Abstract
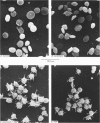
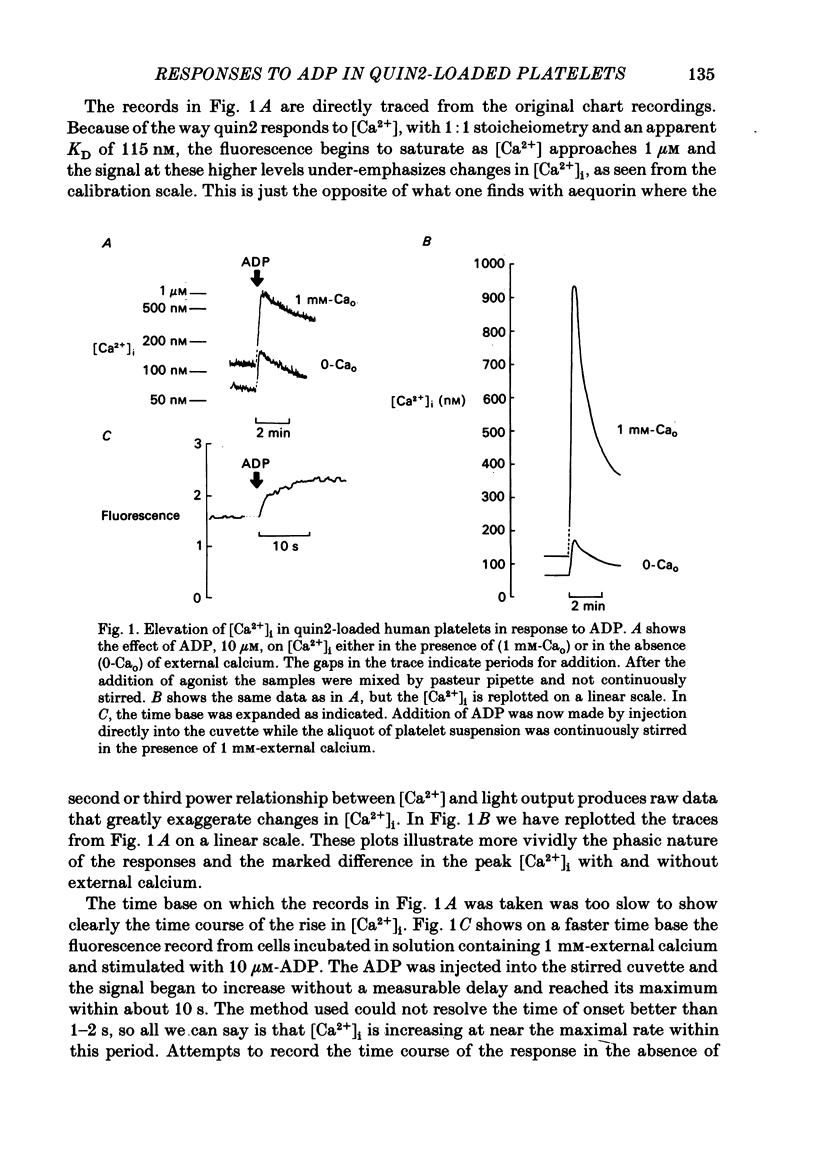
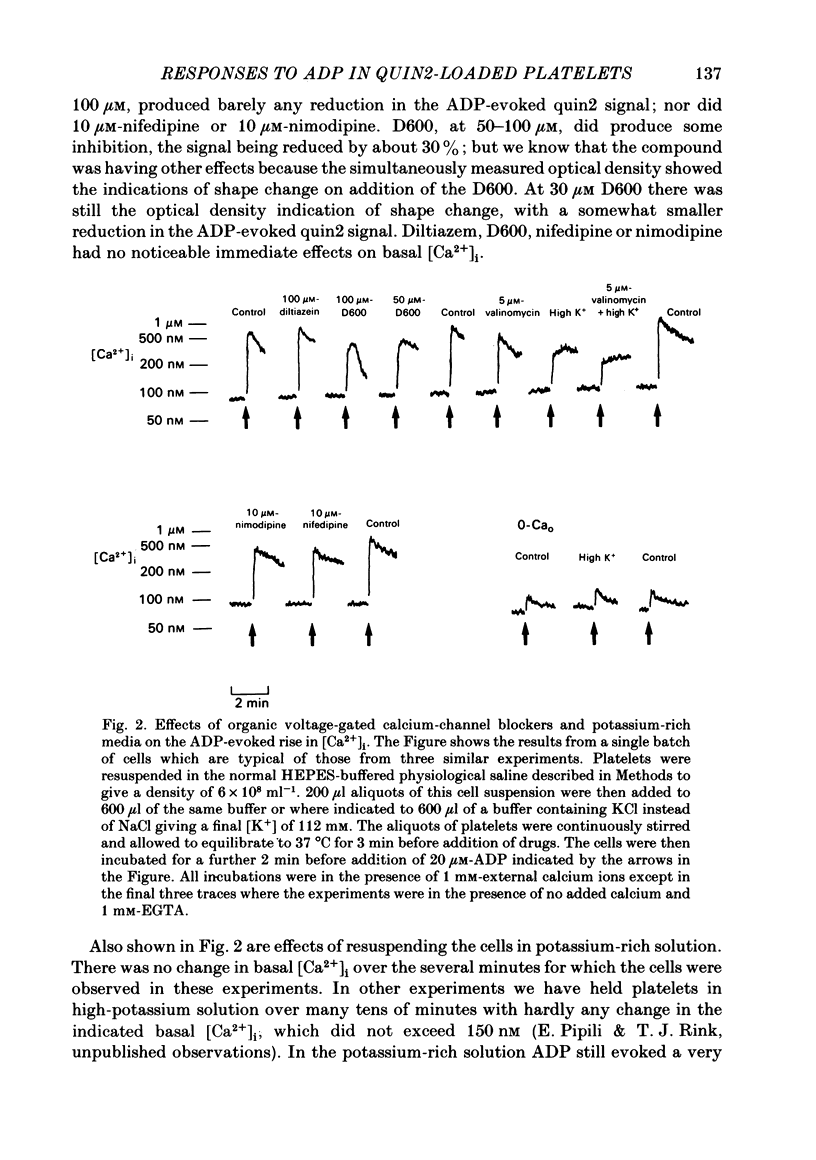
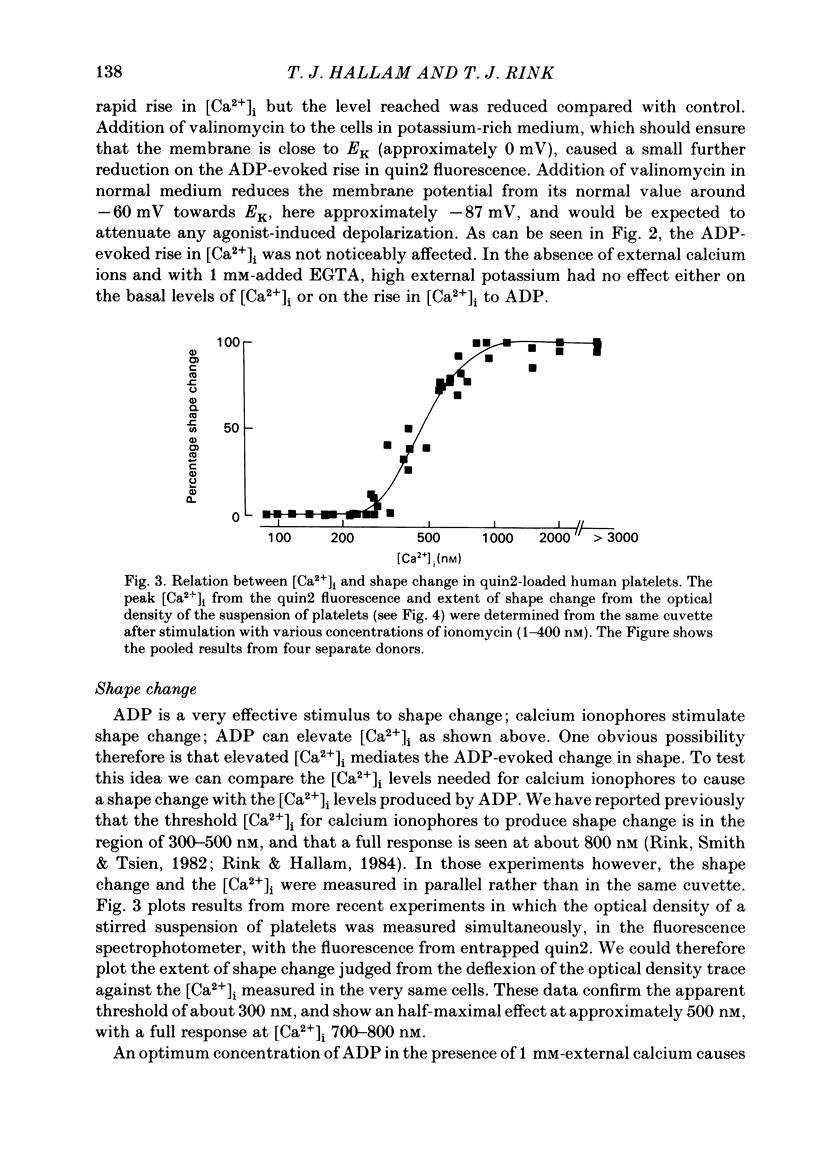
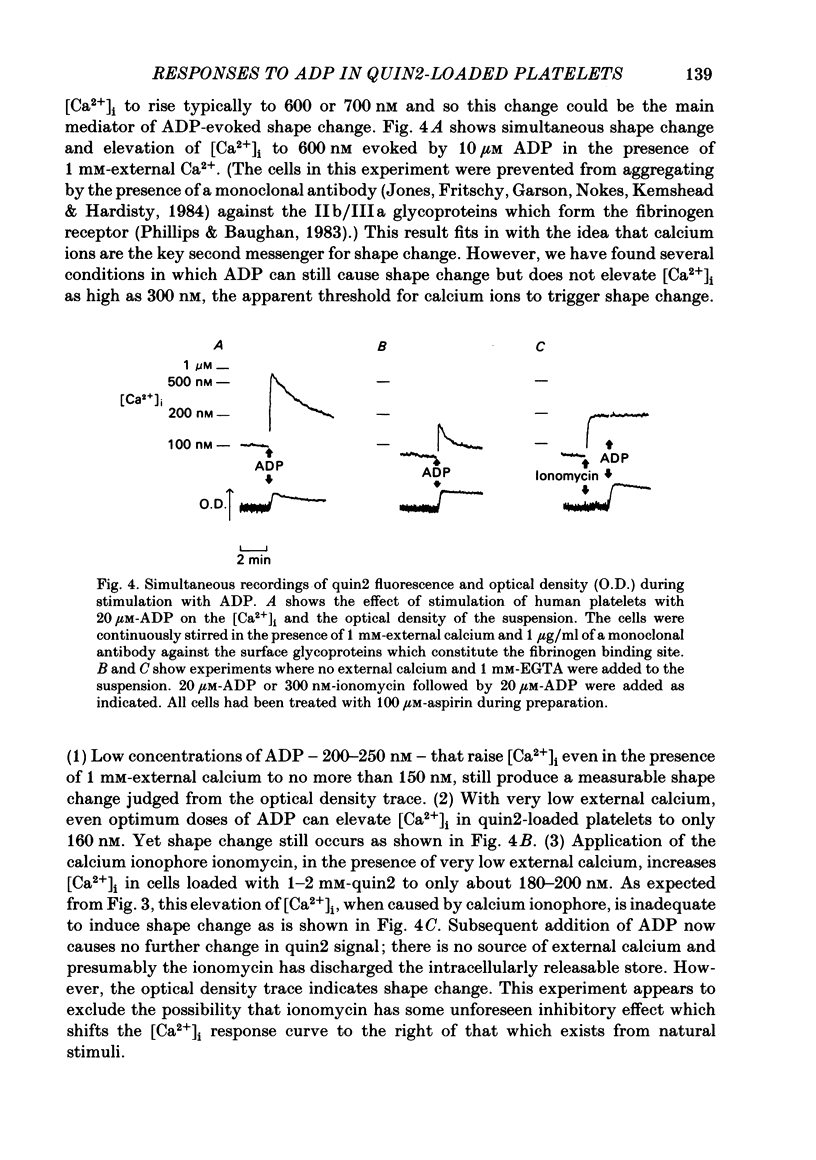
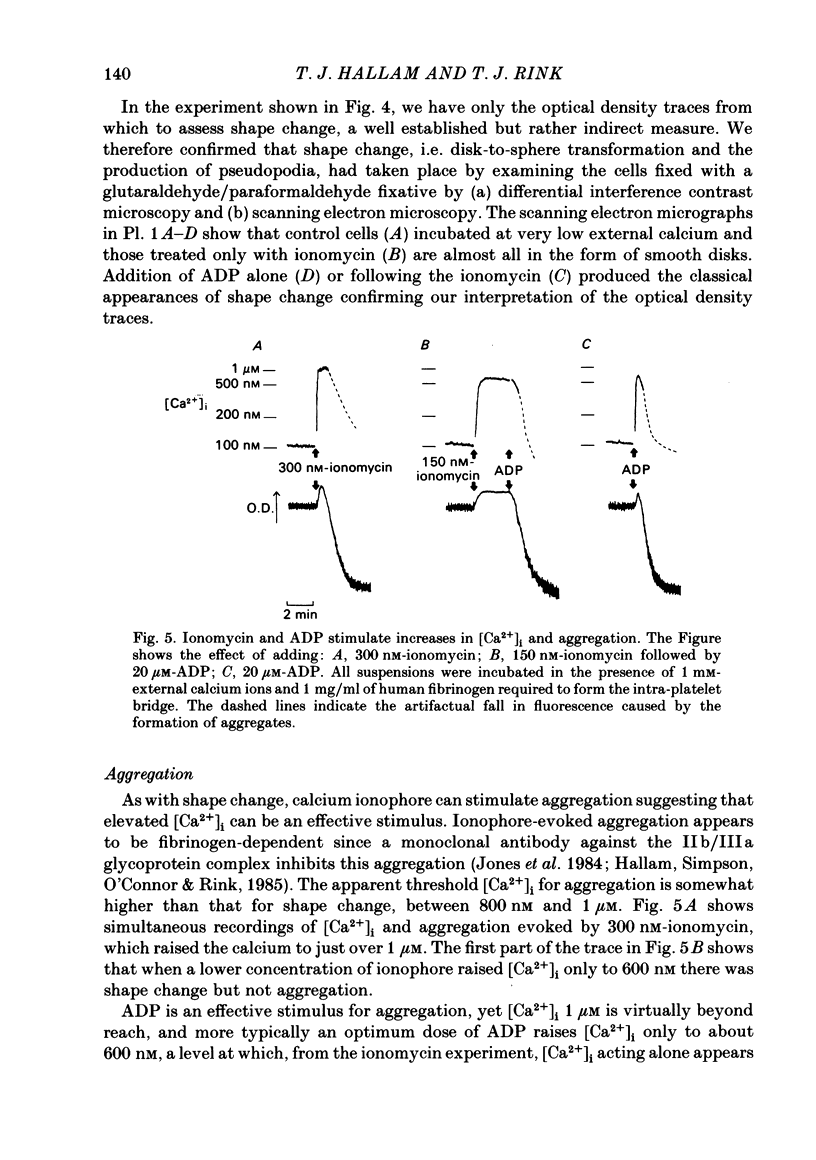
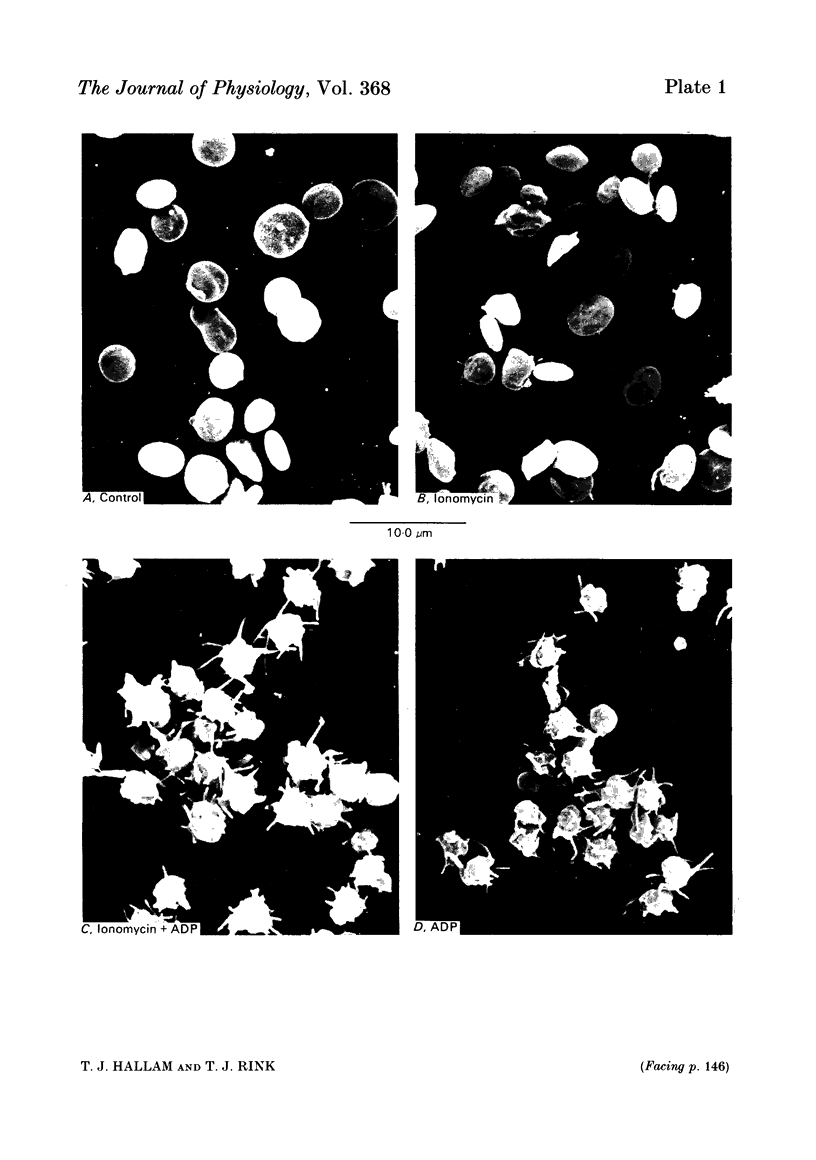
ADP produces a rapid elevation in the concentration of cytoplasmic free calcium, [Ca2+]i, in quin2-loaded human platelets which begins within 1 s of stimulation and peaks after 10 s. In the presence of 1 mM-extracellular calcium, [Ca2+]i peaks at 670 +/- 50 nM in the absence and 610 +/- 30 nM in the presence of a cyclo-oxygenase inhibitor. The production of prostaglandin endoperoxides and thromboxane A2 are not required for stimulation of Ca2+ fluxes by ADP but appear to have a supportive role. In the absence of extracellular calcium ions and with 1 mM-extracellular EGTA, stimulation with ADP caused [Ca2+]i to peak at 160 +/- 20 nM in the absence and 150 +/- 10 nM in the presence of a cyclo-oxygenase inhibitor. ADP can cause the discharge of calcium ions from internal stores and does not require the prior formation of prostaglandin endoperoxides or thromboxane A2. The rise in [Ca2+]i in the presence of extracellular Ca2+ is sixfold larger than in the absence of extracellular Ca2+. This suggests that the major component of the ADP-stimulated rise in [Ca2+]i is caused by the influx of Ca2+ ions across the plasma membrane. Diltiazem, D600, nimodipine and nifedipine had little or no effect on resting or ADP-stimulated [Ca2+]i levels. Depolarization with potassium-rich media alone or in conjunction with valinomycin had no effect on basal [Ca2+]i and only a partial inhibitory effect on ADP-stimulated increases in [Ca2+]i. Depolarization had no effect on the ADP-stimulated rise in [Ca2+]i in Ca2+-free media. Hyperpolarization had no marked effect on the rise in [Ca2+]i produced by ADP in the presence of extracellular calcium. These results are consistent with there being no voltage-dependent channels in the platelet plasma membrane. Using ionomycin, a selective Ca2+ ionophore, and measuring both quin2 fluorescence and optical density of the suspension simultaneously, the threshold [Ca2+]i for shape change was determined to be 300 nM with half-maximal effect at 500 nM and maximal shape change at 800 nM. ADP produced maximal shape change confirmed by scanning electron microscopy with corresponding [Ca2+]i at below 200 nM. The level of [Ca2+]i required to produce aggregation using ionomycin was approximately 1 microM. ADP alone, or following a smaller rise in [Ca2+]i produced by ionomycin to disguise the effect of ADP, produced an aggregatory response at concentrations below 1 microM. These data indicate that excitatory mechanisms are involved producing shape change and aggregation to ADP other than a stimulated rise in [Ca2+]i.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORN G. V. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962 Jun 9;194:927–929. doi: 10.1038/194927b0. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Rink T. J. Catecholamine release from bovine adrenal medulla in response to maintained depolarization. J Physiol. 1975 Dec;253(2):593–620. doi: 10.1113/jphysiol.1975.sp011209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. S., Vilaire G. Exposure of platelet fibrinogen receptors by ADP and epinephrine. J Clin Invest. 1979 Nov;64(5):1393–1401. doi: 10.1172/JCI109597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Best L. C., Holland T. K., Jones P. B., Russell R. G. The interrelationship between thromboxane biosynthesis, aggregation and 5-hydroxytryptamine secretion in human platelets in vitro. Thromb Haemost. 1980 Feb 29;43(1):38–40. [PubMed] [Google Scholar]
- Born G. V. Fluid-mechanical and biochemical interactions in haemostasis. Br Med Bull. 1977 Sep;33(3):193–197. doi: 10.1093/oxfordjournals.bmb.a071435. [DOI] [PubMed] [Google Scholar]
- Born G. V. Observations on the change in shape of blood platelets brought about by adenosine diphosphate. J Physiol. 1970 Aug;209(2):487–511. doi: 10.1113/jphysiol.1970.sp009176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass L. F., Shattil S. J. Changes in surface-bound and exchangeable calcium during platelet activation. J Biol Chem. 1982 Dec 10;257(23):14000–14005. [PubMed] [Google Scholar]
- Feinstein M. B., Fraser C. Human platelet secretion and aggregation induced by calcium ionophores. Inhibition by PGE1 and dibutyryl cyclic AMP. J Gen Physiol. 1975 Nov;66(5):561–581. doi: 10.1085/jgp.66.5.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam T. J., Sanchez A., Rink T. J. Stimulus-response coupling in human platelets. Changes evoked by platelet-activating factor in cytoplasmic free calcium monitored with the fluorescent calcium indicator quin2. Biochem J. 1984 Mar 15;218(3):819–827. doi: 10.1042/bj2180819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam T. J., Thompson N. T., Scrutton M. C., Rink T. J. The role of cytoplasmic free calcium in the responses of quin2-loaded human platelets to vasopressin. Biochem J. 1984 Aug 1;221(3):897–901. doi: 10.1042/bj2210897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D., Fritschy J., Garson J., Nokes T. J., Kemshead J. T., Hardisty R. M. A monoclonal antibody binding to human medulloblastoma cells and to the platelet glycoprotein IIB-IIIA complex. Br J Haematol. 1984 Aug;57(4):621–631. doi: 10.1111/j.1365-2141.1984.tb02939.x. [DOI] [PubMed] [Google Scholar]
- Lee T. C., Malone B., Blank M. L., Snyder F. 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine (platelet-activating factor) stimulates calcium influx in rabbit platelets. Biochem Biophys Res Commun. 1981 Oct 30;102(4):1262–1268. doi: 10.1016/s0006-291x(81)80147-7. [DOI] [PubMed] [Google Scholar]
- Lloyd J. V., Nishizawa E. E., Mustard J. F. Effect of ADP-induced shape change on incorporation of 32P into platelet phosphatidic acid and mono-, di- and triphosphatidyl inositol. Br J Haematol. 1973 Jul;25(1):77–99. doi: 10.1111/j.1365-2141.1973.tb01718.x. [DOI] [PubMed] [Google Scholar]
- MacIntyre D. E., Rink T. J. The role of platelet membrane potential in the initiation of platelet aggregation. Thromb Haemost. 1982 Feb 26;47(1):22–26. [PubMed] [Google Scholar]
- MacIntyre D. E., Shaw A. M. Phospholipid-induced human platelet activation: effects of calcium channel blockers and calcium chelators. Thromb Res. 1983 Sep 15;31(6):833–844. doi: 10.1016/0049-3848(83)90114-7. [DOI] [PubMed] [Google Scholar]
- Massini P., Lüscher E. F. On the significance of the influx of calcium ions into stimulated human blood platelets. Biochim Biophys Acta. 1976 Jul 1;436(3):652–663. doi: 10.1016/0005-2736(76)90447-8. [DOI] [PubMed] [Google Scholar]
- Motulsky H. J., Snavely M. D., Hughes R. J., Insel P. A. Interaction of verapamil and other calcium channel blockers with alpha 1- and alpha 2-adrenergic receptors. Circ Res. 1983 Feb;52(2):226–231. doi: 10.1161/01.res.52.2.226. [DOI] [PubMed] [Google Scholar]
- Mustard J. F., Perry D. W., Ardlie N. G., Packham M. A. Preparation of suspensions of washed platelets from humans. Br J Haematol. 1972 Feb;22(2):193–204. doi: 10.1111/j.1365-2141.1972.tb08800.x. [DOI] [PubMed] [Google Scholar]
- Mustard J. F., Perry D. W., Kinlough-Rathbone R. L., Packham M. A. Factors responsible for ADP-induced release reaction of human platelets. Am J Physiol. 1975 Jun;228(6):1757–1765. doi: 10.1152/ajplegacy.1975.228.6.1757. [DOI] [PubMed] [Google Scholar]
- O'Rourke F. A., Halenda S. P., Zavoico G. B., Feinstein M. B. Inositol 1,4,5-trisphosphate releases Ca2+ from a Ca2+-transporting membrane vesicle fraction derived from human platelets. J Biol Chem. 1985 Jan 25;260(2):956–962. [PubMed] [Google Scholar]
- Owen N. E., Feinberg H., Le Breton G. C. Epinephrine induces Ca2+ uptake in human blood platelets. Am J Physiol. 1980 Oct;239(4):H483–H488. doi: 10.1152/ajpheart.1980.239.4.H483. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Baughan A. K. Fibrinogen binding to human platelet plasma membranes. Identification of two steps requiring divalent cations. J Biol Chem. 1983 Sep 10;258(17):10240–10246. [PubMed] [Google Scholar]
- Rink R. J., Sanchez A., Grinstein S., Rothstein A. Volume restoration in osmotically swollen lymphocytes does not involve changes in free Ca2+ concentration. Biochim Biophys Acta. 1983 Jul 14;762(4):593–596. doi: 10.1016/0167-4889(83)90064-2. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Sanchez A., Hallam T. J. Diacylglycerol and phorbol ester stimulate secretion without raising cytoplasmic free calcium in human platelets. Nature. 1983 Sep 22;305(5932):317–319. doi: 10.1038/305317a0. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Smith S. W., Tsien R. Y. Cytoplasmic free Ca2+ in human platelets: Ca2+ thresholds and Ca-independent activation for shape-change and secretion. FEBS Lett. 1982 Nov 1;148(1):21–26. doi: 10.1016/0014-5793(82)81234-9. [DOI] [PubMed] [Google Scholar]
- Tsien R. W. Calcium channels in excitable cell membranes. Annu Rev Physiol. 1983;45:341–358. doi: 10.1146/annurev.ph.45.030183.002013. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. G. Current concepts of platelet structure. Am J Clin Pathol. 1979 Apr;71(4):363–378. doi: 10.1093/ajcp/71.4.363. [DOI] [PubMed] [Google Scholar]
- White J. G., Rao G. H., Gerrard J. M. Effects of the lonophore A23187 on blood platelets I. Influence on aggregation and secretion. Am J Pathol. 1974 Nov;77(2):135–149. [PMC free article] [PubMed] [Google Scholar]