Abstract
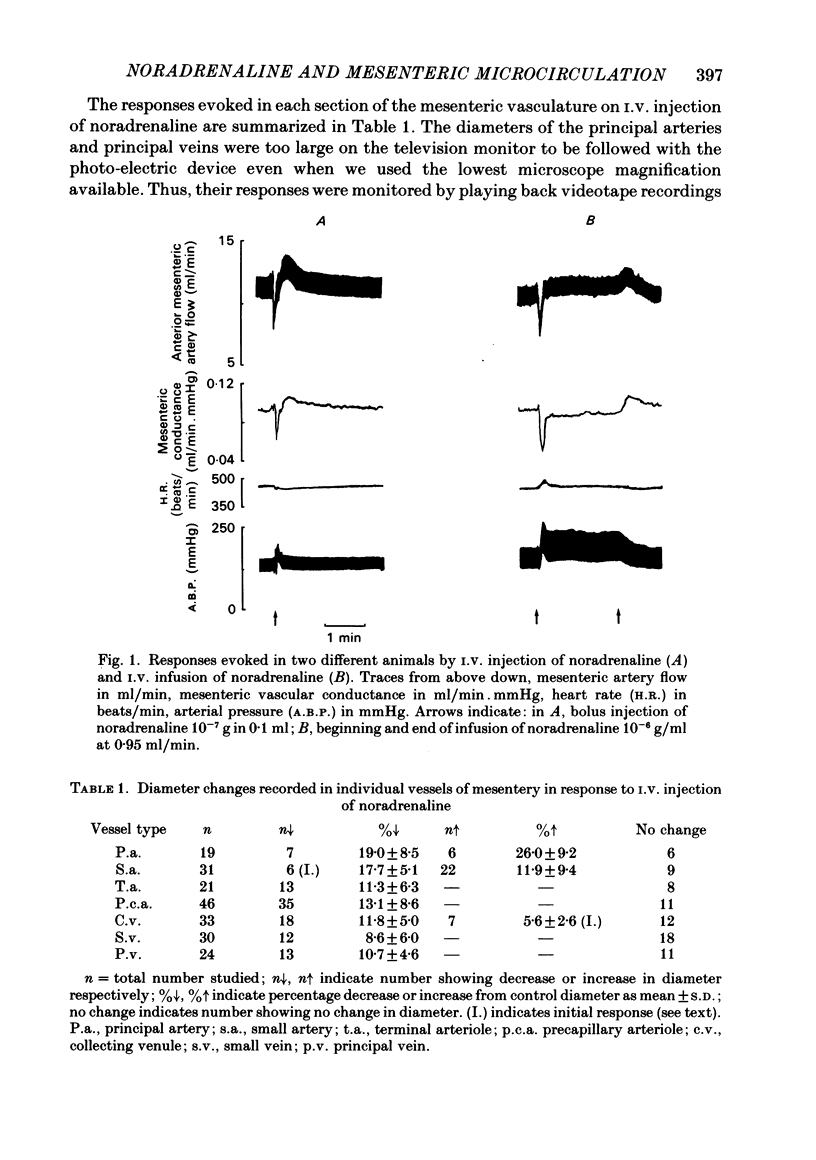
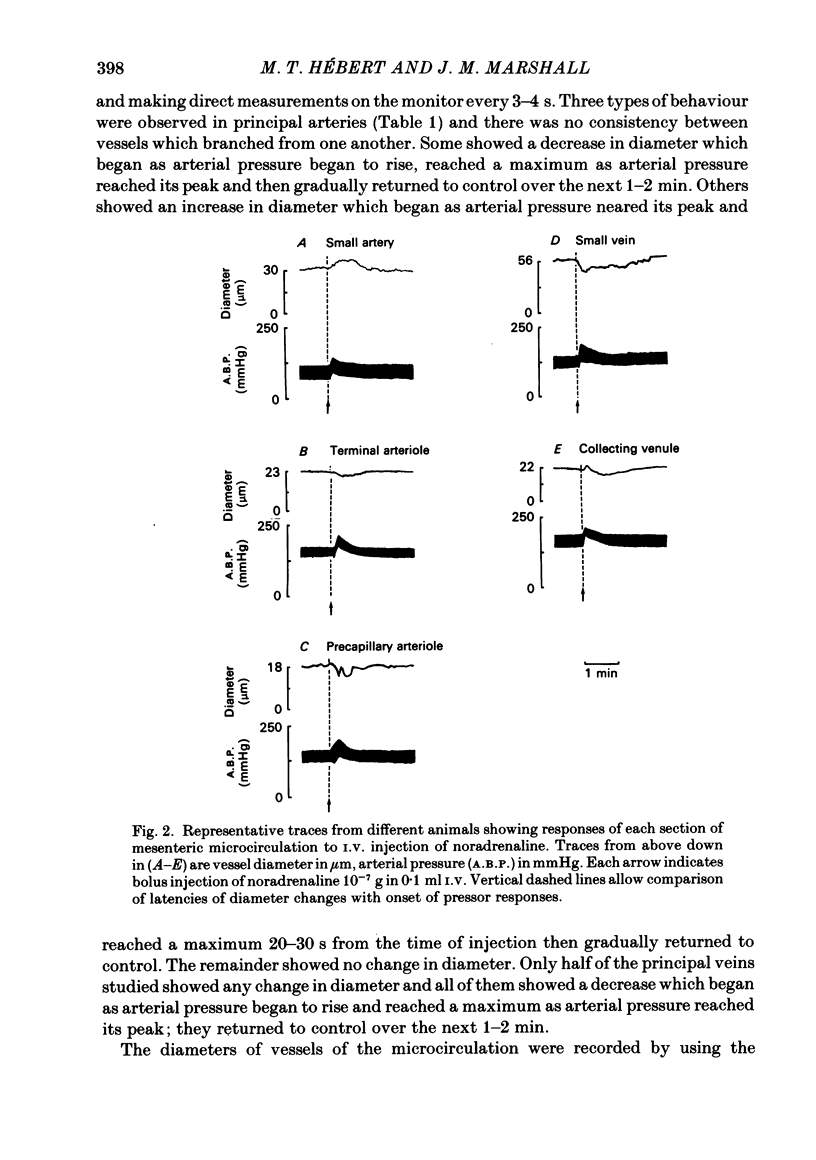
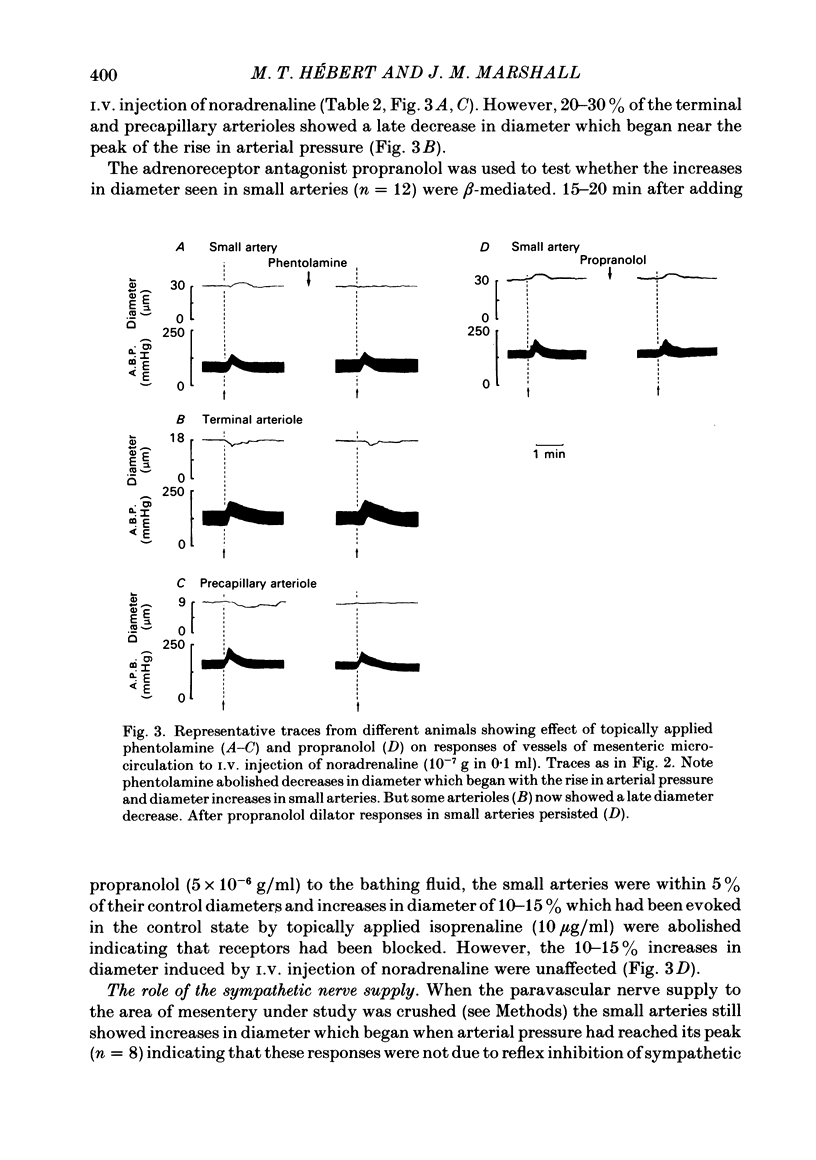
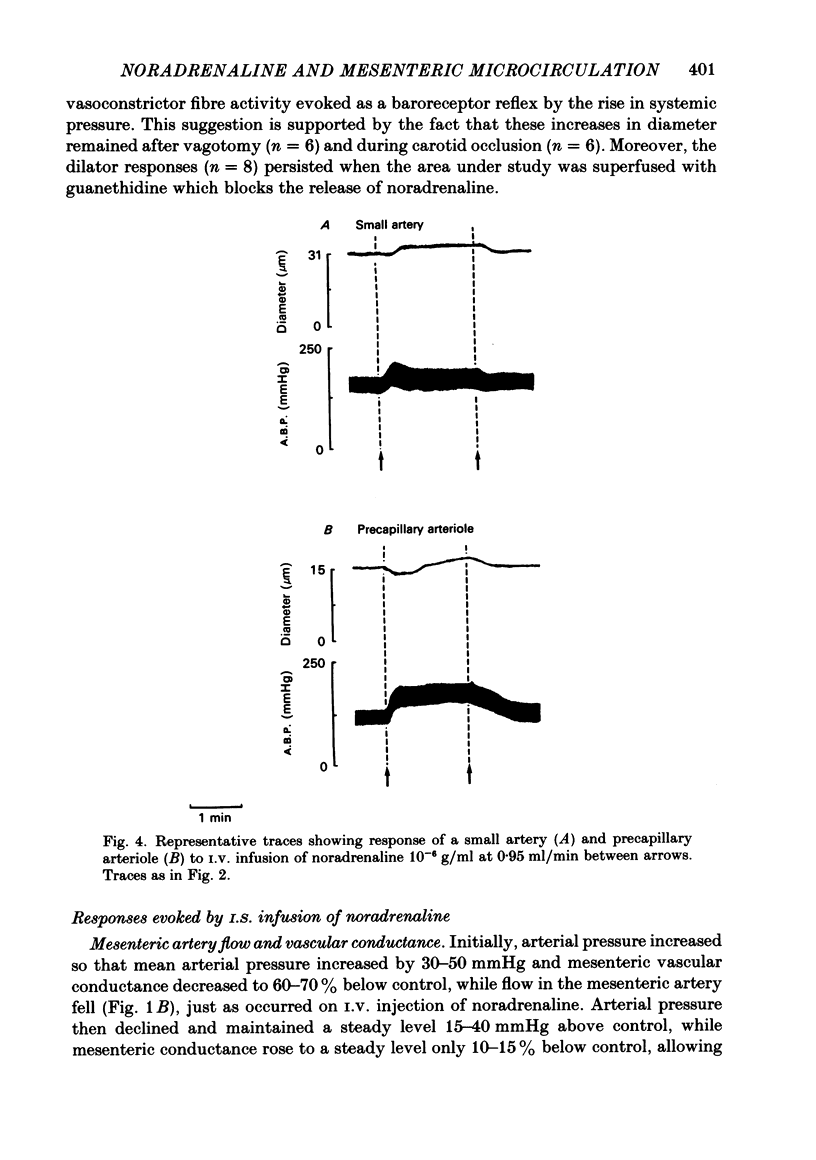
The responses of mesenteric microcirculation of the rat to circulating noradrenaline were studied by in vivo microscopy, using a photo-electric device placed on a television monitor to measure changes in diameter of individual vessels. Blood flow was measured in the anterior mesenteric artery with an electromagnetic flow transducer, mesenteric vascular conductance was computed on-line from mesenteric artery flow and arterial pressure. I.V. injection of noradrenaline induced a decrease in diameter of arterioles (less than 30 microns) which began simultaneously with the rise in arterial pressure and averaged 15% at 15-20 s. By contrast small and principal arteries (30-40 microns and 80-350 microns respectively) generally showed a diameter increase which averaged 10% and began at the peak of the pressor response. Venules and veins (12-560 microns) showed a diameter decrease which averaged 12-15% at 25-30 s. Meanwhile mesenteric vascular conductance fell by 65-75% in 10 s and rose to 30-50% above control in 25-30 s. The diameter increases of small and principal arteries were not reflex dilator responses initiated by the rise in systemic arterial pressure, nor due to beta-adrenoreceptor stimulation. However, local application of phentolamine abolished responses of all sections of the vascular tree indicating that they all depended on activation of alpha-adrenoreceptors. During I.V. infusion of noradrenaline small arteries showed a maintained increase in diameter which began at the peak of the pressor response, while arterioles initially decreased in diameter but then relaxed, often attaining resting diameter before infusion ceased. Meanwhile mesenteric flow and conductance decreased transiently, but then returned to near control levels, i.e. 'autoregulatory escape' occurred. It is argued that noradrenaline induced alpha-mediated contraction of all sections of the vascular tree; the tendency of arteries to constrict was counteracted by the rise in intravascular pressure caused by arteriolar constriction, active constriction of venous vessels may have been augmented by passive collapse secondary to arteriolar constriction. The secondary relaxation of arterial vessels reflects an inherent property of their smooth muscle to relax from the constrictor influence of noradrenaline and is more marked in proximal than distal vessels. It is proposed that the initial decrease in mesenteric vascular conductance in response to circulating noradrenaline may be attributed to active constriction of distal arterioles and the secondary increase in conductance ('escape') to secondary relaxation of more proximal arterial vessels.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altura B. M. Evaluation of neurohumoral substances in local regulation of blood flow. Am J Physiol. 1967 Jun;212(6):1447–1454. doi: 10.1152/ajplegacy.1967.212.6.1447. [DOI] [PubMed] [Google Scholar]
- Bohlen H. G., Henrich H., Gore R. W., Johnson P. C. Intestinal muscle and mucosal blood flow during direct sympathetic stimulation. Am J Physiol. 1978 Jul;235(1):H40–H45. doi: 10.1152/ajpheart.1978.235.1.H40. [DOI] [PubMed] [Google Scholar]
- Cardinal D. C., Higgs G. A. A photometric device for measuring blood vessel diameter in the microcirculation [proceedings]. J Physiol. 1978 Feb;275:5P–7P. [PubMed] [Google Scholar]
- Cardinal D. C., Higgs G. A. A photometric device for measuring blood vessel diameter in the microcirculation. J Pharmacol Methods. 1980 Sep;4(2):109–114. doi: 10.1016/0160-5402(80)90030-3. [DOI] [PubMed] [Google Scholar]
- Chin A. K., Evonuk E. Changes in plasma catecholamine and corticosterone levels after muscular exercise. J Appl Physiol. 1971 Feb;30(2):205–207. doi: 10.1152/jappl.1971.30.2.205. [DOI] [PubMed] [Google Scholar]
- De La Lande I. S., Waterson J. G. Site of action of cocaine on the perfused artery. Nature. 1967 Apr 15;214(5085):313–314. doi: 10.1038/214313a0. [DOI] [PubMed] [Google Scholar]
- Dresel P., Wallentin I. Effects of sympathetic vasoconstrictor fibres, noradrenaline and vasopressin on the intestinal vascular resistance during constant blood flow or blood pressure. Acta Physiol Scand. 1966 Apr;66(4):427–436. doi: 10.1111/j.1748-1716.1966.tb03220.x. [DOI] [PubMed] [Google Scholar]
- FOLKOW B., LEWIS D. H., LUNDGREN O., MELLANDER S., WALLENTIN I. THE EFFECT OF GRADED VASOCONSTRICTOR FIBRE STIMULATION ON THE INTESTINAL RESISTANCE AND CAPACITANCE VESSELS. Acta Physiol Scand. 1964 Aug;61:445–457. [PubMed] [Google Scholar]
- FOLKOW B., LEWIS D. H., LUNDGREN O., MELLANDER S., WALLENTIN I. THE EFFECT OF THE SYMPATHETIC VASOCONSTRICTOR FIBRES ON THE DISTRIBUTION OF CAPILLARY BLOOD FLOW IN THE INTESTINE. Acta Physiol Scand. 1964 Aug;61:458–466. [PubMed] [Google Scholar]
- FOLKOW B., LUNDGREN O., WALLENTIN I. Studies on the relationship between flow resistance, capillary filtration coefficient and regional blood volume in the intestine of the cat. Acta Physiol Scand. 1963 Mar;57:270–283. doi: 10.1111/j.1748-1716.1963.tb02591.x. [DOI] [PubMed] [Google Scholar]
- Fara J. W. Escape from tension induced by noradrenaline or electrical stimulation in isolated mesenteric arteries. Br J Pharmacol. 1971 Dec;43(4):865–867. doi: 10.1111/j.1476-5381.1971.tb07223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness J. B., Marshall J. M. Correlation of the directly observed responses of mesenteric vessles of the rat to nerve stimulation and noradrenaline with the distribution of adrenergic nerves. J Physiol. 1974 May;239(1):75–88. doi: 10.1113/jphysiol.1974.sp010556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore R. W. Wall stress: a determinant of regional differences in response of frog microvessels to norepinephrine. Am J Physiol. 1972 Jan;222(1):82–91. doi: 10.1152/ajplegacy.1972.222.1.82. [DOI] [PubMed] [Google Scholar]
- Greenway C. V., Scott G. D., Zink J. Sites of autoregulatory escape of blood flow in the mesenteric vascular bed. J Physiol. 1976 Jul;259(1):1–12. doi: 10.1113/jphysiol.1976.sp011451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUNDGREN O., WALLENTIN I. LOCAL CHEMICAL AND NERVOUS CONTROL OF CONSECUTIVE VASCULAR SECTIONS IN THE MESENTERIC LYMPH NODES OF THE CAT. Angiologica. 1964;1:284–296. doi: 10.1159/000157592. [DOI] [PubMed] [Google Scholar]
- Marshall J. M., Tandon H. C. Direct observations of muscle arterioles and venules following contraction of skeletal muscle fibres in the rat. J Physiol. 1984 May;350:447–459. doi: 10.1113/jphysiol.1984.sp015211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. M. The effect of uptake by adrenergic nerve terminals on the sensitivity of arterial vessels to topically applied noradrenaline. Br J Pharmacol. 1977 Nov;61(3):429–432. doi: 10.1111/j.1476-5381.1977.tb08436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. M. The influence of the sympathetic nervous system on individual vessels of the microcirculation of skeletal muscle of the rat. J Physiol. 1982 Nov;332:169–186. doi: 10.1113/jphysiol.1982.sp014408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper C. W., Chiueh C. C., Kopin I. J. Plasma catecholamine concentrations in unanesthetized rats during sleep, wakefulness, immobilization and after decapitation. J Pharmacol Exp Ther. 1977 Jul;202(1):144–148. [PubMed] [Google Scholar]
- Richardson D. R., Johnson P. C. Changes in mesenteric capillary flow during norepinephrine infusion. Am J Physiol. 1970 Nov;219(5):1317–1323. doi: 10.1152/ajplegacy.1970.219.5.1317. [DOI] [PubMed] [Google Scholar]
- Richardson D. R., Johnson P. C. Comparison of autoregulatory escape and autoregulation in the intestinal vascular bed. Am J Physiol. 1969 Aug;217(2):586–590. doi: 10.1152/ajplegacy.1969.217.2.586. [DOI] [PubMed] [Google Scholar]
- Richardson D. R., Zweifach B. W. Pressure relationships in the macro- and microcirculation of the mesentery. Microvasc Res. 1970 Oct;2(4):474–488. doi: 10.1016/0026-2862(70)90040-3. [DOI] [PubMed] [Google Scholar]
- Rodigas P., Cession-Fossion A. Evolution de la catécholaminémie, lors de la saignée, chez le rat éveillé. C R Seances Soc Biol Fil. 1972;166(4):747–749. [PubMed] [Google Scholar]
- Ross G. Effects of epinephrine and norepinephrine on the mesenteric circulation of the cat. Am J Physiol. 1967 May;212(5):1037–1042. doi: 10.1152/ajplegacy.1967.212.5.1037. [DOI] [PubMed] [Google Scholar]
- Ross G. Effects of norepinephrine infusions on mesenteric arterial blood flow and its tissue distribution. Proc Soc Exp Biol Med. 1971 Jul;137(3):921–924. doi: 10.3181/00379727-137-35694. [DOI] [PubMed] [Google Scholar]
- Ross G. Norepinephrine vascoconstrictor escape in isolated meseneric arteries. Am J Physiol. 1975 Jun;228(6):1652–1655. doi: 10.1152/ajplegacy.1975.228.6.1652. [DOI] [PubMed] [Google Scholar]
- Ross G., White F. N., Brown A. W., Kolin A. Regional blood flow in the rat. J Appl Physiol. 1966 Jul;21(4):1273–1275. doi: 10.1152/jappl.1966.21.4.1273. [DOI] [PubMed] [Google Scholar]
- Singbartl G., Henrich H. Adrenergic-induced vascular adjustments--initial and escape reactions. II. Role of beta-adrenergic receptors within different sections of the isolated intestinal vascular bed. Pflugers Arch. 1974 Jan 16;346(1):13–24. doi: 10.1007/BF00592646. [DOI] [PubMed] [Google Scholar]


