Abstract
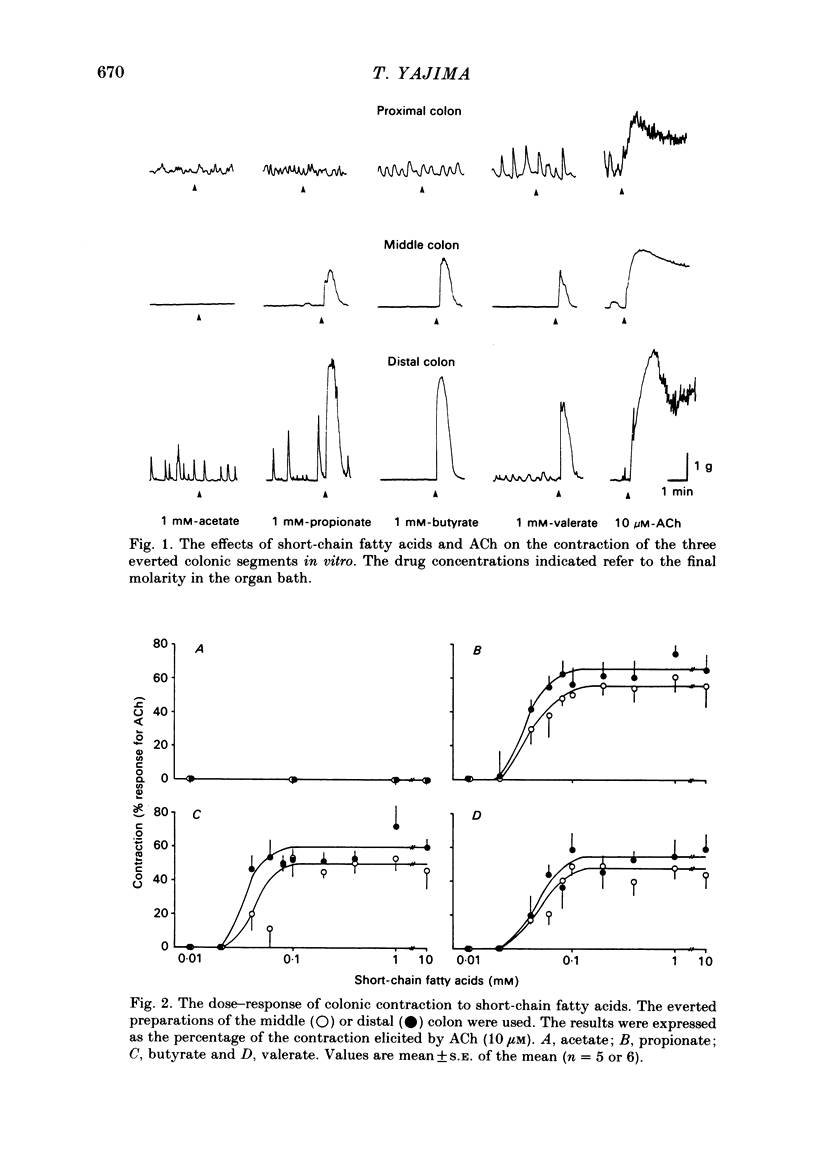
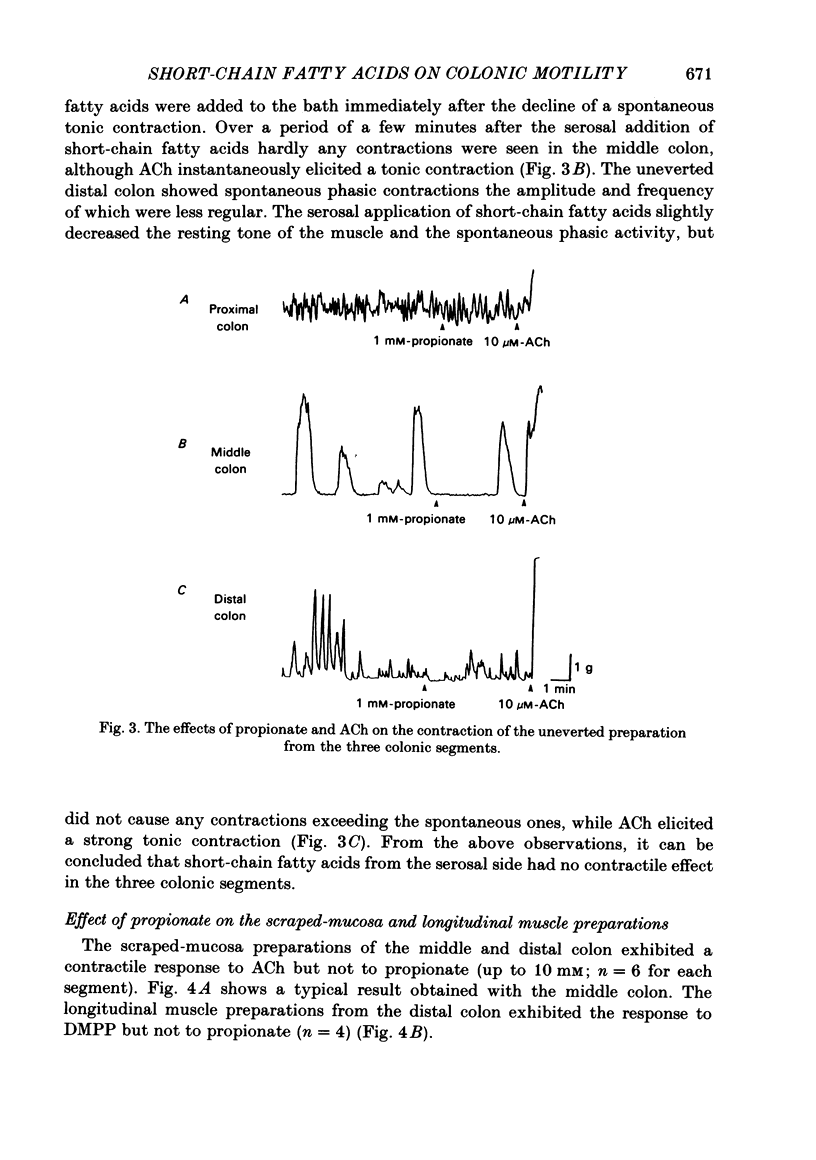
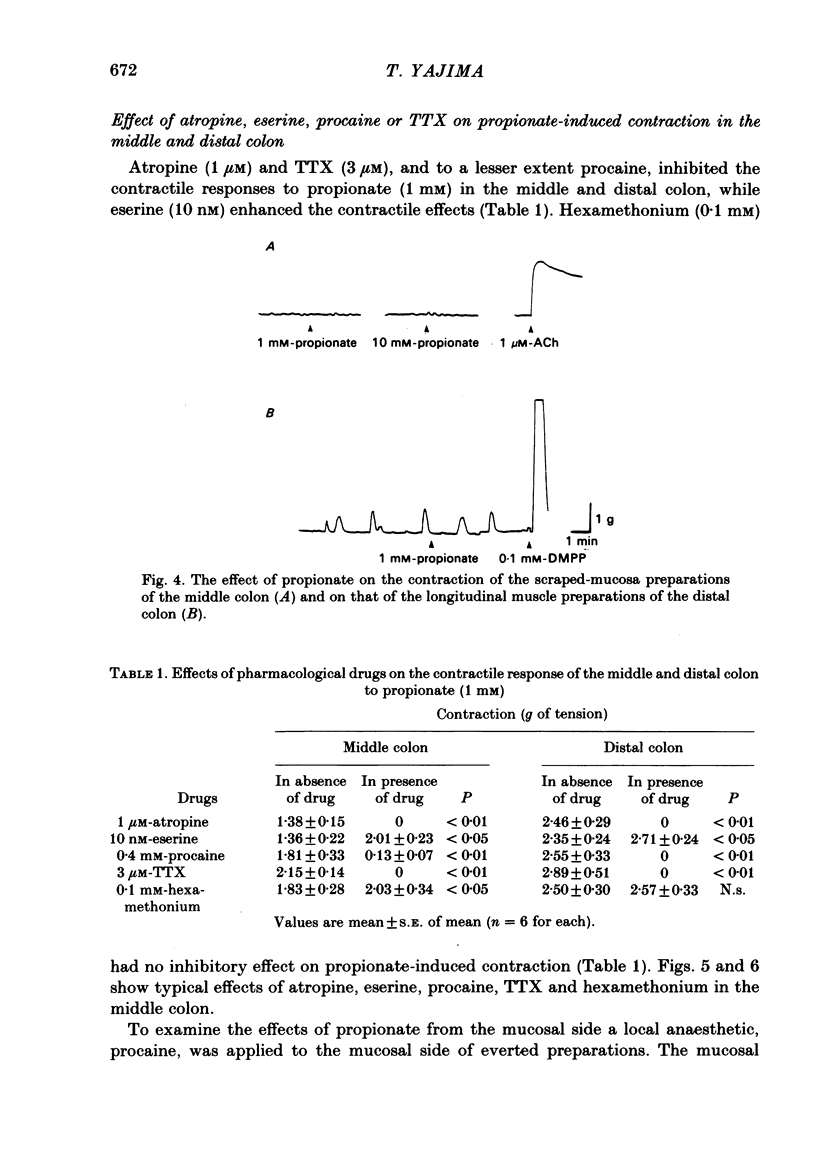
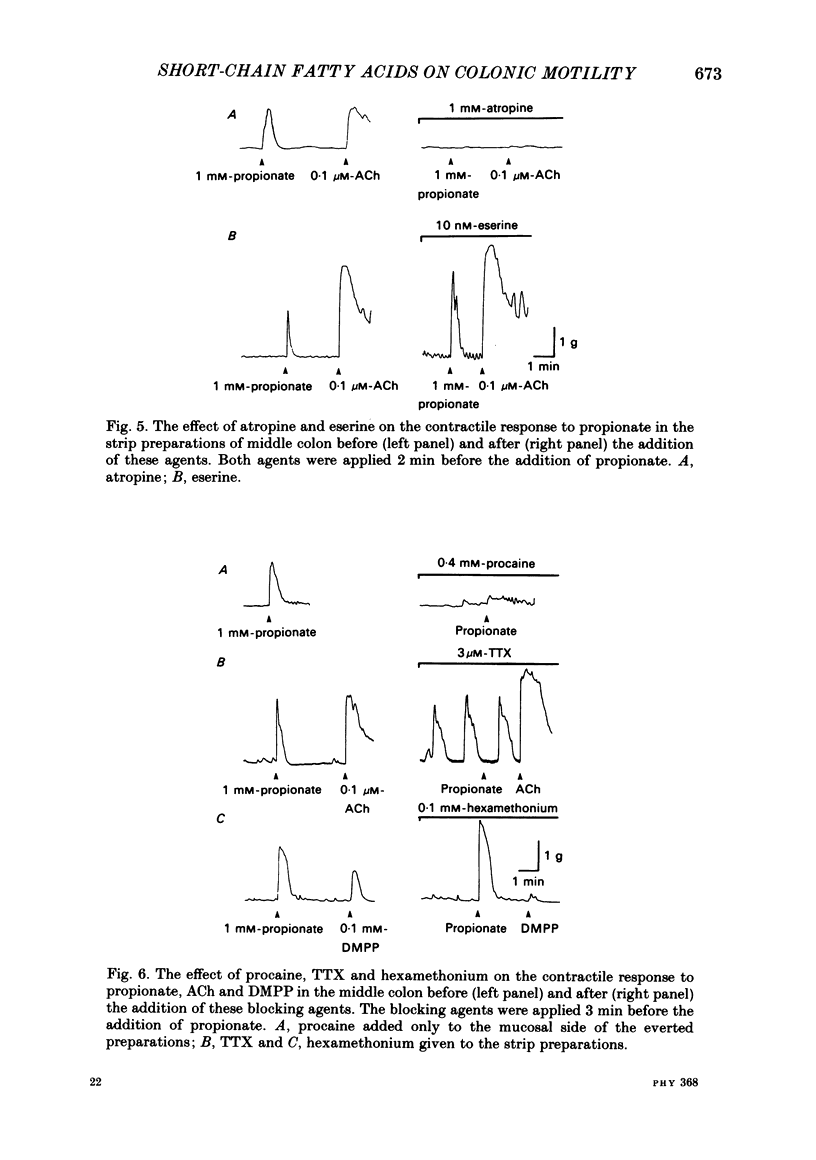
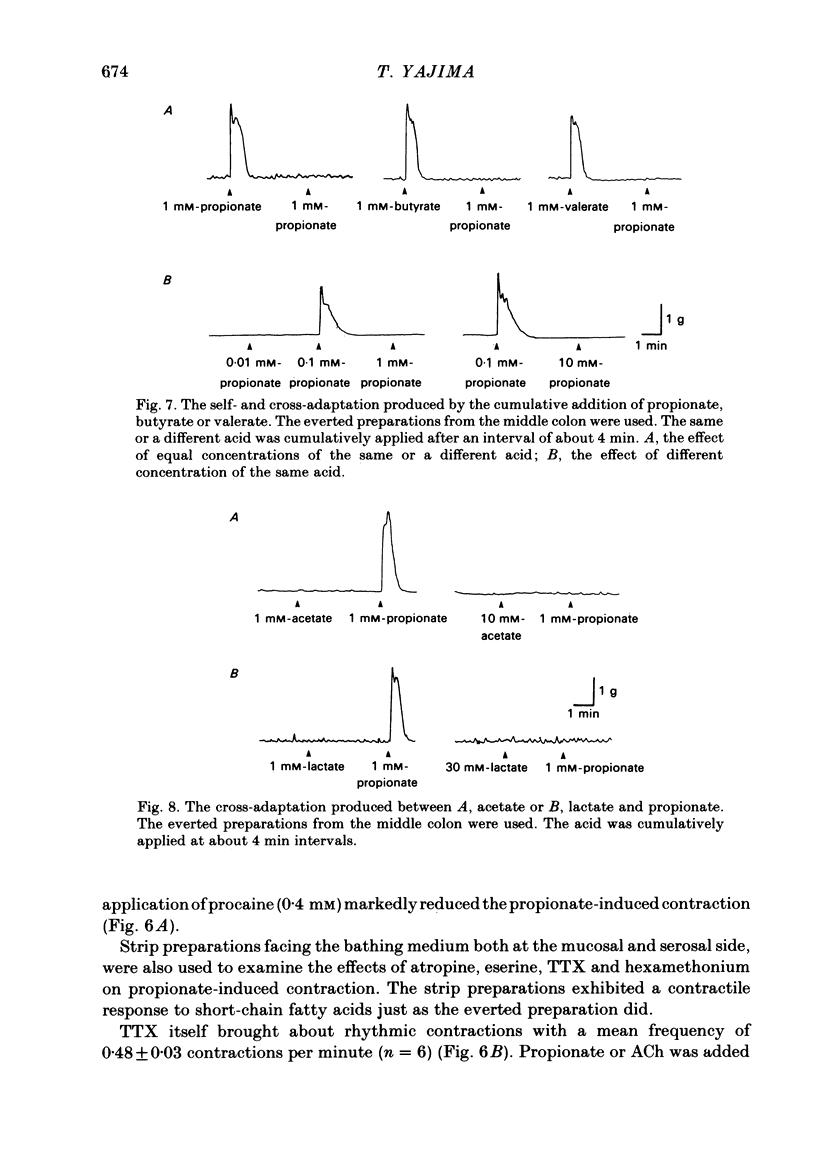
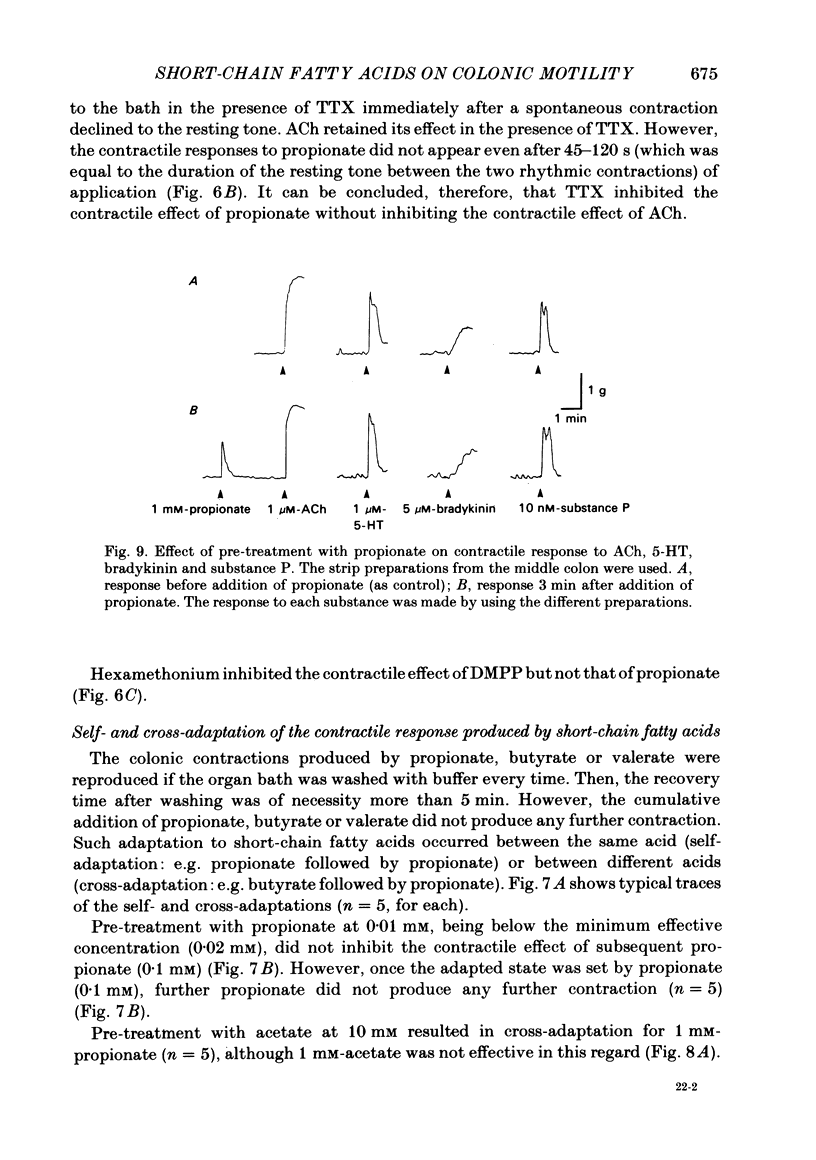
The contractile effect of short-chain fatty acids on proximal, middle and distal segments of the rat colon was studied in vitro. A single contraction of the longitudinal muscle of the everted preparation of the middle and distal but not the proximal colon was induced by mucosal application of propionate, butyrate or valerate. Sigmoid dose-responses were observed between contraction and log dose of propionate, butyrate and valerate. The threshold concentration of short-chain fatty acids was between 0.02 and 0.04 mM. A maximal contraction was induced with 0.1 mM-propionate, butyrate and valerate. While acetate (up to 10 mM) and lactate (up to 30 mM) had no contractile effect at all. Serosal application of short-chain fatty acids was without effect, while the contractile response with up to 10 mM-propionate was abolished in both the middle and distal colon by scraping away the mucosa. Cumulative addition of short-chain fatty acids to the organ bath (without wash-out of the first dose) caused adaptation of the contractile response; thus, the effect of propionate (1 mM) was abolished by prior addition of acetate (10 mM) or lactate (30 mM) or propionate (1 mM) or butyrate (1 mM) or valerate (1 mM). The contractile effect of propionate was also inhibited by atropine (1 microM), procaine (0.4 mM) and tetrodotoxin (3 microM); was unaffected by hexamethonium (0.1 mM) and enhanced by eserine (10 nM). The results suggest that short-chain fatty acids, which are normal constituents of the colon, have the ability to stimulate colonic contractions, probably via an enteric reflex involving local sensory and cholinergic nerves.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anil M. H., Forbes J. M. Feeding in sheep during intraportal infusions of short-chain fatty acids and the effect of liver denervation. J Physiol. 1980 Jan;298:407–414. doi: 10.1113/jphysiol.1980.sp013090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss W. M., Starling E. H. The movements and the innervation of the large intestine. J Physiol. 1900 Dec 31;26(1-2):107–118. doi: 10.1113/jphysiol.1900.sp000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M., Furness J. B. The peristaltic reflex: an analysis of the nerve pathways and their pharmacology. Naunyn Schmiedebergs Arch Pharmacol. 1976 Jul;294(1):47–60. doi: 10.1007/BF00692784. [DOI] [PubMed] [Google Scholar]
- Fioramonti J., Bueno L. Motor activity in the large intestine of the pig related to dietary fibre and retention time. Br J Nutr. 1980 Jan;43(1):155–162. doi: 10.1079/bjn19800074. [DOI] [PubMed] [Google Scholar]
- Gregory P. C. Control of intrinsic reticulo-ruminal motility in the vagotomized sheep. J Physiol. 1984 Jan;346:379–393. doi: 10.1113/jphysiol.1984.sp015029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUKUHARA T., MIYAKE T. The intrinsic reflexes in the colon. Jpn J Physiol. 1959 Mar 25;9(1):49–55. doi: 10.2170/jjphysiol.9.49. [DOI] [PubMed] [Google Scholar]
- HUKUHARA T., NAKAYAMA S., NANBA R. The role of the intrinsic mucosal reflex in the fluid transport through the denervated colonic loop. Jpn J Physiol. 1961 Feb 15;11:71–79. doi: 10.2170/jjphysiol.11.71. [DOI] [PubMed] [Google Scholar]
- Matsuo Y., Seki A. The coordination of gastrointestinal hormones and the autonomic nerves. Am J Gastroenterol. 1978 Jan;69(1):21–50. [PubMed] [Google Scholar]
- Nord E. P., Wright S. H., Kippen I., Wright E. M. Specificity of the Na+-dependent monocarboxylic acid transport pathway in rabbit renal brush border membranes. J Membr Biol. 1983;72(3):213–221. doi: 10.1007/BF01870588. [DOI] [PubMed] [Google Scholar]
- Ritchie J. A. Movement of segmental constrictions in the human colon. Gut. 1971 May;12(5):350–355. doi: 10.1136/gut.12.5.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAPIRO H., WOODWARD E. R. INHIBITION OF THE SECRETIN MECHANISM BY LOCAL ANESTHETICS. Am Surg. 1965 Feb;31:139–141. [PubMed] [Google Scholar]
- Snape W. J., Jr, Shiff S., Cohen S. Effect of deoxycholic acid on colonic motility in the rabbit. Am J Physiol. 1980 Apr;238(4):G321–G325. doi: 10.1152/ajpgi.1980.238.4.G321. [DOI] [PubMed] [Google Scholar]
- Snipes R. L., Clauss W., Weber A., Hörnicke H. Structural and functional differences in various divisions of the rabbit colon. Cell Tissue Res. 1982;225(2):331–346. doi: 10.1007/BF00214686. [DOI] [PubMed] [Google Scholar]
- Yajima T., Kojima K., Tohyama K., Mutai M. Alteration in sensitivity of transmural electrical response to propionate in rat colon after chronic luminal infusion of short-chain fatty acids. Life Sci. 1983 Mar 7;32(10):1073–1079. doi: 10.1016/0024-3205(83)90112-1. [DOI] [PubMed] [Google Scholar]
- Yokokura T., Yajima T., Hashimoto S. Effect of organic acid on gastrointestinal motility of rat in vitro. Life Sci. 1977 Jul 1;21(1):59–62. doi: 10.1016/0024-3205(77)90424-6. [DOI] [PubMed] [Google Scholar]


