Abstract
Purpose of Review
Drug addiction is characterized by compulsive drug seeking and use, accompanied by negative emotional states (hyperkatifeia) and heightened pain sensitivity (hyperalgesia) during withdrawal. Both hyperalgesia and hyperkatifeia are integral components of substance use disorders, negatively impacting treatment and recovery. The underlying neurobiological mechanisms of hyperalgesia and hyperkatifeia involve alterations of brain reward and stress circuits, including the dynorphin/κ-opioid receptor (KOR) system. The dynorphin/KOR system modulates pain perception, negative affect, and addictive behaviors. Here, we review the preclinical evidence of dynorphin/KOR signaling in opioid withdrawal-induced hyperalgesia and hyperkatifeia.
Recent Findings
In opioid dependence models, pharmacological and genetic interventions of the dynorphin/KOR system attenuate somatic and motivational signs of withdrawal and addictive-like behaviors, highlighting its therapeutic potential. Understanding the intricate interplay between dynorphin/KOR signaling, hyperalgesia, hyperkatifeia, and addiction offers novel insights into treatment strategies for opioid use disorder and other substance use disorders.
Summary
Further research is needed to elucidate precise mechanisms of the sexual dimorphism of dynorphin/KOR signaling and identify targeted interventions to mitigate hyperalgesia and hyperkatifeia and facilitate recovery from addiction.
Keywords: Opioid withdrawal-induced hyperalgesia, Hyperkatifeia, Dynorphin, Kappa opioid receptor
Introduction
Drug and alcohol addiction are major contributors to disability-adjusted life years throughout the world. For example, the prevalence of alcohol use disorder was the highest of all substance use disorders (1320.8 per 100,000 people), followed by opioid (353.0 per 100,000 people) and cannabis (289.7 per 100,000 people) use disorders [1]. Opioid use disorder, in particular, afflicts over 30 million individuals (or 0.6% of the world’s population) annually, with a similar prevalence in men and women (World Drug Report 2023; United Nations Office on Drugs and Crime: https://dataunodc.un.org/; accessed May 16, 2024). Opioid use disorder can be defined as a compulsion to seek and take opioids, the loss of control over intake, and the emergence of a negative affect state during withdrawal [2, 3]. It can also be considered within an heuristic framework that consists of three stages, with three domains of dysfunction and three neurocircuitries [4–9].
Hyperalgesia and hyperkatifeia are well-documented symptoms of both acute and prolonged opioid withdrawal. These symptoms reflect opponent processes that hold motivational significance [3]. Opioid withdrawal-induced hyperalgesia and opioid withdrawal-induced hyperkatifeia are clinical phenomena whereby patients who chronically use opioids and individuals with opioid use disorder present higher sensitivity and lower tolerance to painful stimuli and greater emotional pain [2]. The International Association for the Study of Pain defines hyperalgesia as greater pain from a stimulus that normally provokes pain, whereas allodynia refers to pain that is experienced from stimuli that normally do not provoke pain (https://www.iasp-pain.org/resources/terminology/; accessed May 16, 2024). Hyperalgesia and allodynia can co-occur in the context of opioid withdrawal. For simplicity, the present review uses the term “hyperalgesia” to encompass sensitized pain responses. Hyperkatifeia, derived from the Greek katifeia for dejection or negative emotional state, encompasses the hyper-negative emotional state that is observed during withdrawal, characterized by dysphoria, anxiety, sleep disturbances, irritability, and emotional pain [10].
Hyperalgesia is a common response to chronic opioid use in humans, with tolerance, and even sign-reversal occurring in the presence of opioids [11–14]. Importantly, opioids also alleviate emotional pain, which is a key aspect of hyperkatifeia and a driving force for the withdrawal/negative affect stage of the addiction cycle. Chronic opioid use leads to reduced effectiveness for chronic emotional pain due to withdrawal and tolerance. Indeed, there is a significant contribution of negative affect to the perception of “physical pain” and a significant contribution of “physical pain” to negative affect (see [15]). Under this conceptual framework, we and others have hypothesized that it is the pain and misery of hyperalgesia and hyperkatifeia that drive up the dose requirement during chronic pain treatment [3, 16].
Hyperalgesia and hyperkatifeia are complex constructs that are modulated by biopsychosocial factors. The focus of this review is the potential of dynorphin as a biological mediator of these constructs as evidenced in preclinical research. We also broadly review the state-of-the-art clinical evidence of hyperalgesia and hyperkatifeia before discussing the preclinical literature.
Clinical Evidence of Hyperalgesia
Opioids are powerful and effective drugs for relieving pain in humans [17], but opioids are less effective with repeated use, such as for the treatment of chronic pain conditions, particularly neuropathic pain, fibromyalgia, and low-back pain. As a result, the efficacy of long-term treatment with opioids is under significant debate, and the risk of prolonged opioid treatment outweighs the benefits in clinical practice [18–20]. In addition, both physical (somatic) symptoms and hard-to-reverse emotional symptoms are observed with established and continuous opioid therapy [3, 7, 10, 18, 21].
Withdrawal from chronic opioid use produces hyperalgesia, an effect that is most prominent in patients with opioid use disorder than in patients with chronic pain [2]. Hyperalgesia has long been observed in people with a history of opioid addiction [22–24]. Heroin users show greater pain sensitivity while in acute withdrawal and in protracted abstinence [23, 25–27]. Patients in methadone-maintenance therapy have low pain tolerance and report more pain [28–33]. In these individuals, pain is a main trigger of relapse or continued opioid use [28, 34]. Moreover, although buprenorphine maintenance therapy may initially improve hyperalgesia, this improvement is not sustained after prolonged exposure [35]. A recent metanalysis showed that patients with chronic opioid use (methadone, buprenorphine) exhibited lower tolerance to pain, and that tolerance to pain was lower right before a methadone dose than it was immediately after a methadone dose [24].
Individuals with opioid use disorder who underwent a 1-month detoxication program exhibited a shorter latency and lower tolerance in a cold pressor test [36]. Former opioid-dependent patients in abstinence (6–38 months) still exhibited abnormal negative emotion in response to the presentation of noxious stimuli [31], thus underscoring the importance of the aversive aspect of pain over the intensity of stimuli. An interaction between the sensorial component of pain (i.e., nociception) and negative emotional aspect of pain was observed in individuals who were in acute withdrawal (24–72 h) from opioids or in protracted abstinence (30 months), in which negative emotion exacerbated hyperalgesia and caused a further increase in pain sensitivity [27].
Studies showed that non-opioid-dependent individuals who underwent acute exposure to opioids also exhibited hyperalgesia upon the cessation of opioid administration [37–39] or upon the precipitation of withdrawal with an opioid receptor antagonist [40], observations that highlight the rapid recruitment of opponent processes that underlie complex analgesia/hyperalgesia responses to opioids.
Pain neurotransmission is modulated by a balance between ascending and descending systems. The ascending system integrates sensory inputs from primary afferents (the dorsal root ganglion and spinal cord) to the somatosensory cortex through two major pathways: spinothalamic pathway and spino-reticulo-thalamic pathway. The descending system, when activated, inhibits pain responses [41]. Such a descending system is composed of projections from the periaqueductal gray (PAG) to the rostral ventromedial medulla to the spinal cord [42]. The descending system can be activated or inhibited by several cortical and subcortical structures, such as the extended amygdala, and is modulated at least partially by endogenous opioid peptides [43]. Opioid withdrawal-induced hyperalgesia is likely mediated centrally because a peripherally restricted opioid receptor antagonist did not precipitate hyperalgesia [44, 45]. Consistent with the central modulation hypothesis, both central sensitization and descending pain modulation were affected by opioids in individuals with hyperalgesia [38, 46].
Clinical Evidence of Hyperkatifeia
Hyperkatifeia is dissociable from somatic signs of withdrawal and other psychiatric disorders and was described as early as 1880 by Rossbach [47]: “When dependence on opioids finally becomes an illness of itself, opposite effects like restlessness, sleep disturbance, hyperesthesia, neuralgia and irritability become manifest.” Historically, the terms “withdrawal” and “dependence” have been defined differently across various contexts. Withdrawal refers to abstinence from or the cessation of chronic drug use, typically marked by signs and symptoms that are opposite to acute effects of the drug [3].
Dependence is defined as the onset of a withdrawal syndrome when drug use is stopped. According to this definition, any drug, even those without addiction potential, can produce dependence. This concept evolved into the definition of physical dependence, an intense physical disturbance upon discontinuation of drug use. Physical withdrawal syndrome typically presents as opposite effects to that caused by acute administrations of the drug. For example, opioid withdrawal causes pupillary dilation, while opioid intoxication leads to pupillary constriction. Psychological dependence was later defined as a “condition in which a drug produces a feeling of satisfaction and a psychic drive that require periodic or continuous administration of the drug to produce pleasure or to avoid discomfort” [48]. However, both somatic and behavioral symptoms are driven by physiological changes in the body and brain. It is argued that symptoms that are associated with protracted hyperkatifeia hold greater motivational significance for opioid seeking than the somatic signs of withdrawal [3].
This thesis is supported by the strong correlation between dysphoria and spontaneous opioid withdrawal [49]. In studies of heroin-dependent patients undergoing detoxification, the opioid receptor antagonist naloxone increased both somatic signs of withdrawal and dysphoria in a dose-dependent manner [49, 50]. These findings were further supported by community sample studies, which indicated that among individuals with untreated opioid use disorder, withdrawal was perceived as the determining factor in maintaining opioid use and delaying treatment [51]. The author proposed a withdrawal catastrophizing scale as an important clinical measure of hyperkatifeia [52].
A conceptual framework was developed to explain the neural systems thought to mediate hyperkatifeia and drive the motivational aspect of the opponent processes involved in excessive opioid use. This framework involves the downregulation of brain reward circuitry within the system and the recruitment of brain stress circuitry between systems [6, 53]. Within-system neuroadaptation refers to the process where the primary cellular response element to the drug within a specific neurochemical circuit adapts to counteract the effects of the drug. In contrast, between-systems neuroadaptation describes changes in which a different circuit (i.e., stress or antireward circuit) is activated by the reward circuit. The persistence of these opposing effects after the drug is removed is evidenced by the negative emotional withdrawal syndrome.
Consistent with this hyperkatifeia framework, imaging evidence points to an overlap in circuitry that underlies the stress response, reward, and major depressive disorder. Acute stress changes global cortical connectivity and increases striatal connectivity with cortical regions that express genes previously linked to imaging abnormalities in major depression. These regions are abundant in μ- and κ-opioid receptors [54].
Neurocircuitry of Hyperalgesia and Hyperkatifeia (Pre-Clinical)
In animal models, repeated opioid administration produces a long-lasting, gradual, and dose-dependent decrease in nociceptive thresholds [55–61]. As with human studies, hyperalgesia is a consequence of rapid opioid-induced neuroadaptations and can be observed after a single opioid exposure [62–64].
Opioid withdrawal-induced hyperalgesia has been shown to involve a facilitatory mechanism in a descending system, characterized by greater activity of the rostro ventromedial medulla [65, 66]. Recent studies that used immediate early gene expression markers as a proxy of neuronal activation found greater activity in several subcortical and midbrain regions during hyperalgesia in opioid withdrawal. Hyperalgesia that was precipitated by naloxone following a single morphine exposure increased Fos and Zif268 expression in the central nucleus of the amygdala (CeA) capsular and lateral portions, and amygdalo-striatal transition zone, latero-dorsal bed nucleus of the stria terminalis (BNST), and lateral interstitial nucleus of the posterior limb of the anterior commissure in the brain of male rats[67]. Oxycodone-dependent rats exhibited lower pain thresholds and an increase in Fos-positive neurons in the PAG, CeA, locus coeruleus, paraventricular nucleus of the thalamus, agranular insular cortex, BNST, and lateral habenula medial parvocellular region during withdrawal [68]. In heroin-dependent mice, hyperalgesia was associated with higher Fos expression in the lateral hypothalamus, CeA, ventral tegmental area, parabrachial nucleus, dorsal raphe, and locus coeruleus [60].
Although the term hyperkatifeia was coined relatively recently [10], the construct builds from an extensive body of literature on the manifestation of a negative emotional state or negative affect that is associated with drug withdrawal. Current evidence suggests that the extended amygdala is a focal point in neurocircuitry that mediates hyperkatifeia [69]. Here, within- and between-system neuroadaptations of brain reward and stress systems are hypothesized to drive negative emotional states [3]. Although not part of the extended amygdala, the PAG has reciprocal connections with the extended amygdala, and the PAG integrates negative emotions with autonomic, neuroendocrine, and immune responses [70] and participates in the integration of hyperalgesia and hyperkatifeia [71].
Dynorphin and the κ-opioid receptor (KOR) have been implicated in an array of biological processes, including endocrine regulation [72], neuronal myelinization [73], and itch [74], among others. We review evidence of the involvement of dynorphin/KOR system in domains that underlie hyperalgesia and hyperkatifeia.
Dynorphin/KOR System
Dynorphins play a role in multiple functional pathways in the brain. They are encoded by the prodynorphin (Pdyn) gene that is translated into prodynorphin, a 26 kDa protein, which in turn is processed into big-dynorphin by prohormone convertase 1. Big-dynorphin is further processed by prohormone convertase 2 in the presence of carboxypeptidase E to dynorphin A(1–17), dynorphin A(1–8), dynorphin B, α-neoendorphin, and leumorphin (reviewed by [75–78]). Prodynorphin-derived peptides bind preferentially to the KOR [79].
Prodynorphin is found in most brain structures and generally matches the KOR distribution. Prodynorphin is highly concentrated in the nucleus accumbens, whereas its concentration in the thalamus is low. The KOR is highly expressed in cortical, limbic, and brainstem regions of the rodent brain. Particularly high densities are found in the basal anterior forebrain, including the claustrum, endopiriform cortex, olfactory tubercle, striatum (caudate putamen and nucleus accumbens), preoptic area, hypothalamus, and pituitary [80].
The KOR is a Gi-coupled receptor that inhibits cell excitability and neurotransmitter release [81]. The activation of KORs by dynorphin leads to lower dopamine transmission [82]. Electrophysiology evidence shows that KOR activation reduces γ-aminobutyric acid (GABA) and glutamate release in the nucleus accumbens [83, 84], glutamate release from the basolateral amygdala to the BNST [85], and glutamate release from the insular cortex to the substantia nigra [86]. In the CeA, the antagonism or genetic deletion of KORs enhanced GABA release that was caused by corticotropin-releasing factor (CRF), suggesting that CRF activation promotes dynorphin release and subsequent KOR activation, which modulates presynaptic GABA release [87].
Dynorphin and KOR: Pain
Several alterations of the dynorphin/KOR system have been identified in chronic pain. In the spinal cord, the intrathecal administration of high doses of dynorphin produced allodynia in an N-methyl-D-aspartate (NMDA)-dependent manner [88, 89], whereas low doses of dynorphin and KOR agonists produced analgesia [90], an effect that was mediated by adenylyl cyclase 3 [91]. Dynorphin knockout mice showed a faster return to normal nociceptive baseline after a peripheral nerve lesion [92], suggesting a pronociceptive role for dynorphin in chronic pain. κ-Opioid receptor antagonism also enhanced allodynia following sciatic nerve injury. The authors proposed that KOR blockade in the spinal cord shifts dynorphin signaling toward a pronociceptive, NMDA-dependent mechanism [93].
Effects of dynorphin in pain conditions are influenced by sexual hormones. In a model of chronic inflammatory pain that was induced by Complete Freund’s Adjuvant administration, hyperalgesia was higher in proestrus, and dynorphin expression was upregulated in the ipsilateral spinal cord in freely cycling females in diestrus and proestrus. The same study showed that prodynorphin expression was upregulated in the spinal cord in ovariectomized females and downregulated in castrated males compared with intact males [94]. However, in the absence of chronic inflammatory pain, systemic treatment with a KOR agonist produced analgesia in male but not female mice, and the lack of analgesia in females was estrogen-dependent [95]. The intrathecal administration of a KOR agonist induced greater analgesia in males and was estrous phase-dependent in females; in gonadectomized rats, estradiol enhanced analgesia and upregulated KOR expression in females but not males, and testosterone had no effect [90]. Further studies are needed to disentangle the role of estrogen in KOR-induced analgesia in acute and chronic pain.
Chronic pain recruits cortical and subcortical structures through ascending and descending pathways. In chronic pain models, there is higher KOR binding in the CeA [96–98]. The administration of KOR antagonists systemically or directly into the amygdala [97], anterior cingulate cortex [99], and nucleus accumbens shell [100] reversed the aversive component of chronic pain, measured by a decrease in the consumption of, or motivation for, palatable substances (e.g., sucrose). Chronic pain upregulated KOR and dynorphin expression in the nucleus accumbens and midbrain and enhanced KOR agonist-induced aversion in male but not female mice. κ-Opioid receptor antagonism prevented pain-induced aversion in males only but reversed chronic pain-induced anxiety- and depressive-like behaviors in both sexes [101]. In female rats, chronic inflammatory pain led to anhedonia- and anxiety-like behavior, and KOR antagonism in the nucleus accumbens prevented the emergence of anxiety-like responses [102]. However, systemic administration of the KOR antagonist JDtic failed to block conditioned place aversion that was produced by visceral and acetic acid-induced pain in mice [103]. In addition to its effects on motivation and negative affect in chronic pain models, KOR antagonism has been shown to reverse sciatic nerve ligation-induced deficits in total, non-rapid-eye-movement, and fragmented sleep [104].
One suggested mechanism for the effects of dynorphin and KORs in the emotional component of pain is the modulation of dopamine transmission. This is evident in chronic pain models, where systemic KOR antagonism blocked the reduction of dopamine release in the nucleus accumbens in inflammatory pain [105]. However, KOR antagonism did not block lactic acid-induced elevations of brain reward thresholds and the decrease in nucleus accumbens dopamine in rats [106].
In summary, despite some contradictory findings, the dynorphin/KOR system is proposed to be an important mediator of negative affect that is associated with chronic pain. The pain modality and dependent variable that is used to measure negative affect are likely important, as well as the dynorphin neuron subcircuits' opposing actions [107, 108].
Dynorphin and KOR: Negative Affect
Plasma levels of dynorphin positively correlated with the severity of depression [109]. There is an extensive body of work on dysphoric effects of KOR activation and its involvement in depression [110]. The short-acting KOR antagonist aticaprant is being developed for the treatment of anhedonia [111]. The development of KOR antagonists for the treatment of depression is supported by intracranial self-stimulation experiments. κ-Opioid receptor agonists elevated intracranial self-stimulation reward thresholds and decreased the function of the mesolimbic dopamine system [112–116]. κ-Opioid receptor agonists also induced conditioned place aversion [95, 117–119]. κ-Opioid receptor agonism-induced aversion is at least partially mediated by serotonin and dopamine [114, 117, 118] and is less evident in females, an effect that is mediated by estrogen [95]. Anhedonic-like effects of the KOR agonist U50,488 were less evident in females, an effect that was associated with higher tyrosine hydroxylase, an enzyme that is essential for the production of dopamine, in the ventral tegmental area and differential patterns of brain activation, with upregulations in the paraventricular nucleus of the thalamus and BNST [115, 116].
Further evidence of the involvement of KORs in negative affect comes from models of stress-induced anhedonia. κ-Opioid receptor agonism activates the hypothalamic–pituitary–adrenal axis, reflected by an increase in the release of adrenocorticotropic hormone and corticosterone, an increase in the phosphorylation of glucocorticoid receptors, and Fos activation in the thalamus in female rats [120]. Dynorphin and phosphorylated KORs are upregulated in the ventral pallidum and inhibit GABAergic neurons to promote anhedonia-like behavior [121]. The KOR-mediated inhibition of glutamatergic projections from the claustrum to prelimbic cortex was sufficient to promote anhedonia-like behavior [122]. Deleterious effects of chronic social defeat can be prevented by treatment with the long-lasting KOR antagonist norbinaltorphimine [123]. κ-Opioid receptor antagonism also prevented working memory deficits in the face of a stressor in rhesus monkeys [124].
Dynorphin and KORs are also involved in anxiety-like behavior and fear responses. The genetic knockout of KORs selectively in dopaminergic neurons reduced anxiety-like behavior [125]. The deletion of KORs in the CeA and ablation of dynorphin inputs to the CeA increased anxiety-like behavior and impaired conditioned threat discrimination [126]. Dynorphin transmission in the prefrontal cortex suppressed defensive behaviors in response to threat [127]. κ-Opioid receptor stimulation in the caudal portion of the nucleus accumbens shell resulted in the greater inhibition of dopamine release and coincided with the emergence of avoidance behavior [128]. Threat generalization was modulated by KORs in a dorsal raphe-to-ventral tegmental area projection-specific manner [129]. κ-Opioid receptors in the basolateral amygdala regulated anxiety-like behavior and mediated KOR agonist-induced aversion [130]. Upregulation of the dynorphin/KOR system in the amygdala led to the emergence of depression-like behavior following chronic social defeat stress [123]. Altogether, these data suggest the involvement of a dynorphin/KOR system in the dorsal raphe to ventral tegmental area to nucleus accumbens shell and its reciprocal connection with the CeA and basolateral amygdala in the modulation of negative affect.
Dynorphin and KOR: Drug Addiction
It has been hypothesized that the activation of KORs leads to the negative emotional states that are associated with drug withdrawal [131], pain, and particularly pain associated with acute withdrawal. More specific to opioid dependence, animal studies have demonstrated region-specific increases in dynorphin levels after the passive administration of morphine or heroin in the brain [132, 133] and spinal cord [56, 134, 135]. The precursor of dynorphin, prodynorphin, was upregulated after heroin self-administration [136–138], and dynorphin was also upregulated during the anticipation of heroin self-administration [139].
The genetic deletion of KORs prevented the appearance of naloxone-precipitated withdrawal signs and mitigated social interaction deficits observed during withdrawal in male mice [140]. Administering norbinaltorphimine, a selective antagonist of KOR, 5 h before naltrexone-precipitated withdrawal in morphine-dependent rats decreased certain signs of opioid withdrawal during a 30-min withdrawal session and lowered the subsequent conditioned place aversion to the withdrawal chamber 2 days later [141]. Intraventricular and intra-dorsal hippocampus infusions of norbinaltorphimine blocked naloxone-induced conditioned place aversion when given before naloxone [142]. However, opposite effects were observed when norbinaltorphimine was administered before morphine exposure, and they coincided with a decrease in dopamine release in the nucleus accumbens [143].
The KOR antagonist norbinaltorphimine has been shown to improve myriad opioid withdrawal-related behaviors. Administered systemically or intra-nucleus accumbens shell, norbinaltorphimine reduced the development of addiction-like behavior and anxiety-like behavior during withdrawal from heroin in male rats [138]. In male mice, treatment with norbinaltorphimine was sufficient to prevent and reverse heroin abstinence-induced social avoidance [144] and morphine abstinence-induced anhedonia [145]. Systemic KOR antagonism with either norbinaltorphimine or 5’-guanidinonaltrindole reversed heroin withdrawal-induced hyperalgesia in male and female rats [59], an effect that was recapitulated with an intra-CeA infusion of norbinaltorphimine [146].
The reduction of dopamine release caused by dynorphin, particularly in the nucleus accumbens shell, was also hypothesized to modulate aversive/rewarding aspects of drug intake [147]. Acute and protracted withdrawal from morphine upregulated KORs in the nucleus accumbens. The conditional deletion of KORs in the nucleus accumbens prevented withdrawal-induced anhedonia, and a local norbinaltorphimine infusion reversed it [145]. The KOR agonist U50,488H reduced dopamine release in the nucleus accumbens in rats that self-administered heroin, resulting in an increase in immediate heroin intake [148]. κ-Opioid receptor antagonists did not block the acute rewarding effects of opioids but blocked the stress-induced potentiation of opioid reward, the stress-induced reinstatement of opioid-seeking behavior, and the escalation of opioid intake in an extended access model [138].
Other less extensively studied brain regions that may mediate aversive effects of dynorphin and KORs in opioid dependence include the prefrontal cortex, hippocampus, amygdala, and dorsal raphe. For example, morphine withdrawal caused the release of dynorphin in the prelimbic cortex, leading to working memory deficits [149], whereas the inhibition of a dynorphinergic basolateral amygdala-to-ventral hippocampus projection reversed morphine withdrawal-induced anxiety-like behavior [150]. Morphine abstinence activated dynorphin/KOR signaling in the basolateral amygdala, facilitating glutamate transporter 1 upregulation that led to the inhibition of excitatory inputs to the nucleus accumbens. The activation of basolateral amygdala-to-nucleus accumbens inputs blocked depression-like behavior following morphine abstinence [151].
The dynorphin/KOR modulation of serotonergic transmission has been implicated in negative affect-like effects of opioid withdrawal [144, 152, 153]. Protracted abstinence from morphine led to robust social interaction deficits in mice, and this effect was mediated by KORs in the nucleus accumbens shell and by suppressing the release of serotonin [152].
Dynorphin has long been known to be activated by chronic alcohol administration [154] and chronic psychostimulant administration [6, 155]. For alcohol, polymorphisms of the gene that encodes the KOR (Opkr1) have been associated with the severity of alcohol use disorder and depression [156], whereas polymorphisms of the Pdyn gene have been associated with alcohol dependence [157] and the propensity to drink in negative emotional states [158], an effect that appears to be more prominent in men [159].
During acute alcohol withdrawal there was an increase in prodynorphin mRNA expression in the CeA [160] and in the nucleus accumbens [161]. Similar findings are seen in the CeA and hypothalamus in alcohol-preferring rats compared with non-preferring rats after voluntary alcohol consumption [162].
Dynorphin signaling is hypothesized to drive behavioral effects of acute withdrawal from alcohol. A KOR antagonist reversed anxiety-like acute withdrawal effects of exposure to chronic, intermittent alcohol exposure in mice [163] and somatic withdrawal and alcohol self-administration in rats [164]. Again, a prominent, proposed mechanism is that alcohol withdrawal exacerbates the inhibition of dopamine release in the nucleus accumbens, which is normalized by KOR antagonism [154, 163, 165, 166].
Dynorphin/KOR signaling in the extended amygdala also has been shown to mediate compulsive-like drinking in alcohol dependence [167]. A combination of a short-acting KOR antagonist and naltrexone decreased voluntary alcohol drinking in mice [168]. Both systemic and intracerebral KOR antagonist administration blocked high compulsive-like alcohol intake [169, 170]. Antagonism of KORs reduced the stress-induced escalation of intake in mice that were exposed to chronic intermittent alcohol [171–173]. The chemogenetic inhibition of KORs in the CeA reduced drinking in alcohol-dependent rats, similar to microinfusions of norbinaltorphimine in the CeA and BNST [174]. The CeA may also mediate effects of KOR blockade on binge-like drinking in mice [173, 175].
The rostro-caudal location of dynorphin and KOR cells in the nucleus accumbens has been shown to bi-directionally modulate alcohol drinking. The stimulation of KORs in the caudal shell stimulated drinking, whereas stimulation in the rostral shell decreased drinking [176]. The bi-directional differences in alcohol intake coincided with the KOR-mediated inhibition of dopamine release in rostral vs. caudal portions of the shell [177]. κ-Opioid receptor expression was downregulated in the ventral tegmental area following alcohol exposure [178], and KOR overexpression in ventral tegmental area dopaminergic neurons that project to the nucleus accumbens led to compulsive-like alcohol intake [179]. The nucleus accumbens shell also plays a role in the intersection between pain and alcohol, in which chronic inflammatory pain increased alcohol consumption following abstinence in female rats, an effect that was blocked by KOR antagonism [180].
Other regions have been implicated in actions of dynorphin in addiction-like effects of alcohol. Dynorphin deletion and KOR blockade in the insula reduced the escalation of alcohol intake in a two-bottle choice intermittent-access model [181]. κ-Opioid receptor antagonism in the PAG attenuated alcohol withdrawal-induced anxiety- and depressive-like behaviors [182]. Medial prefrontal cortex KORs mediated working memory deficits in alcohol dependence [183].
Most evidence of the involvement of dynorphin and KORs in the neurobiology of stimulant addiction is related to the regulation of dopamine transmission. Binge-like cocaine self-administration upregulated prodynorphin and KOR expression in the nucleus accumbens and ventral tegmental area and increased KOR agonism-induced dopamine release in the nucleus accumbens [184, 185]. A midbrain dopamine circuit was also shown to modulate cocaine-induced conditioned place preference, dynorphin projections from the dorsal raphe to the ventral tegmental area led to dopamine release in response to cocaine [186]. Systemic [187] and intra-nucleus accumbens core [188] KOR antagonist administration prevented the escalation of cocaine intake in an extended-access model in rats, and these treatments reversed cocaine withdrawal-induced anxiety-like behavior [189]. Extended access to methamphetamine upregulated prodynorphin in the nucleus accumbens core and shell, and systemic and intra-nucleus accumbens shell (but not core) KOR antagonist administration prevented the escalation of methamphetamine intake and decreased the motivation to seek the drug [190]. Methamphetamine withdrawal-induced cognitive deficits may also be mediated by KORs in the hippocampus [191] and prelimbic prefrontal cortex [192].
In nicotine dependence, there was an upregulation of KORs and Oprk1 expression in the prefrontal cortex, nucleus accumbens, and hippocampus [193]. Moreover, a KOR agonist promoted the reinstatement of nicotine self-administration and conditioned place preference [194–196]. Evidence also indicates that dynorphin and KORs might play a role in cannabis dependence. Withdrawal from chronic systemic exposure to tetrahydrocannabinol upregulated dynorphin and KORs in the nucleus accumbens [197].
Conclusion
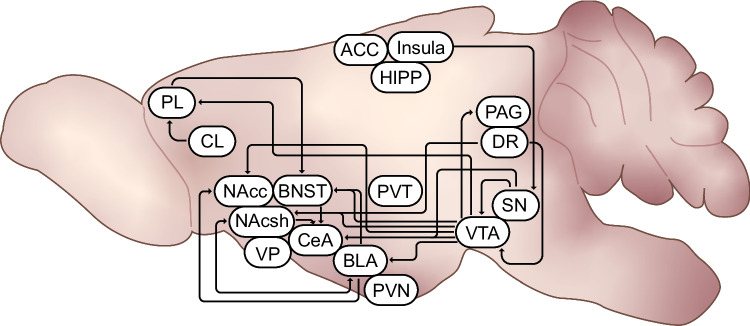
Dynorphin and KORs modulate neurotransmission in the key circuits that are implicated in the interface of chronic pain states, stress, and addiction with a focus on the construct of hyperkatifeia. The circuits and brain regions where KORs and dynorphin modulate behaviors that underlie the hyperalgesia and hyperkatifeia constructs are summarized in Fig. 1. A particular focal point is the modulation of emotional pain or hyperkatifeia by the dynorphin/KOR system via the mesocorticolimbic dopamine system in the context of addiction. Although there is evidence of sexual dimorphism and a role for estrogen in the function of dynorphin and KORs, most published studies have utilized male rodents only. Those that used both male and female subjects frequently presented the data of both sexes combined. In conclusion, we propose that modulation of the dynorphin/KOR system is a promising target for addressing hyperalgesia and hyperkatifeia during opioid withdrawal, which could potentially be used for the treatment of opioid use disorder and addiction more generally.
Fig. 1.
Dynorphin/KOR pathways putatively underlying hyperalgesia and hyperkatifeia. The figure summarizes brain regions and circuits in which dynorphin/KOR signaling has been show to modulate pain, anhedonia, anxiety, motivation, aversion or drug seeking. PL: prelimbic region of prefrontal cortex, CL: claustrum, ACC: anterior cingulate cortex, Insula: insular cortex; HIPP: hippocampus, PAG: periaqueductal grey, DR: dorsal raphe, SN: substantia nigra, VTA: ventral tegmental area, PVN: paraventricular nucleus of the hypothalamus, PVH: paraventricular nucleus of thalamus; CeA: central nucleus of the amygdala, BLA: basolateral amygdala, BNST: bed nucleus of the stria terminalis, NAcc: nucleus accumbens core, NAcsh: nucleus accumbens shell, VP: ventral pallidum
Acknowledgements
We thank National Institute on Drug Abuse Visual Media for their assistance with the illustration and Michael Arends for copyediting.
Author Contributions
RCNM wrote the first manuscript draft and conceptualized the figure. All authors reviewed the manuscript.
Funding
Open access funding provided by the National Institutes of Health. This work was supported by National Institutes of Health Intramural Research Program funding (ZIA-DA000602, National Institute on Drug Abuse, Neurobiology of Addiction Section, PI: GFK; ZIA-DA000644, National Institute on Drug Abuse/National Institute on Alcohol Abuse and Alcoholism, Stress and Addiction Neuroscience Unit, PI: LFV). RCNM received funding from the Center on Compulsive Behaviors, National Institutes of Health, via the National Institutes of Health Shared Resource Subcommittee and Pathway for Independence Award 1K99DA056618—01A1 from the National Institute on Drug Abuse.
Data Availability
No datasets were generated or analyzed during the current study.
Declarations
Human and Animal Rights and Informed Consent
No animal or human subjects by the authors were used in this study.
Competing Interests
The authors declare no competing interests.
Footnotes
The Figure 1 legend was missing in the published article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
3/20/2025
A Correction to this paper has been published: 10.1007/s40429-025-00643-w
References
- 1.Degenhardt L, Charlson F, Ferrari A, et al. The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet Psychiatry. 2018;5:987–1012. 10.1016/S2215-0366(18)30337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Higgins C, Smith BH, Matthews K. Evidence of opioid-induced hyperalgesia in clinical populations after chronic opioid exposure: a systematic review and meta-analysis. Br J Anaesth. 2019;122:e114–26. 10.1016/j.bja.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 3.Koob GF. Neurobiology of opioid addiction: opponent process, hyperkatifeia, and negative reinforcement. Biol Psychiat. 2020;87:44–53. 10.1016/j.biopsych.2019.05.023. [DOI] [PubMed] [Google Scholar]
- 4.Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–8. 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 5.Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the “dark side” of drug addiction. Nat Neurosci. 2005;8:1442–4. 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- 6.Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 7.Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–73. 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwako LE, Momenan R, Litten RZ, et al. Addictions neuroclinical assessment: a neuroscience-based framework for addictive disorders. Biol Psychiatry. 2016;80:179–89. 10.1016/j.biopsych.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwako LE, Schwandt ML, Ramchandani VA, et al. Neurofunctional domains derived from deep behavioral phenotyping in alcohol use disorder. Am J Psychiatry. 2019;176:744–53. 10.1176/appi.ajp.2018.18030357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shurman J, Koob GF, Gutstein HB. Opioids, pain, the brain, and hyperkatifeia: a framework for the rational use of opioids for pain. Pain Med. 2010;11:1092–8. 10.1111/j.1526-4637.2010.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cahill CM, Taylor AM. Neuroinflammation—a co-occurring phenomenon linking chronic pain and opioid dependence. Curr Opin Behav Sci. 2017;13:171–7. 10.1016/j.cobeha.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clin J Pain. 2008;24:479–96. 10.1097/AJP.0b013e31816b2f43. [DOI] [PubMed] [Google Scholar]
- 13.Gallager R, Koob G, Popescu A. The Pathophysiology of Chronic Pain and Clinical Interfaces with Addiction. In: The ASAM principles of addiction medicine. 5th ed. Philadelphia: Wolters Kluwer; 2014. p. 1435–56. [Google Scholar]
- 14.Koob GF. Drug addiction: Hyperkatifeia/negative reinforcement as a framework for medications development. Pharmacol Rev. 2021;73:163–201. 10.1124/pharmrev.120.000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferguson E, Lewis B, Teitelbaum S, et al. Longitudinal associations between pain and substance use disorder treatment outcomes. J Subst Abuse Treat. 2022;143:108892. 10.1016/j.jsat.2022.108892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans CJ, Cahill CM. Neurobiology of opioid dependence in creating addiction vulnerability. F1000Res. 2016;5:1748. 10.12688/f1000research.8369.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lutz P-E, Kieffer BL. Opioid receptors: distinct roles in mood disorders. Trends Neurosci. 2013;36:195–206. 10.1016/j.tins.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ballantyne JC, Sullivan MD, Koob GF. Refractory dependence on opioid analgesics. Pain. 2019;160:2655–60. 10.1097/j.pain.0000000000001680. [DOI] [PubMed] [Google Scholar]
- 19.Dowell D, Ragan KR, Jones CM, et al. CDC clinical practice guideline for prescribing opioids for pain — United States, 2022. MMWR Recomm Rep. 2022;71:1–95. 10.15585/mmwr.rr7103a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ballantyne JC, Koob GF. Allostasis theory in opioid tolerance. Pain. 2021;162:2315–9. 10.1097/j.pain.0000000000002280. [DOI] [PubMed] [Google Scholar]
- 21.Coloma-Carmona A, Carballo JL, Rodríguez-Marín J, Pérez-Carbonell A. Withdrawal symptoms predict prescription opioid dependence in chronic pain patients. Drug Alcohol Depend. 2019;195:27–32. 10.1016/j.drugalcdep.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Ho A, Dole VP. Pain perception in drug-free and in methadone-maintained human ex-addicts. Exp Biol Med. 1979;162:392–5. 10.3181/00379727-162-40689. [DOI] [PubMed] [Google Scholar]
- 23.Ren Z-Y, Shi J, Epstein DH, et al. Abnormal pain response in pain-sensitive opiate addicts after prolonged abstinence predicts increased drug craving. Psychopharmacology. 2009;204:423–9. 10.1007/s00213-009-1472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trøstheim M, Eikemo M. Hyperalgesia in patients with a history of opioid use disorder: a systematic review and meta-analysis. JAMA Psychiat. 2024. 10.1001/jamapsychiatry.2024.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Compton MA. Cold-pressor pain tolerance in opiate and cocaine abusers: Correlates of drug type and use status. J Pain Symptom Manage. 1994;9:462–73. 10.1016/0885-3924(94)90203-8. [DOI] [PubMed] [Google Scholar]
- 26.Liebmann PM, Lehofer M, Moser M, et al. Persistent analgesia in former opiate addicts is resistant to blockade of endogenous opioids. Biol Psychiat. 1997;42:962–4. 10.1016/S0006-3223(97)00337-5. [DOI] [PubMed] [Google Scholar]
- 27.Carcoba LM, Contreras AE, Cepeda-Benito A, Meagher MW. Negative affect heightens opiate withdrawal-induced hyperalgesia in heroin dependent individuals. J Addict Dis. 2011;30:258–70. 10.1080/10550887.2011.581985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Compton P, Charuvastra VC, Ling W. Pain intolerance in opioid-maintained former opiate addicts: effect of long-acting maintenance agent. Drug Alcohol Depend. 2001;63:139–46. 10.1016/S0376-8716(00)00200-3. [DOI] [PubMed] [Google Scholar]
- 29.Doverty M, White JM, Somogyi AA, et al. Hyperalgesic responses in methadone maintenance patients. Pain. 2001;90:91–6. 10.1016/S0304-3959(00)00391-2. [DOI] [PubMed] [Google Scholar]
- 30.Potter JS, Prather K, Weiss RD. Physical pain and associated clinical characteristics in treatment-seeking patients in four substance use disorder treatment modalities. American J Addict. 2008;17:121–5. 10.1080/10550490701862902. [DOI] [PubMed] [Google Scholar]
- 31.Prosser JM, Steinfeld M, Cohen LJ, et al. Abnormal heat and pain perception in remitted heroin dependence months after detoxification from methadone-maintenance. Drug Alcohol Depend. 2008;95:237–44. 10.1016/j.drugalcdep.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 32.Compton P, Canamar CP, Hillhouse M, Ling W. Hyperalgesia in heroin dependent patients and the effects of opioid substitution therapy. J Pain. 2012;13:401–9. 10.1016/j.jpain.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Treister PhDR, Eisenberg MdE, Lawental DE, Pud PhDD. Is opioid-induced hyperalgesia reversible? A study on active and former opioid addicts and drug naïve controls. JOM. 2012;8:343–9. 10.5055/jom.2012.0134. [DOI] [PubMed] [Google Scholar]
- 34.Rieb LM, DeBeck K, Hayashi K, et al. Withdrawal-associated injury site pain prevalence and correlates among opioid-using people who inject drugs in Vancouver, Canada. Drug Alcohol Dependence. 2020;216:108242. 10.1016/j.drugalcdep.2020.108242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wasserman RA, Hassett AL, Harte SE, et al. Pressure sensitivity and phenotypic changes in patients with suspected opioid-induced hyperalgesia being withdrawn from full mu agonists. J Nat Sci. 2017;3:e319. [PMC free article] [PubMed] [Google Scholar]
- 36.Pud D, Cohen D, Lawental E, Eisenberg E. Opioids and abnormal pain perception: New evidence from a study of chronic opioid addicts and healthy subjects. Drug Alcohol Depend. 2006;82:218–23. 10.1016/j.drugalcdep.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 37.Angst MS, Koppert W, Pahl I, et al. Short-term infusion of the μ-opioid agonist remifentanil in humans causes hyperalgesia during withdrawal. Pain. 2003;106:49–57. 10.1016/S0304-3959(03)00276-8. [DOI] [PubMed] [Google Scholar]
- 38.Wanigasekera V, Lee MCH, Rogers R, et al. Neural correlates of an injury-free model of central sensitization induced by opioid withdrawal in humans. J Neurosci. 2011;31:2835–42. 10.1523/JNEUROSCI.5412-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Comelon M, Raeder J, Stubhaug A, et al. Gradual withdrawal of remifentanil infusion may prevent opioid-induced hyperalgesia. Br J Anaesth. 2016;116:524–30. 10.1093/bja/aev547. [DOI] [PubMed] [Google Scholar]
- 40.Compton P, Athanasos P, Elashoff D. Withdrawal hyperalgesia after acute opioid physical dependence in nonaddicted humans: a preliminary study. J Pain. 2003;4:511–9. 10.1016/j.jpain.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 41.Yam M, Loh Y, Tan C, et al. general pathways of pain sensation and the major neurotransmitters involved in pain regulation. IJMS. 2018;19:2164. 10.3390/ijms19082164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hemington KS, Coulombe M-A. The periaqueductal gray and descending pain modulation: why should we study them and what role do they play in chronic pain? J Neurophysiol. 2015;114:2080–3. 10.1152/jn.00998.2014. [DOI] [PubMed] [Google Scholar]
- 43.Bagley EE, Ingram SL. Endogenous opioid peptides in the descending pain modulatory circuit. Neuropharmacology. 2020;173:108131. 10.1016/j.neuropharm.2020.1081312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolff BG, Michelassi F, Gerkin TM, et al. Alvimopan, a novel, peripherally acting μ opioid antagonist: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial of major abdominal surgery and postoperative Ileus. Ann Surg. 2004;240:728–35. 10.1097/01.sla.0000141158.27977.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yuan C-S, Israel RJ. Methylnaltrexone, a novel peripheral opioid receptor antagonist for the treatment of opioid side effects. Expert Opin Investig Drugs. 2006;15:541–52. 10.1517/13543784.15.5.541. [DOI] [PubMed] [Google Scholar]
- 46.Lang-Illievich K, Lang J, Rumpold-Seitlinger G, et al. The dose-response relationship between opioid agonist therapy and alterations in pain pathways in patients with opioid use disorders: a cross-sectional study. CNS Drugs. 2024;38:281–90. 10.1007/s40263-024-01069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rossbach M. Ueber die Gewoehnung an Gifte. Pflugers Arch Gesamte Physiol Menschen. 1880;21:213–25. [Google Scholar]
- 48.Eddy NB, Halbach H, Isbell H, Seevers MH. Drug dependence: its significance and characteristics. Bull World Health Organ. 1965;32:721–33. [PMC free article] [PubMed] [Google Scholar]
- 49.Handelsman L, Aronson MJ, Ness R, et al. The dysphoria of heroin addiction. Am J Drug Alcohol Abuse. 1992;18:275–87. 10.3109/00952999209026067. [DOI] [PubMed] [Google Scholar]
- 50.Kanof PD, Handelsman L, Aronson MJ, et al. Clinical characteristics of naloxone-precipitated withdrawal in human opioid-dependent subjects. J Pharmacol Exp Ther. 1992;260:355–63. [PubMed] [Google Scholar]
- 51.Hall OT, Entrup P, Farabee K, et al. The perceived role of withdrawal in maintaining opioid addiction among adults with untreated opioid use disorder: a survey of syringe exchange program participants. Subst Use Misuse. 2024;59:312–5. 10.1080/10826084.2023.2269571. [DOI] [PubMed] [Google Scholar]
- 52.Hall OT, Vilensky M, Teater JE, et al. Withdrawal catastrophizing scale: initial psychometric properties and implications for the study of opioid use disorder and hyperkatifeia. Am J Drug Alcohol Abuse. 2014;1–13. 10.1080/00952990.2023.2298257. [DOI] [PubMed]
- 53.Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–23. 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- 54.Zhukovsky P, Ironside M, Duda JM, et al. Acute stress increases striatal connectivity with cortical regions enriched for μ- and κ-opioid receptors. Biol Psychiat. 2024. 10.1016/j.biopsych.2024.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Célèrier E, Laulin JP, Corcuff JB, et al. Progressive enhancement of delayed hyperalgesia induced by repeated heroin administration: a sensitization process. J Neurosci. 2001;21:4074–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vanderah TW, Ossipov MH, Lai J, et al. Mechanisms of opioid-induced pain and antinociceptive tolerance: Descending facilitation and spinal dynorphin. Pain. 2001;92:5–9. 10.1016/S0304-3959(01)00311-6. [DOI] [PubMed] [Google Scholar]
- 57.Simonnet G, Rivat C. Opioid-induced hyperalgesia: abnormal or normal pain? NeuroReport. 2003;14:1–7. 10.1097/00001756-200301200-00001. [DOI] [PubMed] [Google Scholar]
- 58.Li Z, Yin P, Chen J, et al. CaMKIIα may modulate fentanyl-induced hyperalgesia via a CeLC-PAG-RVM-spinal cord descending facilitative pain pathway in rats. PLoS ONE. 2017;12:e0177412. 10.1371/journal.pone.0177412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marchette RCN, Gregory-Flores A, Tunstall BJ, et al. κ-Opioid receptor antagonism reverses heroin withdrawal-induced hyperalgesia in male and female rats. Neurobiol Stress. 2021;14:100325. 10.1016/j.ynstr.2021.100325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alvarez-Bagnarol Y, Marchette RCN, Francis C, et al. Neuronal Correlates of Hyperalgesia and Somatic Signs of Heroin Withdrawal in Male and Female Mice. eNeuro. 2022;9:ENEURO.0106–22.2022. 10.1523/ENEURO.0106-22.2022. [DOI] [PMC free article] [PubMed]
- 61.Hastings LE, Frye EV, Carlson ER, et al. Cold nociception as a measure of hyperalgesia during spontaneous heroin withdrawal in mice. Pharmacol Biochem Behav. 2024;235:173694. 10.1016/j.pbb.2023.173694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Laulin J, Larcher A, Célèrier E, et al. Long-lasting increased pain sensitivity in rat following exposure to heroin for the first time. Eur J Neurosci. 1998;10:782–5. 10.1046/j.1460-9568.1998.00083.x. [DOI] [PubMed] [Google Scholar]
- 63.Elhabazi K, Trigo J, Mollereau C, et al. Involvement of neuropeptide FF receptors in neuroadaptive responses to acute and chronic opiate treatments. British J Pharmacology. 2012;165:424–35. 10.1111/j.1476-5381.2011.01563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park PE, Schlosburg JE, Vendruscolo LF, et al. Chronic CRF1 receptor blockade reduces heroin intake escalation and dependence-induced hyperalgesia. Addict Biol. 2015;20:275–84. 10.1111/adb.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaplan H, Fields HL. Hyperalgesia during acute opioid abstinence: evidence for a nociceptive facilitating function of the rostral ventromedial medulla. J Neurosci. 1991;11:1433–9. 10.1523/JNEUROSCI.11-05-01433.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vera-Portocarrero LP, Ossipov MH, Lai J, et al. Descending facilitatory pathways from the rostroventromedial medulla mediate naloxone-precipitated withdrawal in morphine-dependent rats. J Pain. 2011;12:667–76. 10.1016/j.jpain.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hamlin AS, McNally GP, Osborne PB. Induction of c-Fos and zif268 in the nociceptive amygdala parallel abstinence hyperalgesia in rats briefly exposed to morphine. Neuropharmacology. 2007;53:330–43. 10.1016/j.neuropharm.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 68.Simpson S, Kimbrough A, Boomhower B et al. Depletion of the Microbiome Alters the Recruitment of Neuronal Ensembles of Oxycodone Intoxication and Withdrawal. 2020; eNeuro 7:ENEURO.0312–19.2020. 10.1523/ENEURO.0312-19.2020. [DOI] [PMC free article] [PubMed]
- 69.Harris GC, Aston-Jones G. Activation in extended amygdala corresponds to altered hedonic processing during protracted morphine withdrawal. Behav Brain Res. 2007;176:251–8. 10.1016/j.bbr.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.George DT, Ameli R, Koob GF. Periaqueductal gray sheds light on dark areas of psychopathology. Trends Neurosci. 2019;42:349–60. 10.1016/j.tins.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 71.Vázquez-León P, Miranda-Páez A, Chávez-Reyes J, et al. The periaqueductal gray and its extended participation in drug addiction phenomena. Neurosci Bull. 2021. 10.1007/s12264-021-00756-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsuchida H, Nonogaki M, Inoue N, et al. Dynorphin-κ-opioid receptor signaling, but not µ-opioid receptor signaling, partly mediates the suppression of luteinizing hormone release during late lactation in rats. Neurosci Lett. 2022;791:136920. 10.1016/j.neulet.2022.136920. [DOI] [PubMed] [Google Scholar]
- 73.Ding X, Rasband MN. Dynorphin, won’t you myelinate my neighbor? Neuron. 2021;109:3537–9. 10.1016/j.neuron.2021.10.027. [DOI] [PubMed] [Google Scholar]
- 74.Beck TC, Wilson EM, Wilkes E, et al. Kappa opioid agonists in the treatment of itch: just scratching the surface? Itch. 2023;8:e0072. 10.1097/itx.0000000000000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schwarzer C. 30 years of dynorphins — New insights on their functions in neuropsychiatric diseases. Pharmacol Ther. 2009;123:353–70. 10.1016/j.pharmthera.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chavkin C. Dynorphin–still an extraordinarily potent opioid peptide. Mol Pharmacol. 2013;83:729–36. 10.1124/mol.112.083337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Margolis EB, Moulton MG, Lambeth PS, O’Meara MJ. The life and times of endogenous opioid peptides: Updated understanding of synthesis, spatiotemporal dynamics, and the clinical impact in alcohol use disorder. Neuropharmacology. 2022;225:109376. 10.1016/j.neuropharm.2022.109376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ballouze R, Salhimi SM, Mohtar N, Fazalul Rahiman SS. Dynorphin 1–17 biotransformation peptides: properties, challenges and solutions for future therapeutics development. Future Med Chem. 2023. 10.4155/fmc-2023-0016. [DOI] [PubMed] [Google Scholar]
- 79.Chavkin C, James IF, Goldstein A. Dynorphin Is a Specific Endogenous Ligand of the κ Opioid Receptor. Science. 1982;215:413–5. 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- 80.Le Merrer J, Becker JAJ, Befort K, Kieffer BL. Reward Processing by the Opioid System in the Brain. Physiol Rev. 2009;89:1379–412. 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eguchi M. Recent advances in selective opioid receptor agonists and antagonists. Med Res Rev. 2004;24:182–212. 10.1002/med.10059. [DOI] [PubMed] [Google Scholar]
- 82.Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther. 2007;116:306–21. 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hjelmstad GO, Fields HL. Kappa opioid receptor activation in the nucleus accumbens inhibits glutamate and GABA release through different mechanisms. J Neurophysiol. 2003;89:2389–95. 10.1152/jn.01115.2002. [DOI] [PubMed] [Google Scholar]
- 84.Tejeda HA, Wu J, Kornspun AR, et al. Pathway- and cell-specific kappa-opioid receptor modulation of excitation-inhibition balance differentially gates D1 and D2 accumbens neuron activity. Neuron. 2017;93:147–63. 10.1016/j.neuron.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Crowley NA, Bloodgood DW, Hardaway JA, et al. Dynorphin controls the gain of an amygdalar anxiety circuit. Cell Rep. 2016;14:2774–83. 10.1016/j.celrep.2016.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pina MM, Pati D, Hwa LS, et al. The kappa opioid receptor modulates GABA neuron excitability and synaptic transmission in midbrain projections from the insular cortex. Neuropharmacology. 2020;165:107831. 10.1016/j.neuropharm.2019.107831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kang-Park M, Kieffer BL, Roberts AJ, et al. Interaction of CRF and kappa opioid systems on gabaergic neurotransmission in the mouse central amygdala. J Pharmacol Exp Ther. 2015;355:206–11. 10.1124/jpet.115.225870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vandera TW, Laughlin T, Lashbrook JM, et al. Single intrathecal injections of dynorphin A or des-Tyr-dynorphins produce long-lasting allodynia in rats: blockade by MK-801 but not naloxone. Pain. 1996;68:275–81. 10.1016/S0304-3959(96)03225-3. [DOI] [PubMed] [Google Scholar]
- 89.Laughlin TM, Vanderah TW, Lashbrook J, et al. Spinally administered dynorphin A produces long-lasting allodynia: involvement of NMDA but not opioid receptors. Pain. 1997;72:253–60. 10.1016/S0304-3959(97)00046-8. [DOI] [PubMed] [Google Scholar]
- 90.Lawson K, Nag S, Thompson A, Mokha S. Sex-specificity and estrogen-dependence of kappa opioid receptor-mediated antinociception and antihyperalgesia. Pain. 2010;151:806–15. 10.1016/j.pain.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang W-W, Cao H, Li Y, et al. Peripheral ablation of type III adenylyl cyclase induces hyperalgesia and eliminates KOR-mediated analgesia in mice. JCI Insight. 2022;7:e153191. 10.1172/jci.insight.153191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Z, Gardell LR, Ossipov MH, et al. Pronociceptive actions of dynorphin maintain chronic neuropathic pain. J Neurosci. 2001;21:1779–86. 10.1523/JNEUROSCI.21-05-01779.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Obara I, Mika J, Schäfer MK-H, Przewlocka B. Antagonists of the κ-opioid receptor enhance allodynia in rats and mice after sciatic nerve ligation. Br J Pharmacol. 2003;140:538–46. 10.1038/sj.bjp.0705427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bradshaw H, Miller J, Ling Q, et al. Sex differences and phases of the estrous cycle alter the response of spinal cord dynorphin neurons to peripheral inflammation and hyperalgesia. Pain. 2000;85:93–9. 10.1016/S0304-3959(99)00253-5. [DOI] [PubMed] [Google Scholar]
- 95.Abraham AD, Schattauer SS, Reichard KL, et al. Estrogen regulation of GRK2 inactivates kappa opioid receptor signaling mediating analgesia, but not aversion. J Neurosci. 2018;38:8031–43. 10.1523/JNEUROSCI.0653-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Narita M, Kaneko C, Miyoshi K, et al. Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharmacology. 2006;31:739–50. 10.1038/sj.npp.1300858. [DOI] [PubMed] [Google Scholar]
- 97.Nation KM, De Felice M, Hernandez PI, et al. Lateralized kappa opioid receptor signaling from the amygdala central nucleus promotes stress-induced functional pain. Pain. 2018;159:919–28. 10.1097/j.pain.0000000000001167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Navratilova E, Ji G, Phelps C, et al. Kappa opioid signaling in the central nucleus of the amygdala promotes disinhibition and aversiveness of chronic neuropathic pain. Pain. 2019;160:824–32. 10.1097/j.pain.0000000000001458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Navratilova E, Qu C, Ji G, et al. Opposing effects on descending control of nociception by µ and κ opioid receptors in the anterior cingulate cortex. Anesthesiology. 2024;140:272–83. 10.1097/ALN.0000000000004773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Massaly N, Copits BA, Wilson-Poe AR, et al. Pain-induced negative affect is mediated via recruitment of the nucleus accumbens kappa opioid system. Neuron. 2019;102:564-573.e6. 10.1016/j.neuron.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu S (Steve), Pickens S, Burma NE, et al. Kappa opioid receptors drive a tonic aversive component of chronic pain. J Neurosci. 2019. 39:4162–4178.10.1523/JNEUROSCI.0274-19.2019. [DOI] [PMC free article] [PubMed]
- 102.Lorente JD, Cuitavi J, Rullo L, et al. Sex-dependent effect of inflammatory pain on negative affective states is prevented by kappa opioid receptors blockade in the nucleus accumbens shell. Neuropharmacology. 2024;242:109764. 10.1016/j.neuropharm.2023.109764. [DOI] [PubMed] [Google Scholar]
- 103.Bagdas D, Muldoon PP, AlSharari S, et al. Expression and pharmacological modulation of visceral pain-induced conditioned place aversion in mice. Neuropharmacology. 2016;102:236–43. 10.1016/j.neuropharm.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ito H, Navratilova E, Vagnerova B, et al. Chronic pain recruits hypothalamic dynorphin/kappa opioid receptor signalling to promote wakefulness and vigilance. Brain. 2023;146:1186–99. 10.1093/brain/awac153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Narita M, Kishimoto Y, Ise Y, et al. Direct evidence for the involvement of the mesolimbic κ -opioid system in the morphine-induced rewarding effect under an inflammatory pain-like state. Neuropsychopharmacology. 2005;30:111–8. 10.1038/sj.npp.1300527. [DOI] [PubMed] [Google Scholar]
- 106.Leitl MD, Onvani S, Bowers MS, et al. Pain-related depression of the mesolimbic dopamine system in rats: expression, blockade by analgesics, and role of endogenous κ-opioids. Neuropsychopharmacol. 2014;39:614–24. 10.1038/npp.2013.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cahill CM, Taylor AMW, Cook C, et al. Does the kappa opioid receptor system contribute to pain aversion? Front Pharmacol. 2014;5:253. 10.3389/fphar.2014.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Massaly N, Morón JA, Al-Hasani R. A trigger for opioid misuse: chronic pain and stress dysregulate the mesolimbic pathway and kappa opioid system. Front Neurosci. 2016;10:480. 10.3389/fnins.2016.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang Y, Zhou B, Fang S, et al. Dynorphin participates in interaction between depression and non-erosive reflux disease. Esophagus. 2022. 10.1007/s10388-022-00955-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dagher M, Cahill CM, Evans CJ. Opioid systems and depression: the relationship is strengthening. Biol Psychiat. 2022;92:920–2. 10.1016/j.biopsych.2022.09.020. [DOI] [PubMed] [Google Scholar]
- 111.Krystal AD, Pizzagalli DA, Smoski M, et al. A randomized proof-of-mechanism trial applying the ‘fast-fail’ approach to evaluating κ-opioid antagonism as a treatment for anhedonia. Nat Med. 2020;26:760–8. 10.1038/s41591-020-0806-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA. Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology. 2004;172:463–70. 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- 113.Potter DN, Damez-Werno D, Carlezon WA, et al. Repeated exposure to the κ-opioid receptor agonist salvinorin a modulates extracellular signal-regulated kinase and reward sensitivity. Biol Psychiat. 2011;70:744–53. 10.1016/j.biopsych.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chefer VI, Bäckman CM, Gigante ED, Shippenberg TS. Kappa opioid receptors on dopaminergic neurons are necessary for kappa-mediated place aversion. Neuropsychopharmacol. 2013;38:2623–31. 10.1038/npp.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Russell SE, Rachlin AB, Smith KL, et al. Sex differences in sensitivity to the depressive-like effects of the kappa opioid receptor agonist U-50488 in rats. Biol Psychiat. 2014;76:213–22. 10.1016/j.biopsych.2013.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Conway SM, Puttick D, Russell S, et al. Females are less sensitive than males to the motivational- and dopamine-suppressing effects of kappa opioid receptor activation. Neuropharmacology. 2019;146:231–41. 10.1016/j.neuropharm.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Land BB, Bruchas MR, Schattauer S, et al. Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. PNAS. 2009;106:19168–73. 10.1073/pnas.0910705106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ehrich JM, Messinger DI, Knakal CR, et al. Kappa opioid receptor-induced aversion requires p38 MAPK activation in VTA dopamine neurons. J Neurosci. 2015;35:12917–31. 10.1523/JNEUROSCI.2444-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu JJ, Sharma K, Zangrandi L, et al. In vivo brain GPCR signaling elucidated by phosphoproteomics. Science. 2018;360:eaao4927. 10.1126/science.aao4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Varastehmoradi B, Smith KL, Müller HK, et al. Kappa opioid activation changes protein profiles in different regions of the brain relevant to depression. Eur Neuropsychopharmacol. 2023;72:9–17. 10.1016/j.euroneuro.2023.03.010. [DOI] [PubMed] [Google Scholar]
- 121.Ji M-J, Gao Z-Q, Yang J, et al. Dynorphin promotes stress-induced depressive behaviors by inhibiting ventral pallidal neurons in rats. Acta Physiol. 2022;236:e13882. 10.1111/apha.13882. [DOI] [PubMed] [Google Scholar]
- 122.Wang Y-J, Zan G-Y, Xu C, et al. The claustrum-prelimbic cortex circuit through dynorphin/κ-opioid receptor signaling underlies depression-like behaviors associated with social stress etiology. Nat Commun. 2023;14:7903. 10.1038/s41467-023-43636-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zan G, Sun X, Wang Y, et al. Amygdala dynorphin/κ opioid receptor system modulates depressive-like behavior in mice following chronic social defeat stress. Acta Pharmacol Sin. 2022;43:577–87. 10.1038/s41401-021-00677-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wallace TL, Martin WJ, Arnsten AFT. Kappa opioid receptor antagonism protects working memory performance from mild stress exposure in Rhesus macaques. Neurobiology of Stress. 2022;21:100493. 10.1016/j.ynstr.2022.100493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Van’t Veer A, Bechtholt AJ, Onvani S, et al. Ablation of kappa-opioid receptors from brain dopamine neurons has anxiolytic-like effects and enhances cocaine-induced plasticity. Neuropsychopharmacol. 2013;38:1585–97. 10.1038/npp.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Baird MA, Hsu TY, Wang R, et al. κ Opioid receptor-dynorphin signaling in the central amygdala regulates conditioned threat discrimination and anxiety. eNeuro. 2021;8:ENEURO.0370–20.2020. 10.1523/ENEURO.0370-20.2020. [DOI] [PMC free article] [PubMed]
- 127.Wang H, Flores RJ, Yarur HE, et al. Prefrontal cortical dynorphin peptidergic transmission constrains threat-driven behavioral and network states. 2014; Neuron S0896627324001934. 10.1016/j.neuron.2024.03.015. [DOI] [PMC free article] [PubMed]
- 128.Pirino BE, Spodnick MB, Gargiulo AT, et al. Kappa-opioid receptor-dependent changes in dopamine and anxiety-like or approach-avoidance behavior occur differentially across the nucleus accumbens shell rostro-caudal axis. Neuropharmacology. 2020;181:108341. 10.1016/j.neuropharm.2020.108341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fellinger L, Jo YS, Hunker AC, et al. A midbrain dynorphin circuit promotes threat generalization. Curr Biol. 2021;31:4388-4396.e5. 10.1016/j.cub.2021.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Paliarin F, Duplantis C, Jones AF, et al. A Cre Driver Line for Genetic Targeting of Kappa Opioid Receptor Expressing Cells. eNeuro. 2023;10:ENEURO.0043–23.2023. 10.1523/ENEURO.0043-23.2023. [DOI] [PMC free article] [PubMed]
- 131.Chavkin C, Koob GF. Dynorphin, dysphoria, and dependence: the stress of addiction. Neuropsychopharmacology. 2016;41:373–4. 10.1038/npp.2015.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Avi Weissman B, Zamir N. Differential effects of heroin on opioid levels in the rat brain. Eur J Pharmacol. 1987;139:121–3. 10.1016/0014-2999(87)90506-1. [DOI] [PubMed] [Google Scholar]
- 133.Nylander I, Vlaskovska M, Terenius L. Brain dynorphin and enkephalin systems in Fischer and Lewis rats: effects of morphine tolerance and withdrawal. Brain Res. 1995;683:25–35. 10.1016/0006-8993(95)00279-Y. [DOI] [PubMed] [Google Scholar]
- 134.Gardell LR, Ossipov MH, Vanderah TW, et al. Dynorphin-independent spinal cannabinoid antinociception. Pain. 2002;100:243–8. 10.1016/S0304-3959(02)00173-2. [DOI] [PubMed] [Google Scholar]
- 135.Gardell LR, King T, Ossipov MH, et al. Opioid receptor-mediated hyperalgesia and antinociceptive tolerance induced by sustained opiate delivery. Neurosci Lett. 2006;396:44–9. 10.1016/j.neulet.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 136.Solecki W, Ziolkowska B, Krowka T, et al. Alterations of prodynorphin gene expression in the rat mesocorticolimbic system during heroin self-administration. Brain Res. 2009;1255:113–21. 10.1016/j.brainres.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 137.Zhou Q, Carlsson A, Hallberg M, Nyberg F. Substance P N-terminal fragment SP(1–7) attenuates chronic morphine tolerance and affects dynorphin B and nociceptin in rats. Peptides. 2011;32:1661–5. 10.1016/j.peptides.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 138.Schlosburg JE, Whitfield TW, Park PE, et al. Long-term antagonism of κ opioid receptors prevents escalation of and increased motivation for heroin intake. J Neurosci. 2013;33:19384–92. 10.1523/JNEUROSCI.1979-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cappendijk SLT, Hurd YL, Nylander I, et al. A heroin-, but not a cocaine-expecting, self-administration state preferentially alters endogenous brain peptides. Eur J Pharmacol. 1999;365:175–82. 10.1016/S0014-2999(98)00874-7. [DOI] [PubMed] [Google Scholar]
- 140.Lutz P-E, Ayranci G, Chu-Sin-Chung P, et al. Distinct mu, delta, and kappa opioid receptor mechanisms underlie low sociability and depressive-like behaviors during heroin abstinence. Neuropsychopharmacol. 2014;39:2694–705. 10.1038/npp.2014.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kelsey JE, Verhaak AMS, Schierberl KC. The kappa-opioid receptor antagonist, nor-binaltorphimine (nor-BNI), decreases morphine withdrawal and the consequent conditioned place aversion in rats. Behav Brain Res. 2015;283:16–21. 10.1016/j.bbr.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 142.Chen Y, Wang C, Zan G, et al. Upregulation of dynorphin/kappa opioid receptor system in the dorsal hippocampus contributes to morphine withdrawal-induced place aversion. Acta Pharmacol Sin. 2023;44:538–45. 10.1038/s41401-022-00987-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Spanagel R, Almeida OF, Bartl C, Shippenberg TS. Endogenous kappa-opioid systems in opiate withdrawal: role in aversion and accompanying changes in mesolimbic dopamine release. Psychopharmacology. 1994;115:121–7. 10.1007/BF02244761. [DOI] [PubMed] [Google Scholar]
- 144.Lalanne L, Ayranci G, Filliol D, et al. Kappa opioid receptor antagonism and chronic antidepressant treatment have beneficial activities on social interactions and grooming deficits during heroin abstinence. Addict Biol. 2017;22:1010–21. 10.1111/adb.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang J, Lu Y, Jia M, et al. Kappa opioid receptor in nucleus accumbens regulates depressive-like behaviors following prolonged morphine withdrawal in mice. iScience. 2023;26:107536. 10.1016/j.isci.2023.107536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yakhnitsa V, Ji G, Hein M, et al. Kappa opioid receptor blockade in the amygdala mitigates pain like-behaviors by inhibiting corticotropin releasing factor neurons in a rat model of functional pain. Front Pharmacol. 2022;13:903978. 10.3389/fphar.2022.903978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Carlezon WA, Haile CN, Coppersmith R, et al. Distinct sites of opiate reward and aversion within the midbrain identified using a herpes simplex virus vector expressing gluR1. J Neurosci. 2000;20:RC62–RC62. 10.1523/JNEUROSCI.20-05-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xi ZX, Fuller SA, Stein EA. Dopamine release in the nucleus accumbens during heroin self-administration is modulated by kappa opioid receptors: an in vivo fast-cyclic voltammetry study. J Pharmacol Exp Ther. 1998;284:151–61. [PubMed] [Google Scholar]
- 149.Abraham AD, Casello SM, Schattauer SS, et al. Release of endogenous dynorphin opioids in the prefrontal cortex disrupts cognition. Neuropsychopharmacol. 2021;46:2330–9. 10.1038/s41386-021-01168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Deji C, Yan P, Ji Y, et al. The basolateral amygdala to ventral hippocampus circuit controls anxiety-like behaviors induced by morphine withdrawal. Front Cell Neurosci. 2022;16:894886. 10.3389/fncel.2022.894886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zan G-Y, Wang Y-J, Li X-P, et al. Amygdalar κ-opioid receptor-dependent upregulating glutamate transporter 1 mediates depressive-like behaviors of opioid abstinence. Cell Rep. 2021;37:109913. 10.1016/j.celrep.2021.109913. [DOI] [PubMed] [Google Scholar]
- 152.Pomrenze MB, Cardozo Pinto DF, Neumann PA, et al. Modulation of 5-HT release by dynorphin mediates social deficits during opioid withdrawal. Neuron. 2022. 10.1016/j.neuron.2022.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.West AM, Holleran KM, Jones SR. Kappa opioid receptors reduce serotonin uptake and escitalopram efficacy in the mouse substantia nigra pars reticulata. Int J Mol Sci. 2023;24:2080. 10.3390/ijms24032080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Karkhanis AN, Al-Hasani R. Dynorphin and its role in alcohol use disorder. Brain Res. 2020;1735:146742. 10.1016/j.brainres.2020.146742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nestler EJ. Historical review: Molecular and cellular mechanisms of opiate and cocaine addiction. Trends Pharmacol Sci. 2004;25:210–8. 10.1016/j.tips.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 156.Özkan-Kotiloğlu S, Kaya-Akyüzlü D, Yurdakul R, et al. Effect of PDYN and OPRK1 polymorphisms on the risk of alcohol use disorder and the intensity of depressive symptoms. Alcohol Alcoholism. 2023;agad036. 10.1093/alcalc/agad036. [DOI] [PubMed]
- 157.Xuei X, Dick D, Flury-Wetherill L, et al. Association of the κ-opioid system with alcohol dependence. Mol Psychiatry. 2006;11:1016–24. 10.1038/sj.mp.4001882. [DOI] [PubMed] [Google Scholar]
- 158.Karpyak VM, Winham SJ, Preuss UW, et al. Association of the PDYN gene with alcohol dependence and the propensity to drink in negative emotional states. Int J Neuropsychopharmacol. 2013;16:975–85. 10.1017/S1461145712001137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Winham SJ, Preuss UW, Geske JR, et al. Associations of prodynorphin sequence variation with alcohol dependence and related traits are phenotype-specific and sex-dependent. Sci Rep. 2015;5:15670. 10.1038/srep15670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kissler JL, Sirohi S, Reis DJ, et al. The one-two punch of alcoholism: role of central amygdala dynorphins/kappa-opioid receptors. Biol Psychiat. 2014;75:774–82. 10.1016/j.biopsych.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Przewłocka B, Turchan J, Lasoń W, Przewłocki R. Ethanol withdrawal enhances the prodynorphin system activity in the rat nucleus accumbens. Neurosci Lett. 1997;238:13–6. 10.1016/S0304-3940(97)00829-X. [DOI] [PubMed] [Google Scholar]
- 162.Zhou Y, Colombo G, Gessa GL, Kreek MJ. Effects of voluntary alcohol drinking on corticotropin-releasing factor and preprodynorphin mRNA levels in the central amygdala of Sardinian alcohol-preferring rats. Neurosci Lett. 2013;554:110–4. 10.1016/j.neulet.2013.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rose JH, Karkhanis AN, Chen R, et al. Supersensitive Kappa Opioid Receptors Promotes Ethanol Withdrawal-Related Behaviors and Reduce Dopamine Signaling in the Nucleus Accumbens. IJNPPY. 2016;19:pyv127. 10.1093/ijnp/pyv127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Flores-Ramirez FJ, Illenberger JM, Pascasio G, et al. LY2444296, a κ-opioid receptor antagonist, selectively reduces alcohol drinking in male and female Wistar rats with a history of alcohol dependence. Sci Rep. 2024;14:5804. 10.1038/s41598-024-56500-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Karkhanis AN, Huggins KN, Rose JH, Jones SR. Switch from excitatory to inhibitory actions of ethanol on dopamine levels after chronic exposure: Role of kappa opioid receptors. Neuropharmacology. 2016;110:190–7. 10.1016/j.neuropharm.2016.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Spodnick MB, Amirault RT, Towner TT, et al. Adolescent intermittent ethanol exposure effects on kappa opioid receptor mediated dopamine transmission: sex and age of exposure matter. Brain Sci. 2020;10:472. 10.3390/brainsci10080472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Borrego MB, Chan AE, Ozburn AR. Regulation of alcohol drinking by ventral striatum and extended amygdala circuitry. Neuropharmacology. 2022;212:109074. 10.1016/j.neuropharm.2022.109074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhou Y, Zhou D, Kreek M. Aticaprant (Clinically Developed Kappa-Opioid Receptor Antagonist) Combined With Naltrexone Prevents Alcohol “Relapse” Drinking. J Pharm Pharmacol (Los Angel). 2022; 9. 10.13188/2327-204x.1000032. [DOI] [PMC free article] [PubMed]
- 169.Hölter SM, Henniger MSH, Lipkowski AW, Spanagel R. Kappa-opioid receptors and relapse-like drinking in long-term ethanol-experienced rats. Psychopharmacology. 2000;153:93–102. 10.1007/s002130000601. [DOI] [PubMed] [Google Scholar]
- 170.Walker BM, Koob GF. Pharmacological evidence for a motivational role of κ-opioid systems in ethanol dependence. Neuropsychopharmacol. 2008;33:643–52. 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Anderson RI, Lopez MF, Becker HC. Forced swim stress increases ethanol consumption in C57BL/6J mice with a history of chronic intermittent ethanol exposure. Psychopharmacology. 2016;233:2035–43. 10.1007/s00213-016-4257-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Anderson RI, Lopez MF, Becker HC. Stress-induced enhancement of ethanol intake in C57BL/6J mice with a history of chronic ethanol exposure: involvement of kappa opioid receptors. Front Cell Neurosci. 2016;10:45. 10.3389/fncel.2016.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Anderson RI, Lopez MF, Griffin WC, et al. Dynorphin-kappa opioid receptor activity in the central amygdala modulates binge-like alcohol drinking in mice. Neuropsychopharmacol. 2019;44:1084–92. 10.1038/s41386-018-0294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Haun HL, Lebonville CL, Solomon MG, et al. Dynorphin/kappa opioid receptor activity within the extended amygdala contributes to stress-enhanced alcohol drinking in mice. Biol Psychiat. 2022;91:1019–28. 10.1016/j.biopsych.2022.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lebonville CL, Rinker JA, O’Hara K, et al. Real-time activity of dynorphin-expressing neurons in mouse central amygdala during alcohol drinking. 2024. 10.1101/2024.02.18.580880.
- 176.Pirino BE, Hawks A, Carpenter BA, et al. Kappa-opioid receptor stimulation in the nucleus accumbens shell and ethanol drinking: Differential effects by rostro-caudal location and level of drinking. Neuropsychopharmacology. 2024. 10.1038/s41386-024-01850-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Karkhanis AN, West AM, Jones SR. Kappa opioid receptor agonist U50,488 inhibits dopamine more in caudal than rostral nucleus accumbens core. Basic Clin Pharma Tox. 2023;133:526–34. 10.1111/bcpt.13929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rosin Å, Lindholm S, Franck J, Georgieva J. Downregulation of kappa opioid receptor mRNA levels by chronic ethanol and repetitive cocaine in rat ventral tegmentum and nucleus accumbens. Neurosci Lett. 1999;275:1–4. 10.1016/S0304-3940(99)00675-8. [DOI] [PubMed] [Google Scholar]
- 179.Lepreux G, Shinn GE, Wei G, et al. Recapitulating phenotypes of alcohol dependence via overexpression of Oprk1 in the ventral tegmental area of non-dependent TH: Cre rats. Neuropharmacology. 2023;228:109457. 10.1016/j.neuropharm.2023.109457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lorente JD, Cuitavi J, Campos-Jurado Y, et al. Kappa opioid receptor blockade in the nucleus accumbens shell prevents sex-dependent alcohol deprivation effect induced by inflammatory pain. Pain. 2022;163:e137. 10.1097/j.pain.0000000000002332. [DOI] [PubMed] [Google Scholar]
- 181.Pina MM, Pati D, Neira S, et al. Insula dynorphin and kappa opioid receptor systems regulate alcohol drinking in a sex-specific manner in mice. J Neurosci JN-RM-0406–22. 2023. 10.1523/jneurosci.0406-22.2023. [DOI] [PMC free article] [PubMed]
- 182.Vázquez-León P, Miranda-Páez A, Sánchez-Castillo H, Marichal-Cancino BA. Pharmacologic hyperreactivity of kappa opioid receptors in periaqueductal gray matter during alcohol withdrawal syndrome in rats. Pharmacol Rep. 2023. 10.1007/s43440-023-00522-z. [DOI] [PubMed] [Google Scholar]
- 183.Wei G, Sirohi S, Walker BM. Dysregulated kappa-opioid receptors in the medial prefrontal cortex contribute to working memory deficits in alcohol dependence. Addict Biol. 2022;27:e13138. 10.1111/adb.13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Estave PM, Sun H, Peck EG, et al. Cocaine self-administration augments kappa opioid receptor system-mediated inhibition of dopamine activity in the mesolimbic dopamine system. IBRO Neurosci Rep. 2023;14:129–37. 10.1016/j.ibneur.2023.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Estave PM, Albertson SE, Karkhanis AN, Jones SR. Co-targeting the kappa opioid receptor and dopamine transporter reduces motivation to self-administer cocaine and partially reverses dopamine system dysregulation. Sci Rep. 2024;14:6509. 10.1038/s41598-024-53463-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Abraham AD, Casello SM, Land BB, Chavkin C. Optogenetic stimulation of dynorphinergic neurons within the dorsal raphe activate kappa opioid receptors in the ventral tegmental area and ablation of dorsal raphe prodynorphin or kappa receptors in dopamine neurons blocks stress potentiation of cocaine reward. Addict Neurosci. 2022;1:100005. 10.1016/j.addicn.2022.100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lord J, Schmeichel B. Dynorphin blockade attenuates cocaine seeking in male rats. Appalachian Student Research Forum. 2024.
- 188.Gordon-Fennell L, Farero RD, Burgeno LM, et al. Kappa Opioid Receptors in Mesolimbic Terminals Mediate Escalation of Cocaine Consumption. 2023. bioRxiv. 10.1101/2023.12.21.57284.
- 189.Akins NS, Salahuddin MF, Pandey P, et al. Alleviation of cocaine withdrawal and pertinent interactions between salvinorin-based antagonists and kappa opioid receptor. ACS Chem Neurosci. 2023;14:958–76. 10.1021/acschemneuro.2c00806. [DOI] [PubMed] [Google Scholar]
- 190.Whitfield TW, Schlosburg JE, Wee S, et al. κ Opioid receptors in the nucleus accumbens shell mediate escalation of methamphetamine intake. J Neurosci. 2015;35:4296–305. 10.1523/JNEUROSCI.1978-13.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Avila JA, Memos N, Aslan A, et al. Voluntary oral methamphetamine increases memory deficits and contextual sensitization during abstinence associated with decreased PKMζ and increased κOR in the hippocampus of female mice. J Psychopharmacol. 2021;35:1240–52. 10.1177/02698811211048285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cheng Y, Deng Y, Deng D, et al. Prelimbic cortex dynorphin/κ opioid receptor system modulates methamphetamine-induced cognitive impairment. Addict Biol. 2023;28:e13323. 10.1111/adb.13323. [DOI] [PubMed] [Google Scholar]
- 193.Shao J, Fei Y, Xiao J, et al. The role of miRNA-144-3p/Oprk1/KOR in nicotine dependence and nicotine withdrawal in male rats. Nicotine Tob Res. 2023;25:1856–64. 10.1093/ntr/ntad118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Al-Hasani R, McCall JG, Bruchas MR. Exposure to chronic mild stress prevents kappa opioid-mediated reinstatement of cocaine and nicotine place preference. Front Pharmacol. 2013;4:96. 10.3389/fphar.2013.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Grella SL, Funk D, Coen K, et al. Role of the kappa-opioid receptor system in stress-induced reinstatement of nicotine seeking in rats. Behav Brain Res. 2014;265:188–97. 10.1016/j.bbr.2014.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gowrishankar R, Gomez A, Waliki M, Bruchas MR. Kappa-opioid receptor activation reinstates nicotine self-administration in mice. Addiction Neuroscience. 2022;2:100017. 10.1016/j.addicn.2022.100017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hasbi A, Madras BK, George SR. Daily Δ9-tetrahydrocannabinol and withdrawal increase dopamine D1–D2 receptor heteromer to mediate anhedonia- and anxiogenic-like behavior through a dynorphin and kappa opioid receptor mechanism. Biol Psychiatr Global Open Sci. 2023;3:550–66. 10.1016/j.bpsgos.2022.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during the current study.