Abstract
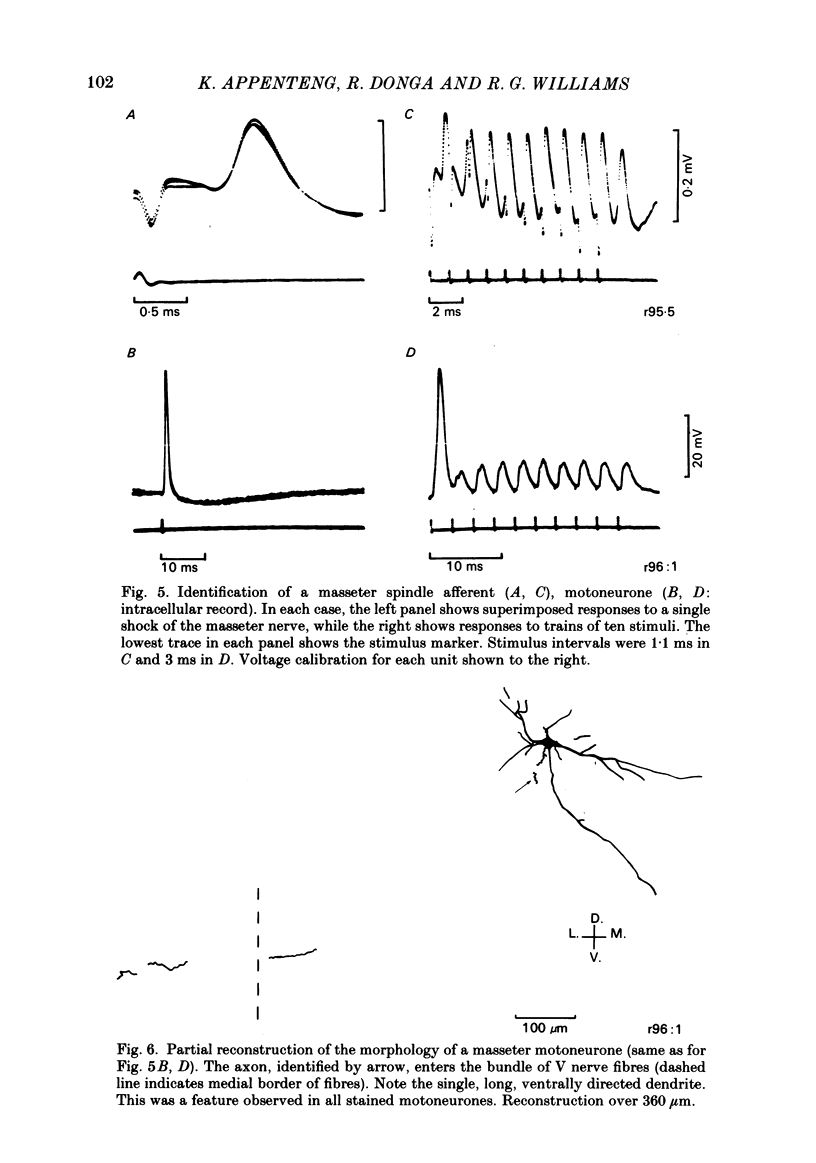
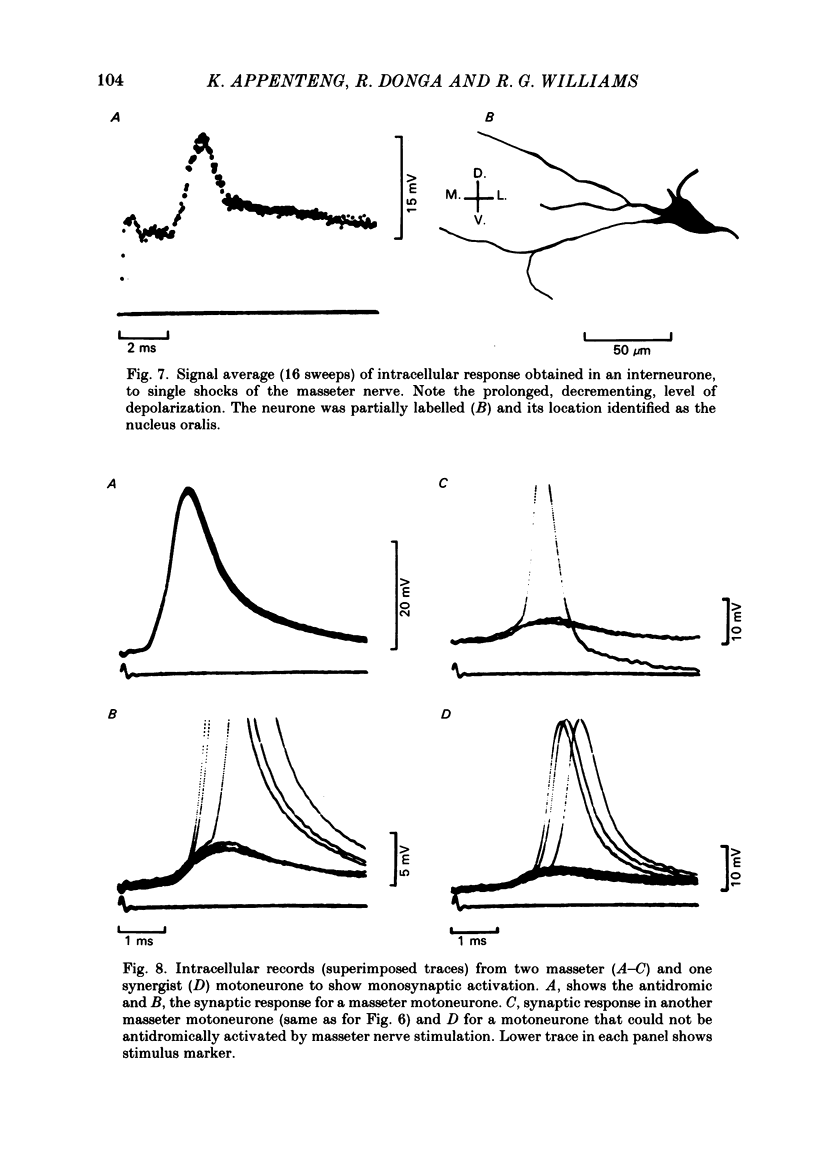
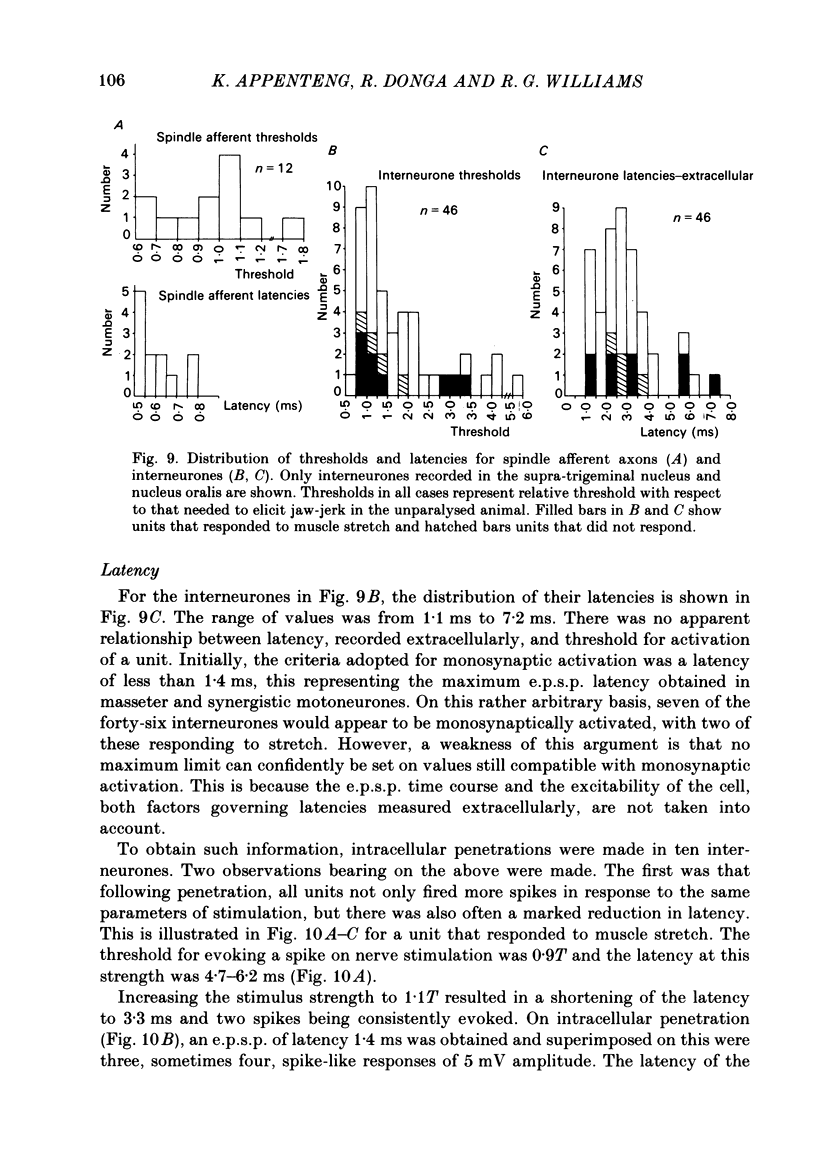
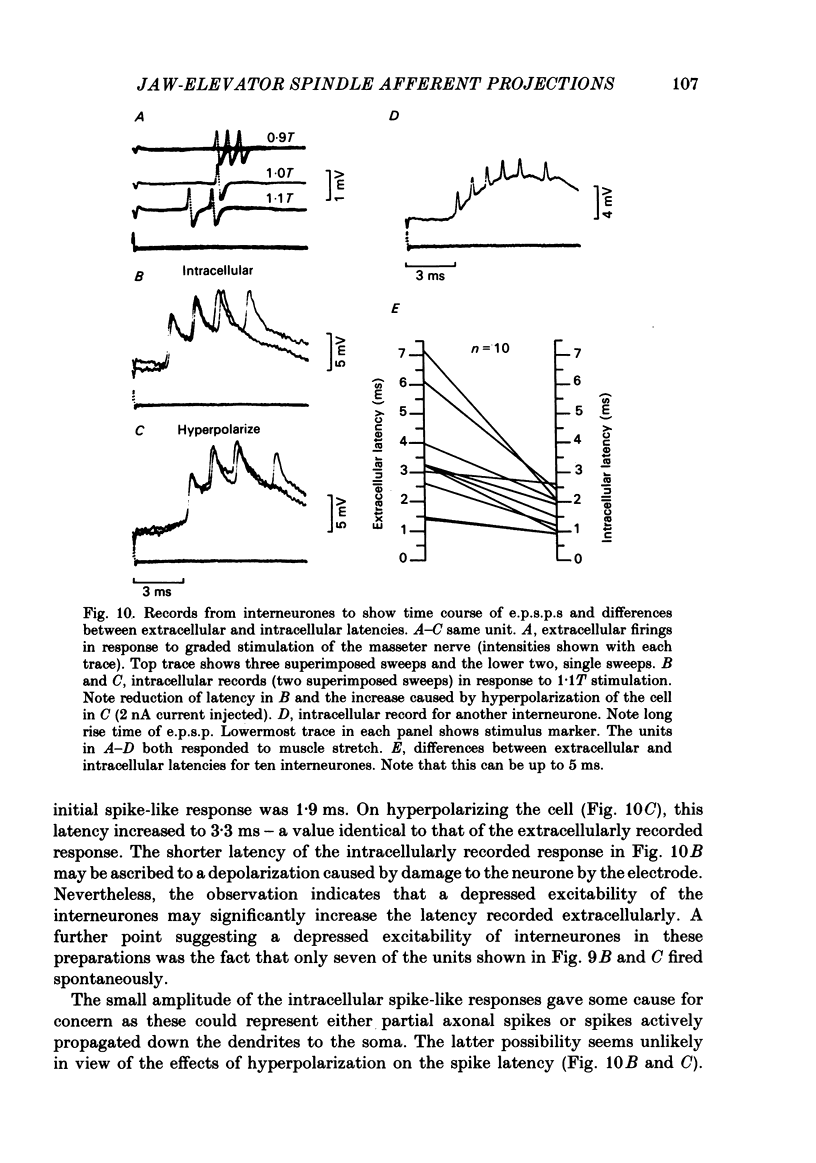
The fluorescent compound Lucifer Yellow was injected into the somata of nine identified jaw-elevator muscle spindle afferents, located in the V mesencephalic nucleus. Reconstructions of the central course of their axons were subsequently made from serial, transverse, sections to identify sites of projection. Three sites of termination were identified on the basis of collaterals that ended in varicosities and/or boutons. All afferents projected to the V nucleus oralis and, all but one, also to the V motor nucleus. Two out of nine afferents had terminations in the supra-trigeminal nucleus, though a further four appeared to send collaterals to this area. The relative density of projection, judged by the number of collaterals supplied to each area, decreased in the order: V nucleus oralis, V motor nucleus and supra-trigeminal nucleus. The central course of the afferent axons was such that impulses from the periphery would arrive first at the V motor nucleus, then the V nucleus oralis, the supra-trigeminal nucleus, and finally the afferent somata in the V mesencephalic nucleus. In animals in which the masseter nerve was exposed in-continuity for electrical stimulation, electrophysiological recordings were made in the three areas described above to identify units that received a monosynaptic input from spindles in the masseter muscle. Criteria were formulated on the basis of the pattern of responses on stimulation of the masseter nerve, and the morphology of labelled neurones, for differentiating between afferents, interneurones, and motoneurones. In the V motor nucleus, monosynaptic excitatory post-synaptic potentials (e.p.s.p.s) were obtained in both synergist and masseter motoneurones. These were assumed to arise from a masseter muscle spindle input as the thresholds for exciting such afferents and eliciting e.p.s.p.s were similar. Some interneurones, chiefly in the V nucleus oralis, were activated at thresholds close to that of muscle spindle afferents and could also fire in response to muscle stretch. As their latencies (measured extracellularly) were similar to that of e.p.s.p.s in motoneurones, they were assumed to receive a monosynaptic muscle spindle input. However, most interneurones were activated at longer latencies (up to 7 ms) and some also fired to muscle stretch. Arguments are advanced, based on the long rise time of e.p.s.p.s recorded in some, that the majority of these may also be candidates for monosynaptic activation.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



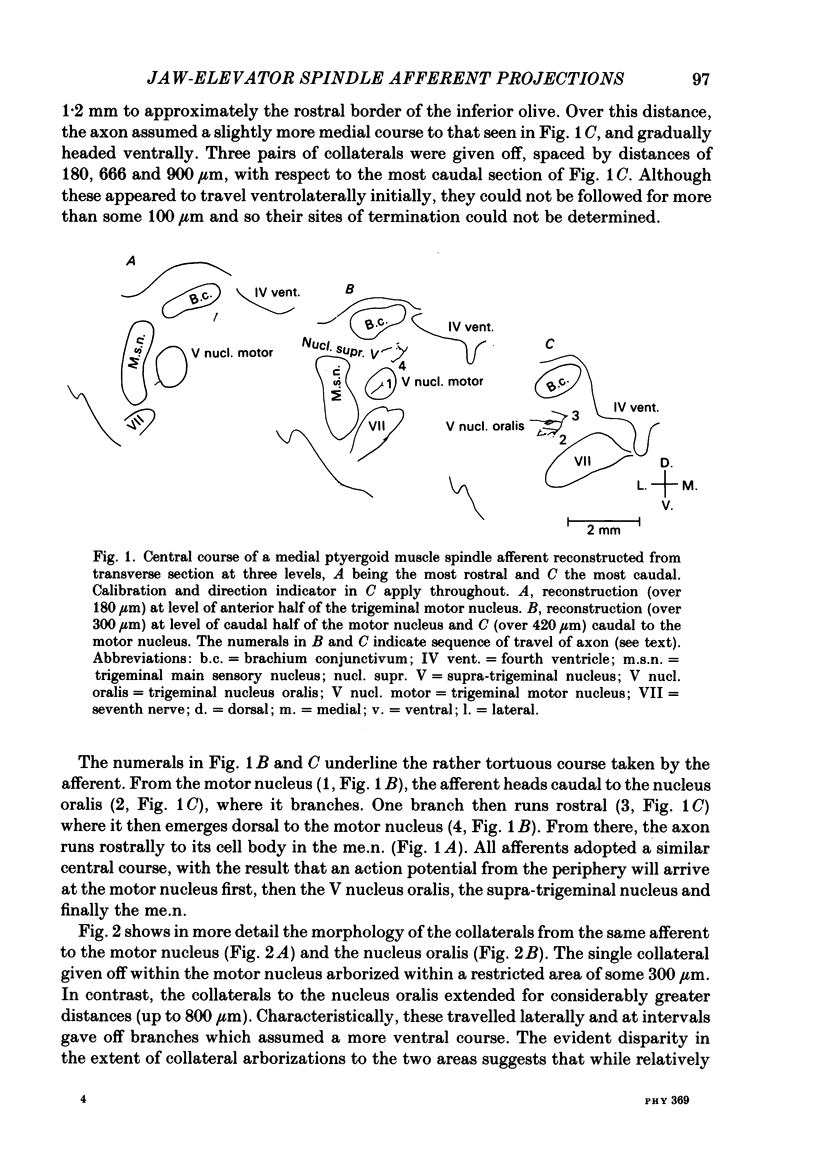
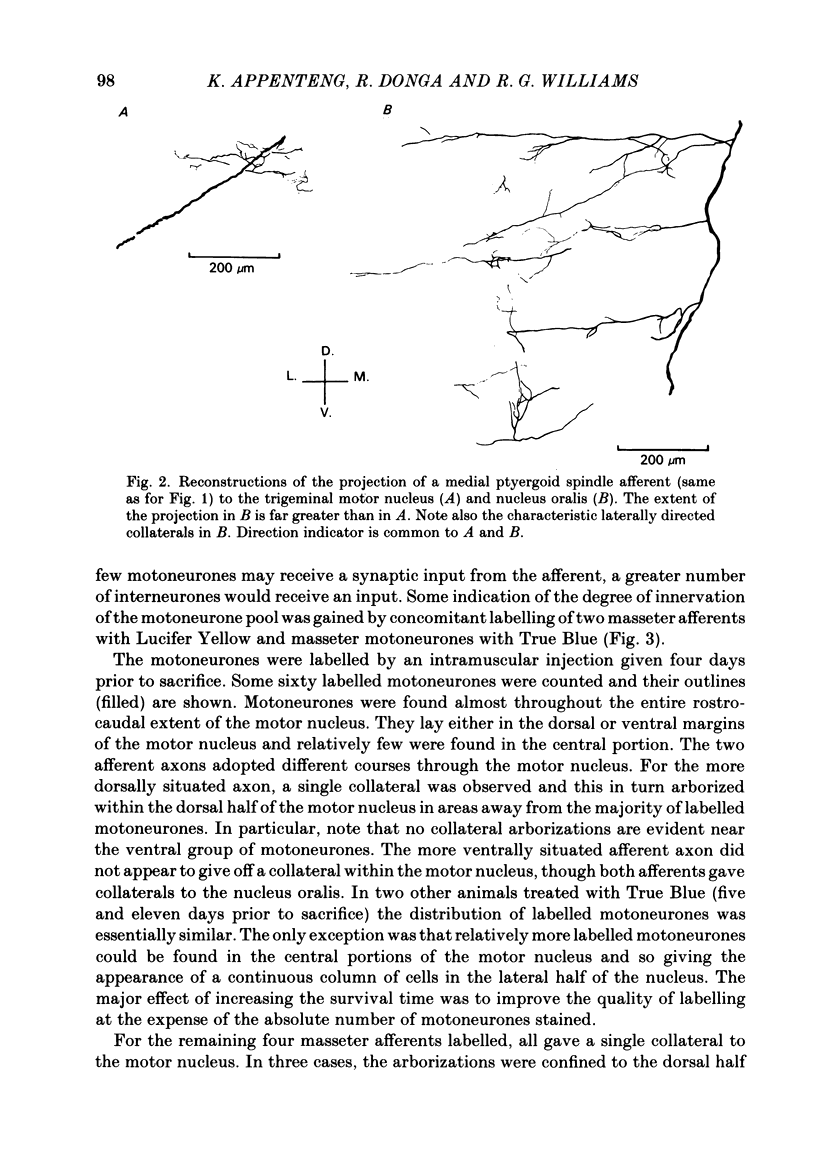
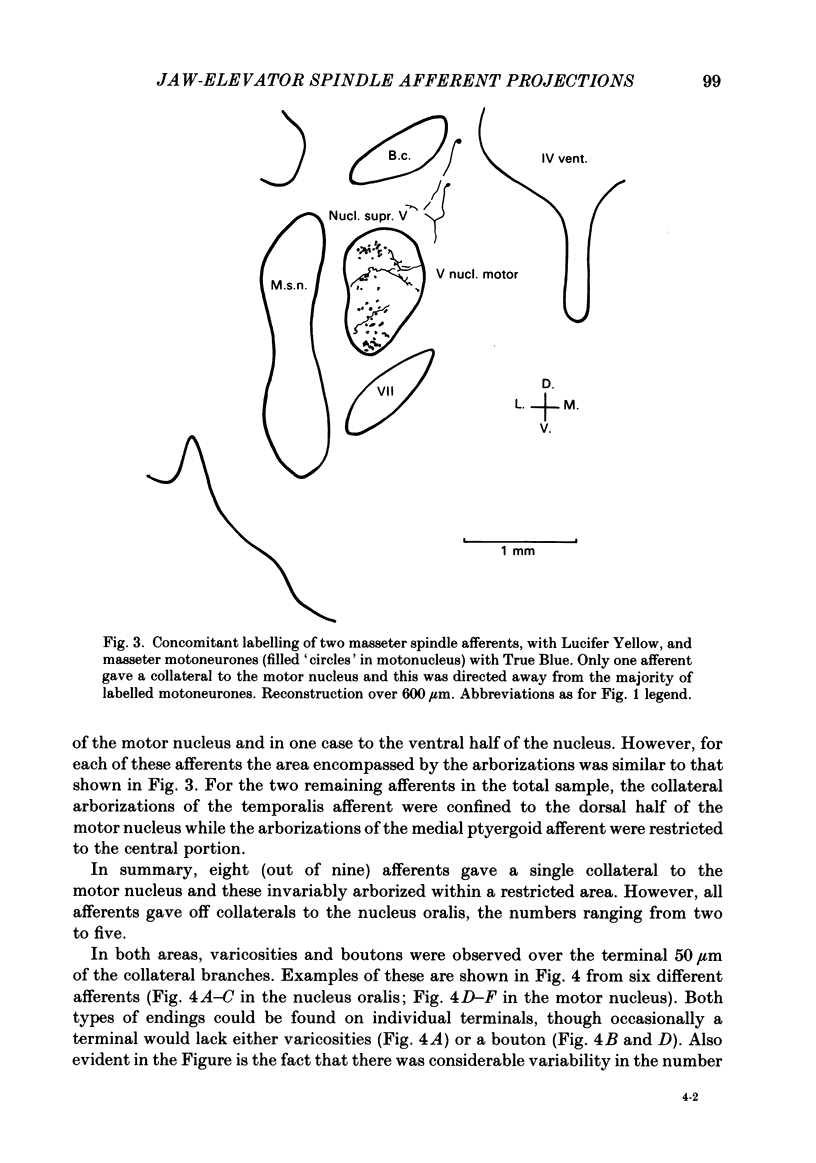
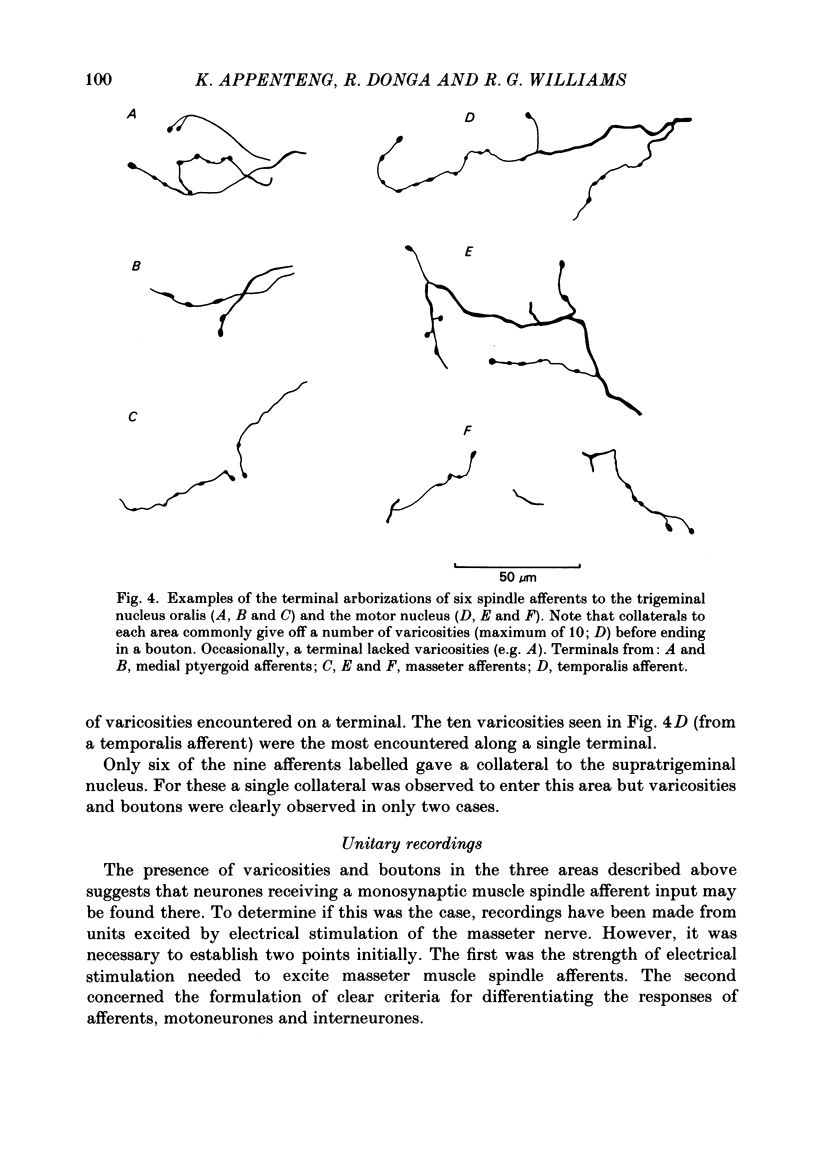









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appenteng K., Morimoto T., Taylor A. Methods used to compare monosynaptic and multisynaptic projection of spindle afferents to jaw elevator motoneurones of different types [proceedings]. J Physiol. 1979 Aug;293:13P–13P. [PubMed] [Google Scholar]
- Appenteng K., O'Donovan M. J., Somjen G., Stephens J. A., Taylor A. The projection of jaw elevator muscle spindle afferents to fifth nerve motoneurones in the cat. J Physiol. 1978 Jun;279:409–423. doi: 10.1113/jphysiol.1978.sp012353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E. The morphology of group Ia afferent fibre collaterals in the spinal cord of the cat. J Physiol. 1978 Jan;274:111–127. doi: 10.1113/jphysiol.1978.sp012137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., CURTIS D. R., ECCLES J. C. The interpretation of spike potentials of motoneurones. J Physiol. 1957 Dec 3;139(2):198–231. doi: 10.1113/jphysiol.1957.sp005887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody F. W., Harrison L. M., Taylor A. Analysis of activity of muscle spindles of the jaw-closing muscles during normal movements in the cat. J Physiol. 1975 Dec;253(2):565–582. doi: 10.1113/jphysiol.1975.sp011207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody F. W., Lee R. W., Taylor A. A functional analysis of the components of the mesencephalic nucleus of the fifth nerve in the cat. J Physiol. 1972 Oct;226(1):249–261. doi: 10.1113/jphysiol.1972.sp009983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand J., Gogan P., Guéritaud J. P., Horcholle-Bossavit G., Tyc-Dumont S. Morphological and electrophysiological properties of trigeminal neurones projecting to the accessory abducens nucleus of the cat. Exp Brain Res. 1983;53(1):118–128. doi: 10.1007/BF00239404. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., IGGO A., LUNDBERG A. Electrophysiological investigations on Renshaw cells. J Physiol. 1961 Dec;159:461–478. doi: 10.1113/jphysiol.1961.sp006821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., FATT P., KOKETSU K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. J Physiol. 1954 Dec 10;126(3):524–562. doi: 10.1113/jphysiol.1954.sp005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyffe R. E. The morphology of group II muscle afferent fibre collaterals [proceedings]. J Physiol. 1979 Nov;296:39P–40P. [PubMed] [Google Scholar]
- Inoue H., Morimoto T., Kawamura Y. Response characteristics an classification of muscle spindles of the masseter muscle in the cat. Exp Neurol. 1981 Nov;74(2):548–560. doi: 10.1016/0014-4886(81)90190-4. [DOI] [PubMed] [Google Scholar]
- Ishizuka N., Mannen H., Hongo T., Sasaki S. Trajectory of group Ia afferent fibers stained with horseradish peroxidase in the lumbosacral spinal cord of the cat: three dimensional reconstructions from serial sections. J Comp Neurol. 1979 Jul 15;186(2):189–211. doi: 10.1002/cne.901860206. [DOI] [PubMed] [Google Scholar]
- Jacquin M. F., Semba K., Egger M. D., Rhoades R. W. Organization of HRP-labeled trigeminal mandibular primary afferent neurons in the rat. J Comp Neurol. 1983 Apr 20;215(4):397–420. doi: 10.1002/cne.902150405. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Rastad J., Westman J. Intracellular application of horseradish peroxidase and its light and electron microscopical appearance in spinocervical tract cells. Brain Res. 1976 Apr 9;105(3):557–562. doi: 10.1016/0006-8993(76)90603-x. [DOI] [PubMed] [Google Scholar]
- Karlsen K. Fibre calibre spectra of nerves to the masticatory muscles in the cat. Acta Odontol Scand. 1969 Jun;27(3):263–270. doi: 10.3109/00016356909008955. [DOI] [PubMed] [Google Scholar]
- Kidokoro Y., Kubota K., Shuto S., Sumino R. Possible interneurons responsible for reflex inhibition of motoneurons of jaw-closing muscles from the inferior dental nerve. J Neurophysiol. 1968 Sep;31(5):709–716. doi: 10.1152/jn.1968.31.5.709. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. Excitatory post-synaptic potentials from single muscle spindle afferents in external intercostal motoneurones of the cat. J Physiol. 1982 Jan;322:287–314. doi: 10.1113/jphysiol.1982.sp014038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer W. J., Spindler A. J., Eisner D. A. Thick slurry bevelling: a new technique for bevelling extremely fine microelectrodes and micropipettes. Pflugers Arch. 1979 Sep;381(3):287–288. doi: 10.1007/BF00583261. [DOI] [PubMed] [Google Scholar]
- Loeb G. E., Duysens J. Activity patterns in individual hindlimb primary and secondary muscle spindle afferents during normal movements in unrestrained cats. J Neurophysiol. 1979 Mar;42(2):420–440. doi: 10.1152/jn.1979.42.2.420. [DOI] [PubMed] [Google Scholar]
- Lynch R. A qualitative investigation of the topographical representation of masticatory muscles within the motor trigeminal nucleus of the rat: a horseradish peroxidase study. Brain Res. 1985 Feb 18;327(1-2):354–358. doi: 10.1016/0006-8993(85)91535-5. [DOI] [PubMed] [Google Scholar]
- Matesz C. Peripheral and central distribution of fibres of the mesencephalic trigeminal root in the rat. Neurosci Lett. 1981 Nov 18;27(1):13–17. doi: 10.1016/0304-3940(81)90198-1. [DOI] [PubMed] [Google Scholar]
- Mizuno N., Konishi A., Sato M. Localization of masticatory motoneurons in the cat and rat by means of retrograde axonal transport of horseradish peroxidase. J Comp Neurol. 1975 Nov 1;164(1):105–115. doi: 10.1002/cne.901640109. [DOI] [PubMed] [Google Scholar]
- Mizuno N., Yasui Y., Nomura S., Itoh K., Konishi A., Takada M., Kudo M. A light and electron microscopic study of premotor neurons for the trigeminal motor nucleus. J Comp Neurol. 1983 Apr 10;215(3):290–298. doi: 10.1002/cne.902150305. [DOI] [PubMed] [Google Scholar]
- Morimoto T., Inoue H., Kawamura Y. Diameter spectra of sensory and motor fibers in nerves to jaw-closing and jaw-opening muscles in the cat. Jpn J Physiol. 1982;32(2):171–182. doi: 10.2170/jjphysiol.32.171. [DOI] [PubMed] [Google Scholar]
- Prochazka A., Westerman R. A., Ziccone S. P. Discharges of single hindlimb afferents in the freely moving cat. J Neurophysiol. 1976 Sep;39(5):1090–1104. doi: 10.1152/jn.1976.39.5.1090. [DOI] [PubMed] [Google Scholar]
- Snow P. J., Rose P. K., Brown A. G. Tracing axons and axon collaterals of spinal neurons using intracellular injection of horseradish peroxidase. Science. 1976 Jan 23;191(4224):312–313. doi: 10.1126/science.54936. [DOI] [PubMed] [Google Scholar]
- Stewart W. W. Functional connections between cells as revealed by dye-coupling with a highly fluorescent naphthalimide tracer. Cell. 1978 Jul;14(3):741–759. doi: 10.1016/0092-8674(78)90256-8. [DOI] [PubMed] [Google Scholar]
- Walmsley B., Tracey D. J. An intracellular study of Renshaw cells. Brain Res. 1981 Oct 26;223(1):170–175. doi: 10.1016/0006-8993(81)90818-0. [DOI] [PubMed] [Google Scholar]


