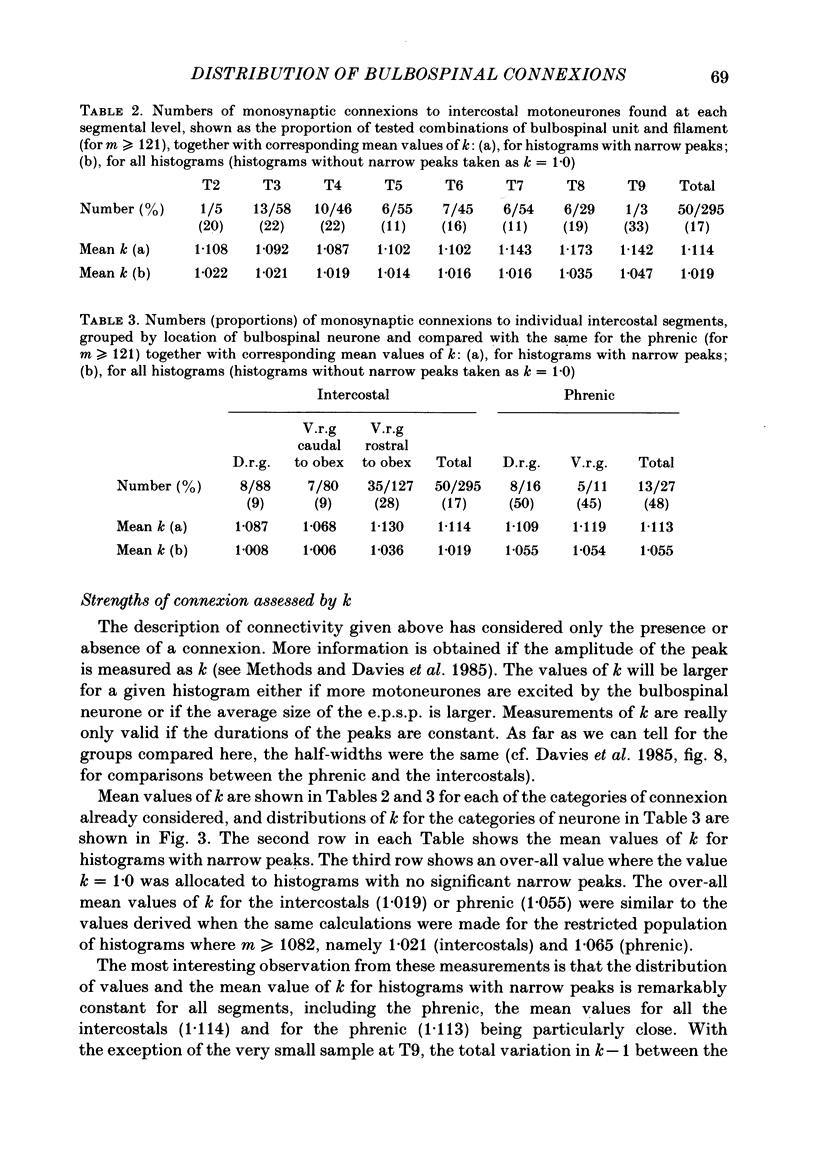
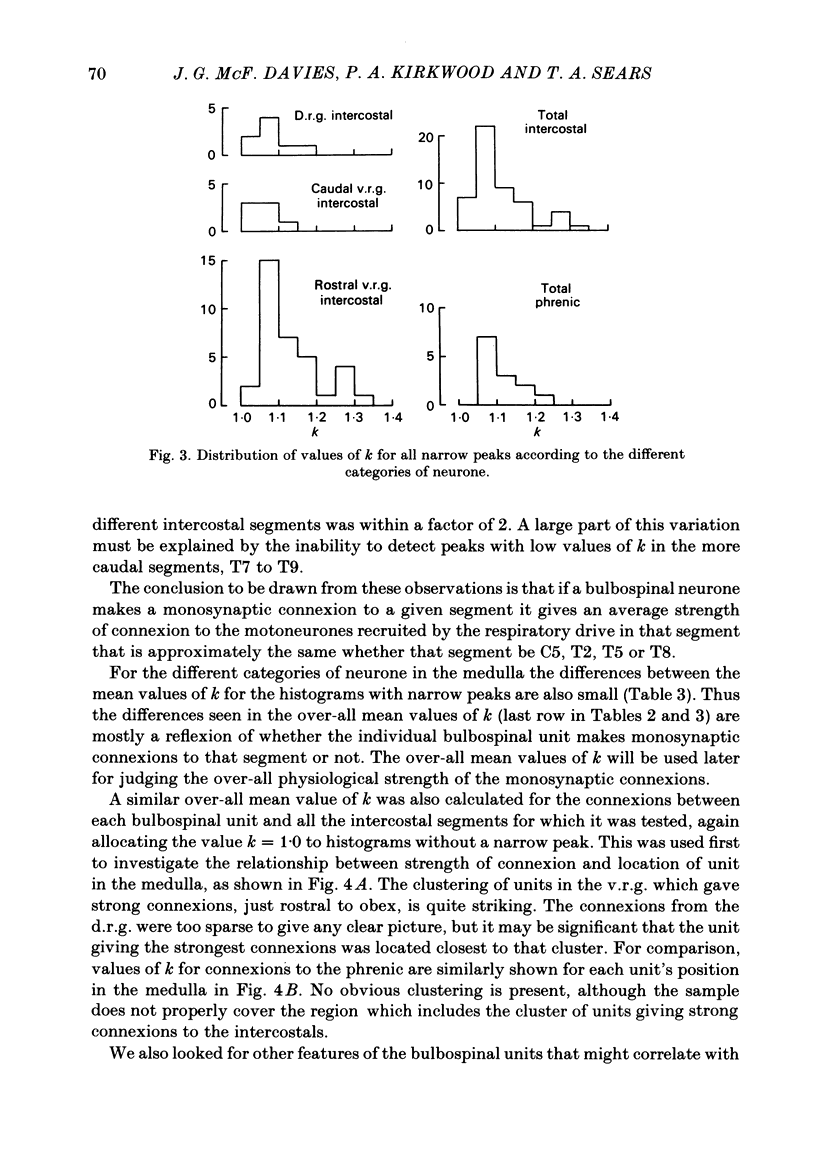
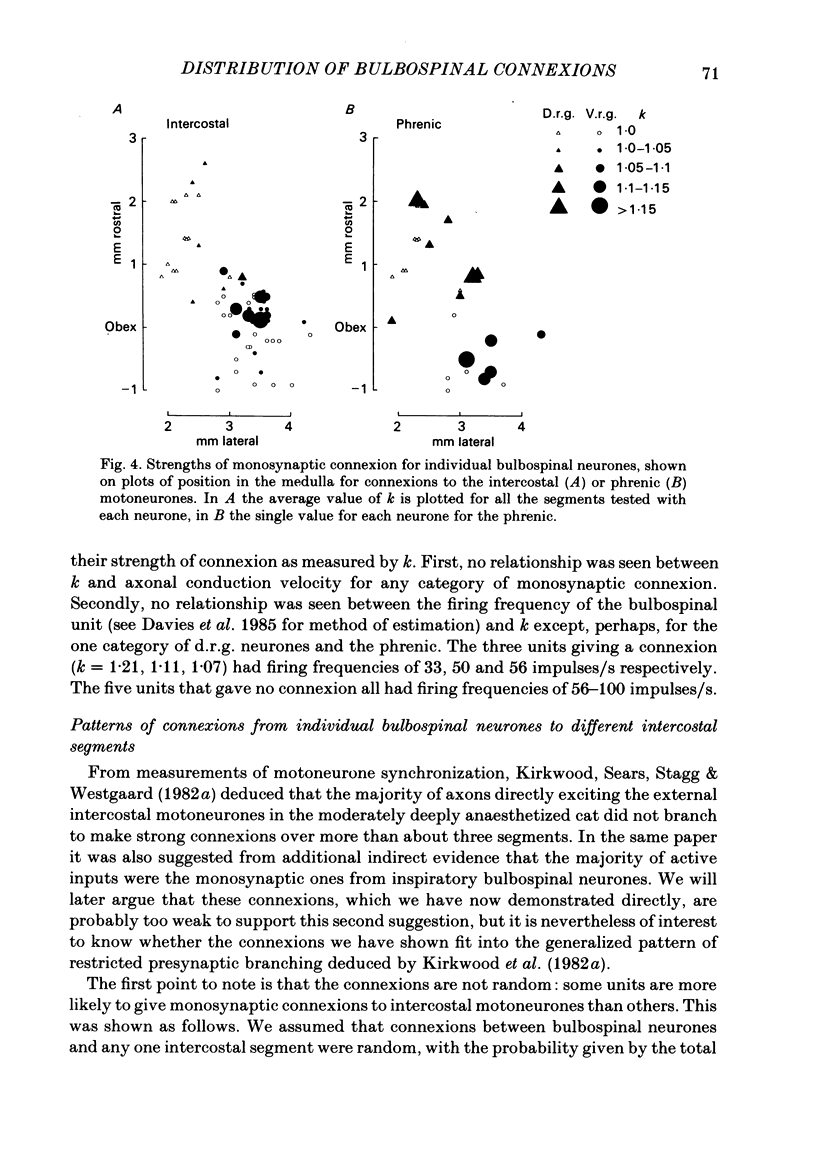
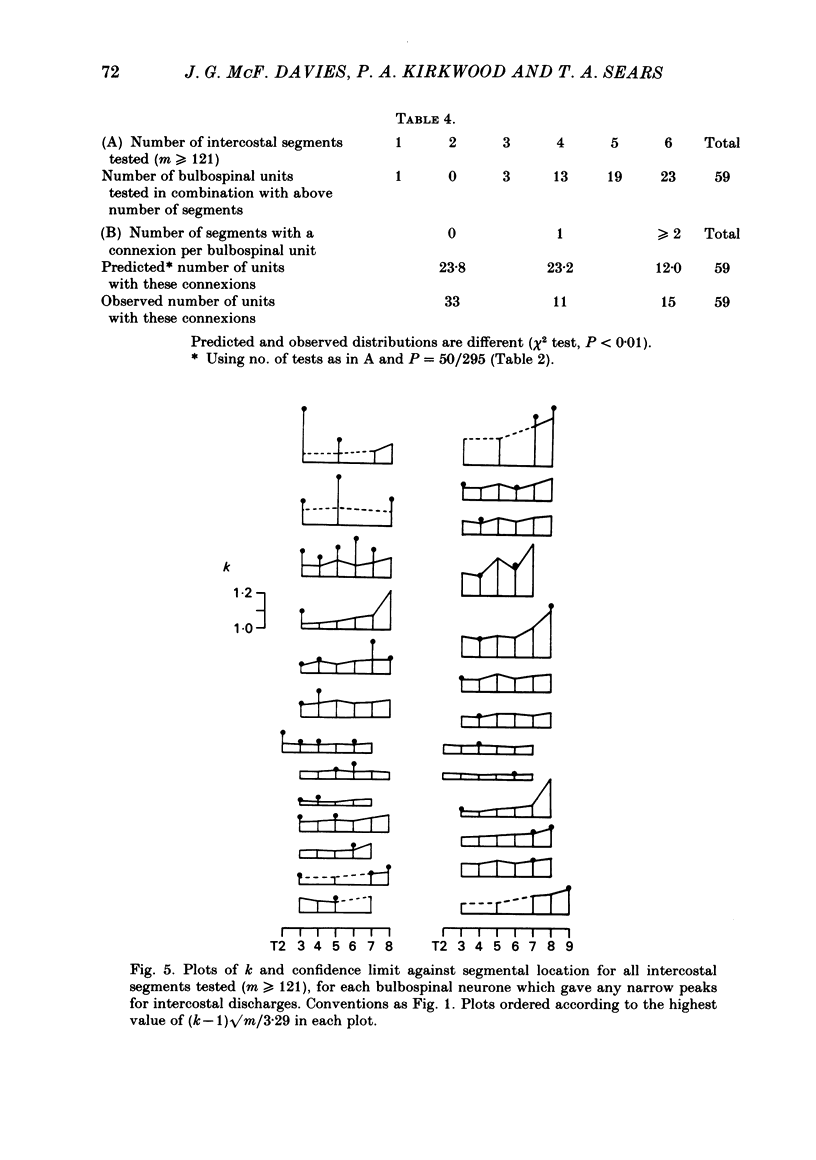
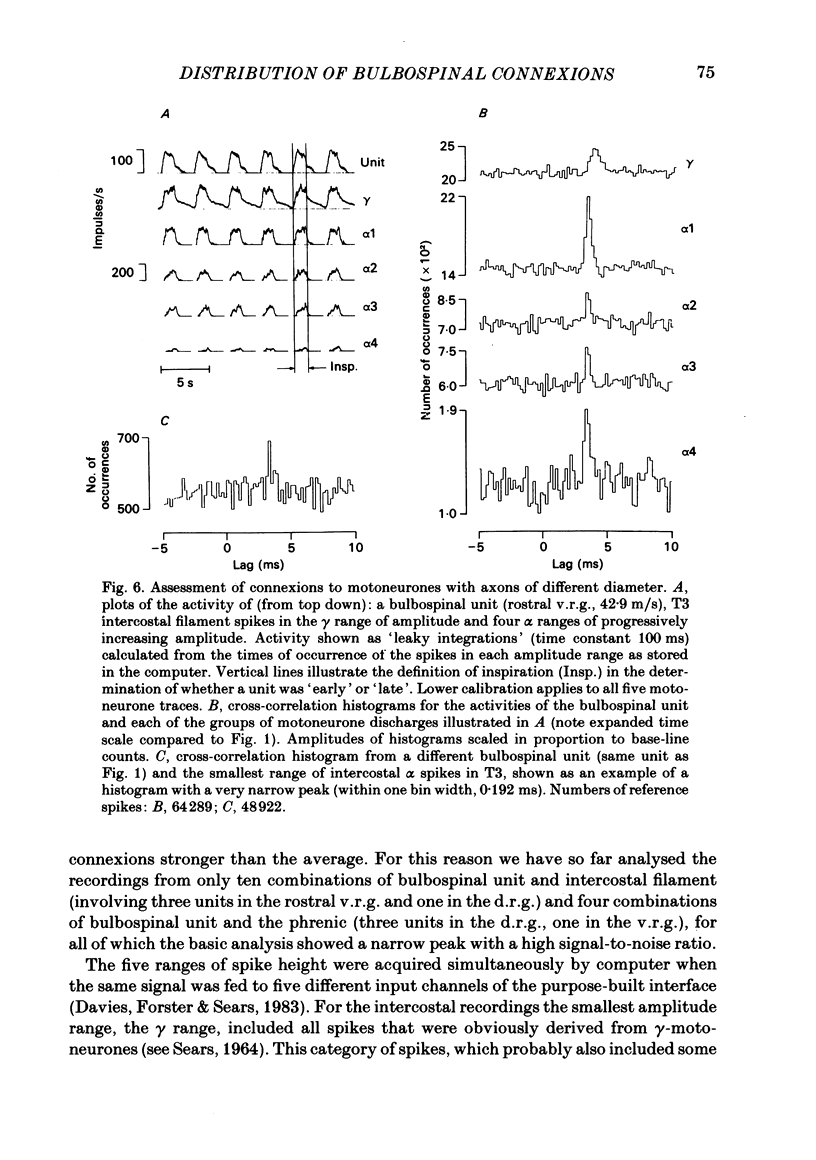
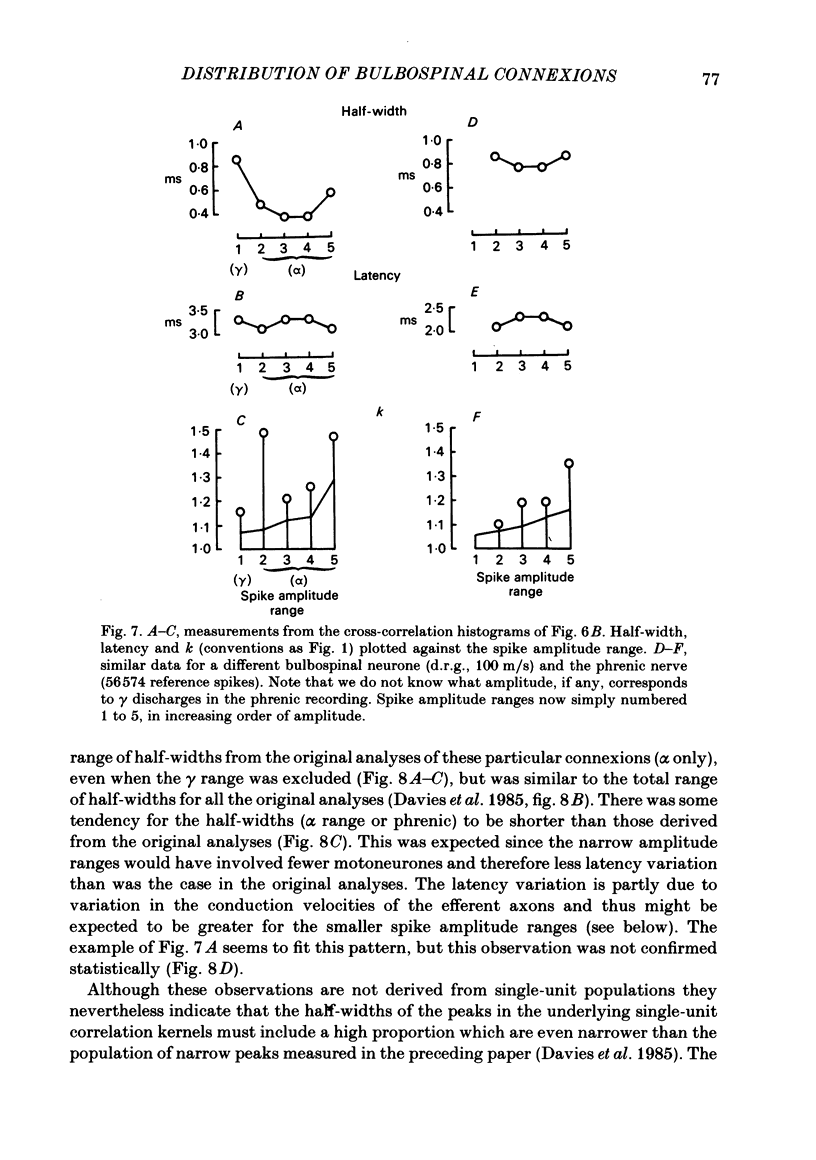
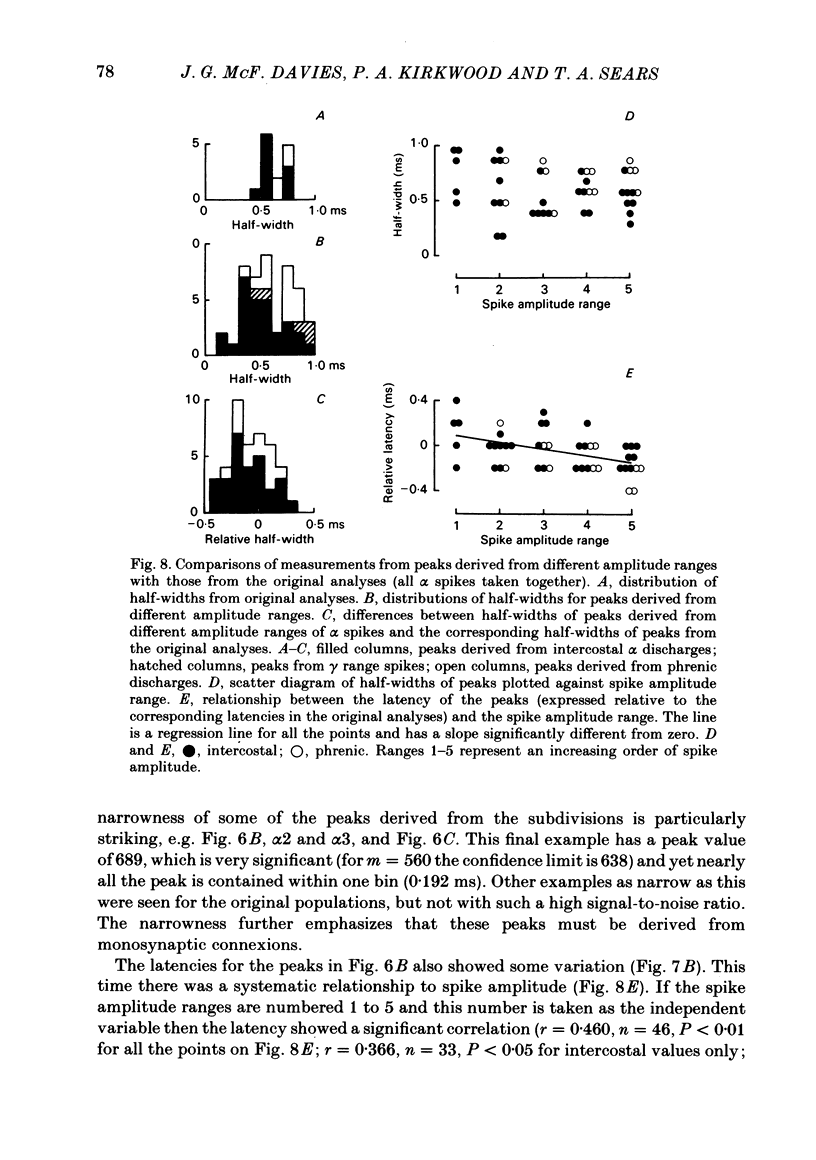
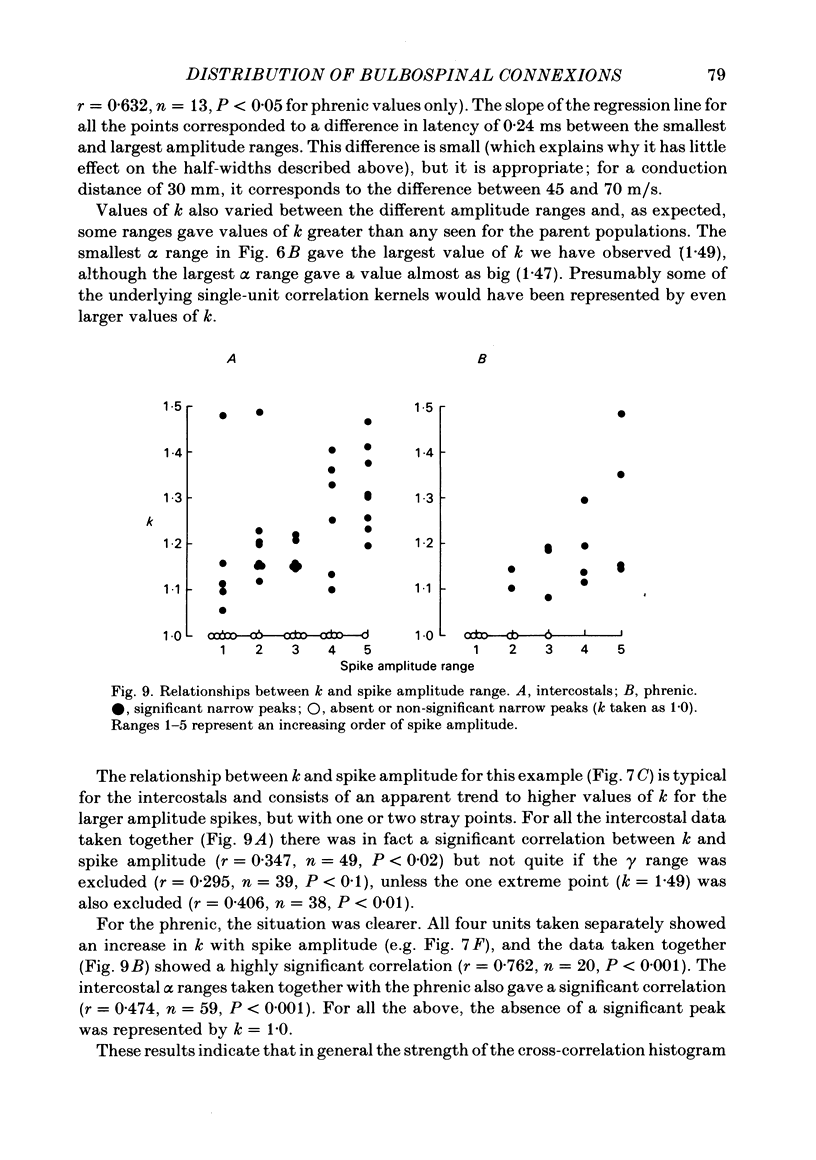
Monosynaptic connexions were sought between bulbospinal inspiratory neurones and inspiratory motoneurones of the C5 phrenic nerve or the intercostal nerves of up to six segments in the anaesthetized cat; they were identified by the presence of narrow peaks in cross-correlation histograms constructed from the spontaneous discharges of these neurones. About half of the bulbospinal neurones tested with the phrenic gave a monosynaptic connexion, as did just under half of those tested with the intercostal nerves. Some neurones gave connexions to both. For pairs of bulbospinal neurones and intercostal nerves, connexions were seen in 50 out of 295 instances (17%). No evidence was found of systematic patterns of connexions to different segments. Some neurones gave connexions to adjacent segments, others up to five segments apart. Bulbospinal neurones were not equipotent at giving connexions to the intercostal motoneurones. If a neurone gave a connexion to one segment, it was more likely to give connexion to another segment than chance would predict. The neurones with strong connexions were concentrated rostral to obex in the ventral respiratory group; neurones in both the caudal part of the ventral group and in the dorsal group gave weaker connexions. Regional variation within the medulla in the proportions of neurones giving connexions to the phrenic motoneurones was not seen, in particular neurones of the dorsal group (eight out of sixteen) and the ventral group (five out of eleven) were equipotent. When the strength of the connexions was assessed by k, the ratio of the peak count in the cross-correlation histogram to the base-line count, the mean strength was similar for the peaks seen for the phrenic (k = 1.113) and the intercostal (k = 1.114) motoneurones. However, because, on a segment-by-segment basis, fewer connexions were seen for the intercostals, the over-all mean value of k (absence of a narrow peak taken as k = 1.0) was stronger for the phrenic (k = 1.055) than for the intercostals (k = 1.019). For the phrenic, the apparent strength of connexion (assessed by k) was considerably stronger for the motoneurones giving large spikes than for those giving small ones. The same result was probably also true for the intercostals. With several assumptions, the total depolarization during inspiration derived monosynaptically in phrenic or intercostal motoneurones was calculated and found to be low, about 1 mV for the intercostals and 3-4 mV for the phrenic.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aminoff M. J., Sears T. A. Spinal integration of segmental, cortical and breathing inputs to thoracic respiratory motoneurones. J Physiol. 1971 Jun;215(2):557–575. doi: 10.1113/jphysiol.1971.sp009485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averill D. B., Cameron W. E., Berger A. J. Monosynaptic excitation of dorsal medullary respiratory neurons by slowly adapting pulmonary stretch receptors. J Neurophysiol. 1984 Oct;52(4):771–785. doi: 10.1152/jn.1984.52.4.771. [DOI] [PubMed] [Google Scholar]
- Backman S. B., Anders C., Ballantyne D., Röhrig N., Camerer H., Mifflin S., Jordan D., Dickhaus H., Spyer K. M., Richter D. W. Evidence for a monosynaptic connection between slowly adapting pulmonary stretch receptor afferents and inspiratory beta neurones. Pflugers Arch. 1984 Oct;402(2):129–136. doi: 10.1007/BF00583324. [DOI] [PubMed] [Google Scholar]
- Berger A. J., Averill D. B., Cameron W. E. Morphology of inspiratory neurons located in the ventrolateral nucleus of the tractus solitarius of the cat. J Comp Neurol. 1984 Mar 20;224(1):60–70. doi: 10.1002/cne.902240106. [DOI] [PubMed] [Google Scholar]
- Bianchi A. L. Localisation et étude des neurones respiratoires bulbaires. Mise en jeu antidromique par stimulation spinale ou vagale. J Physiol (Paris) 1971 Jan-Feb;63(1):5–40. [PubMed] [Google Scholar]
- Davies J. G., Kirkwood P. A., Sears T. A. The detection of monosynaptic connexions from inspiratory bulbospinal neurones to inspiratory motoneurones in the cat. J Physiol. 1985 Nov;368:33–62. doi: 10.1113/jphysiol.1985.sp015845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duron B., Jung-Caillol M. C., Marlot D. Myelinated nerve fiber supply and muscle spindles in the respiratory muscles of cat: quantitative study. Anat Embryol (Berl) 1978 Feb 20;152(2):171–192. doi: 10.1007/BF00315923. [DOI] [PubMed] [Google Scholar]
- Duron B. Postural and ventilatory functions of intercostal muscles. Acta Neurobiol Exp (Wars) 1973;33(1):355–380. [PubMed] [Google Scholar]
- Ellaway P. H., Murthy K. S. The origins and characteristics of cross-correlated activity between gamma-motoneurones in the cat. Q J Exp Physiol. 1985 Apr;70(2):219–232. doi: 10.1113/expphysiol.1985.sp002905. [DOI] [PubMed] [Google Scholar]
- Fedorko L., Merrill E. G., Lipski J. Two descending medullary inspiratory pathways to phrenic motoneurones. Neurosci Lett. 1983 Dec 30;43(2-3):285–291. doi: 10.1016/0304-3940(83)90202-1. [DOI] [PubMed] [Google Scholar]
- Gustafsson B., McCrea D. Influence of stretch-evoked synaptic potentials on firing probability of cat spinal motoneurones. J Physiol. 1984 Feb;347:431–451. doi: 10.1113/jphysiol.1984.sp015074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENNEMAN E., SOMJEN G., CARPENTER D. O. FUNCTIONAL SIGNIFICANCE OF CELL SIZE IN SPINAL MOTONEURONS. J Neurophysiol. 1965 May;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- Hilaire G., Monteau R. Connexions entre les neurones inspiratoires bulbaires et les motoneurones phréniques et intercostaux. J Physiol (Paris) 1976;72(8):987–1000. [PubMed] [Google Scholar]
- Hilaire G., Monteau R. Facteurs déterminant l'order de recrutement des motoneurones phréniques. J Physiol (Paris) 1979;75(7):765–781. [PubMed] [Google Scholar]
- Holstege G., Kuypers H. G. The anatomy of brain stem pathways to the spinal cord in cat. A labeled amino acid tracing study. Prog Brain Res. 1982;57:145–175. doi: 10.1016/S0079-6123(08)64128-X. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J., Hultborn H., Jespersen B., Kiehn O. Intrinsic membrane properties causing a bistable behaviour of alpha-motoneurones. Exp Brain Res. 1984;55(2):391–394. doi: 10.1007/BF00237290. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A. On the use and interpretation of cross-correlations measurements in the mammalian central nervous system. J Neurosci Methods. 1979 Aug;1(2):107–132. doi: 10.1016/0165-0270(79)90009-8. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A., Stagg D., Westgaard R. H. The spatial distribution of synchronization of intercostal motoneurones in the cat. J Physiol. 1982 Jun;327:137–155. doi: 10.1113/jphysiol.1982.sp014224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. The effects of single afferent impulses on the probability of firing of external intercostal motoneurones in the cat. J Physiol. 1982 Jan;322:315–336. doi: 10.1113/jphysiol.1982.sp014039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. The synaptic connexions to intercostal motoneurones as revealed by the average common excitation potential. J Physiol. 1978 Feb;275:103–134. doi: 10.1113/jphysiol.1978.sp012180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A., Tuck D. L., Westgaard R. H. Variations in the time course of the synchronization of intercostal motoneurones in the cat. J Physiol. 1982 Jun;327:105–135. doi: 10.1113/jphysiol.1982.sp014223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipski J., Kubin L., Jodkowski J. Synaptic action of R beta neurons on phrenic motoneurons studied with spike-triggered averaging. Brain Res. 1983 Dec 12;288(1-2):105–118. doi: 10.1016/0006-8993(83)90085-9. [DOI] [PubMed] [Google Scholar]
- Loewy A. D., Burton H. Nuclei of the solitary tract: efferent projections to the lower brain stem and spinal cord of the cat. J Comp Neurol. 1978 Sep 15;181(2):421–449. doi: 10.1002/cne.901810211. [DOI] [PubMed] [Google Scholar]
- Rikard-Bell G. C., Bystrzycka E. K., Nail B. S. Brainstem projections to the phrenic nucleus: a HRP study in the cat. Brain Res Bull. 1984 May;12(5):469–477. doi: 10.1016/0361-9230(84)90162-x. [DOI] [PubMed] [Google Scholar]
- SEARS T. A. EFFERENT DISCHARGES IN ALPHA AND FUSIMOTOR FIBRES OF INTERCOSTAL NERVES OF THE CAT. J Physiol. 1964 Nov;174:295–315. doi: 10.1113/jphysiol.1964.sp007488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUMI T. Organization of spinal respiratory neurons. Ann N Y Acad Sci. 1963 Jun 24;109:561–570. doi: 10.1111/j.1749-6632.1963.tb13487.x. [DOI] [PubMed] [Google Scholar]
- Sears T. A. The respiratory motoneuron and apneusis. Fed Proc. 1977 Sep;36(10):2412–2420. [PubMed] [Google Scholar]


