Abstract
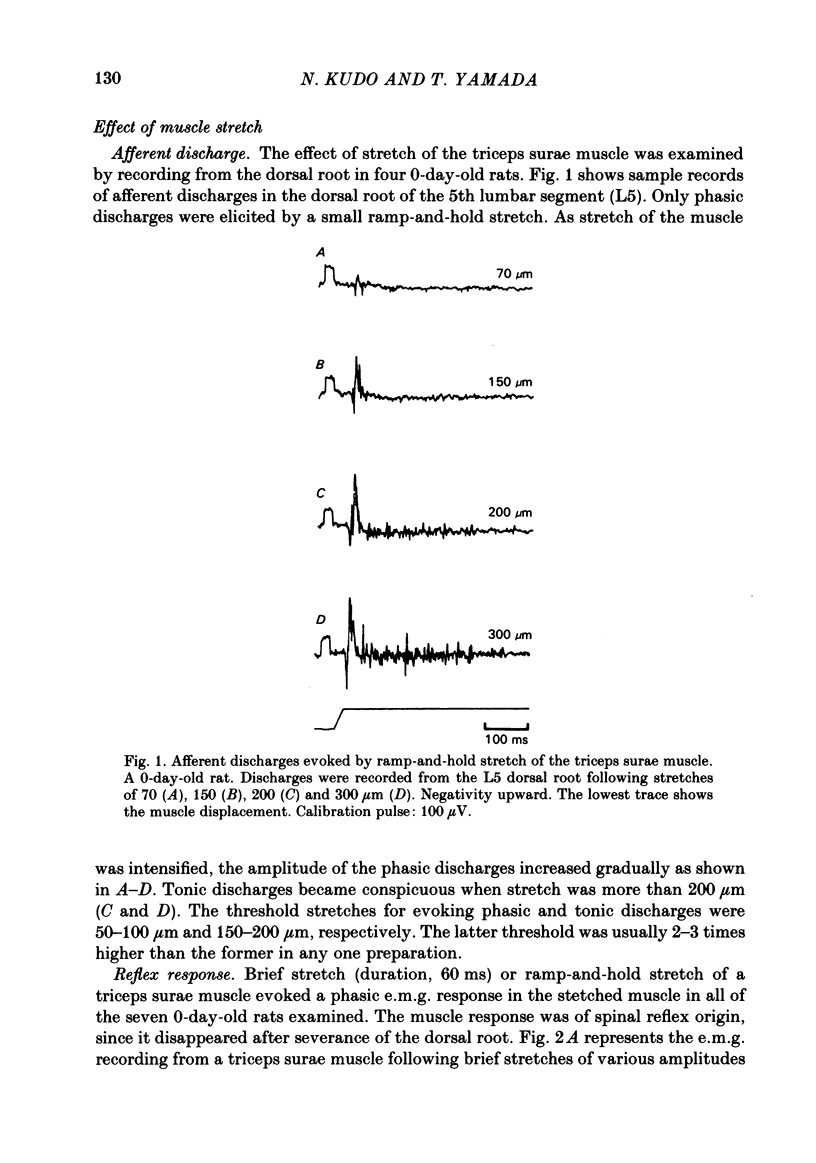
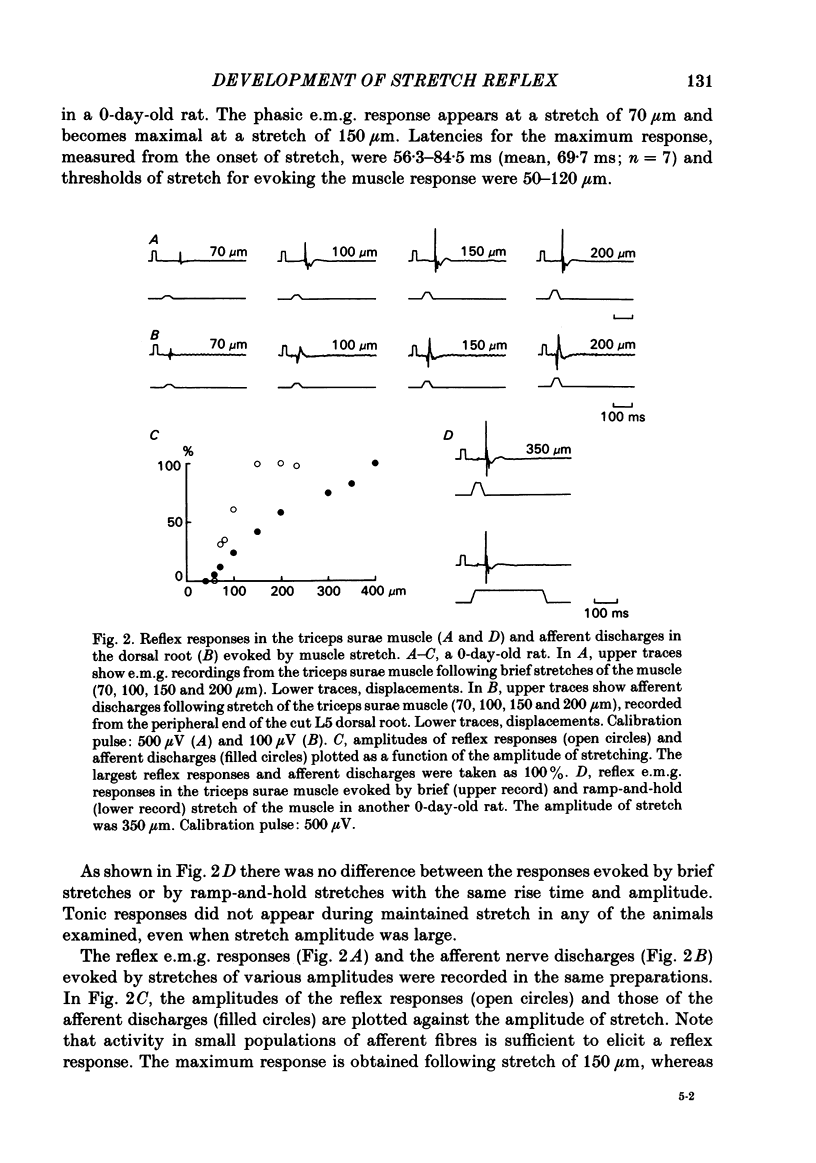
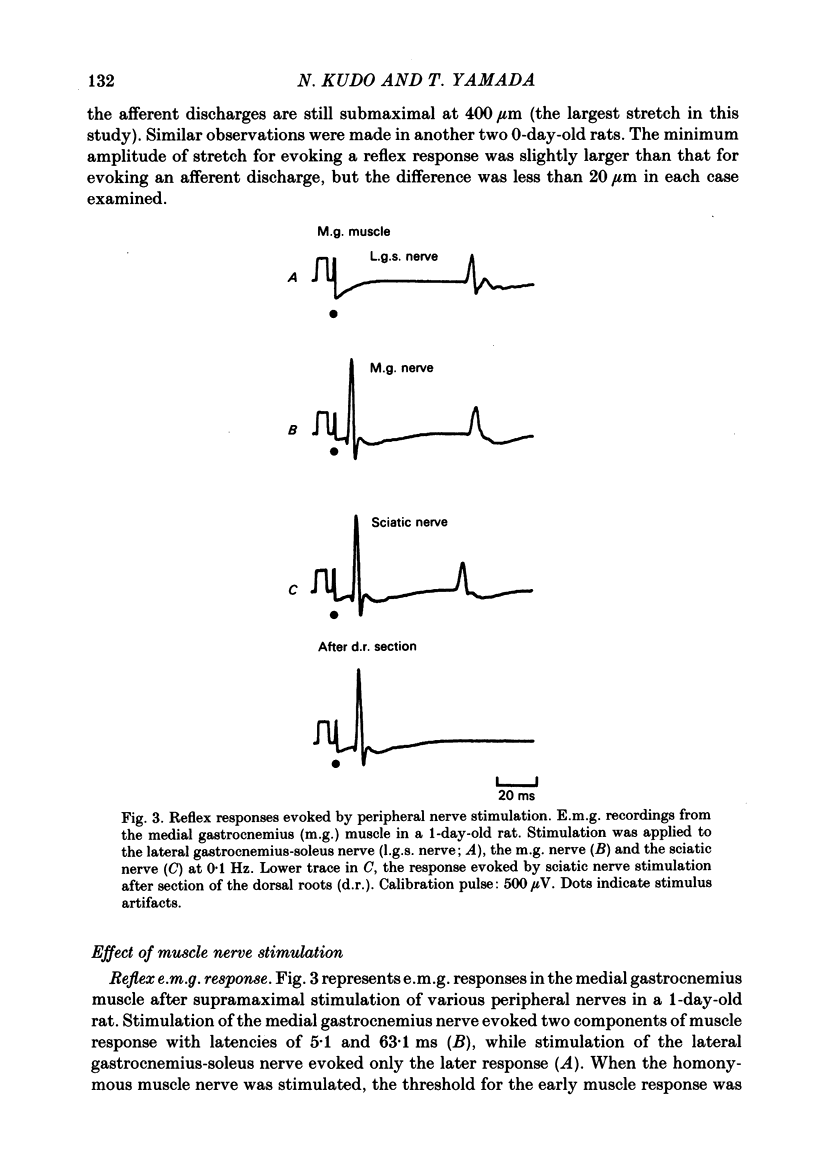
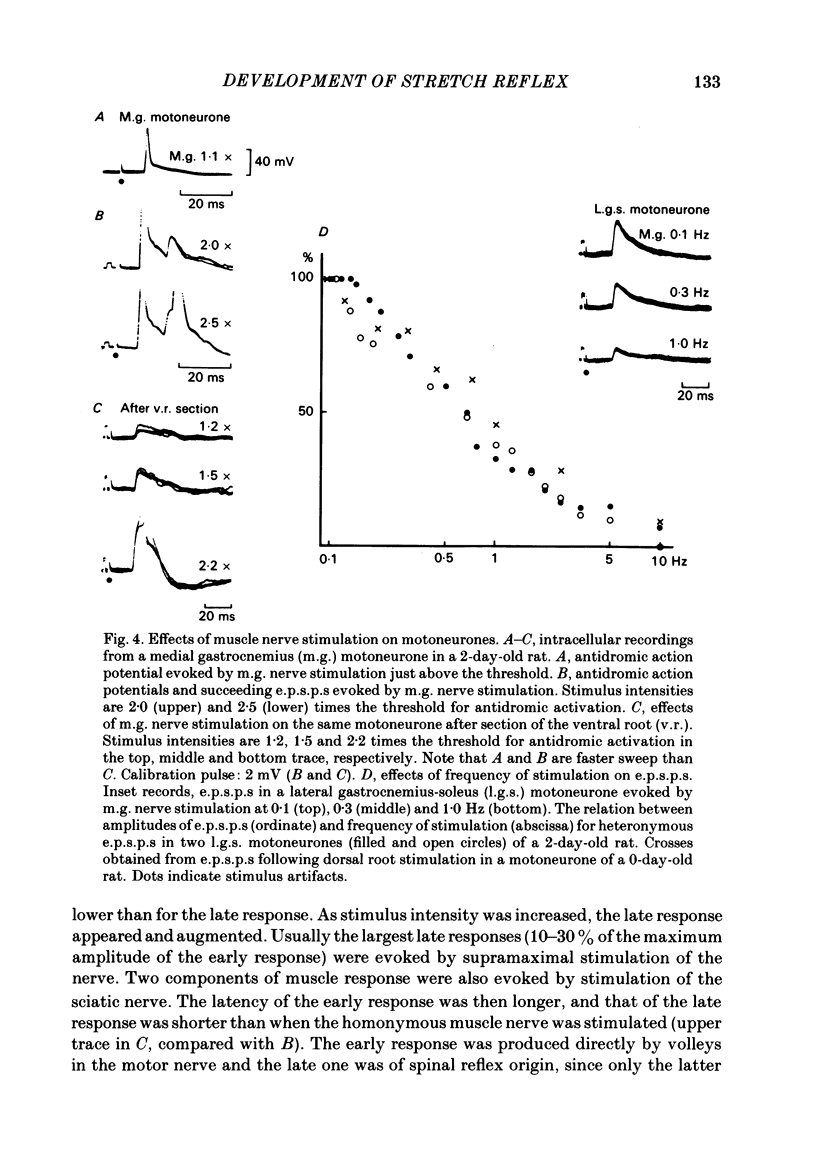
The properties and development of the stretch reflex pathway were investigated in new-born and fetal rats using the isolated spinal cord-hind limb preparation. Muscle afferent discharge was elicited by small stretch of the triceps surae muscle in the new-born rat and in the fetus. It appeared as early as embryonic day 18.5. Ramp-and-hold stretch elicited only phasic discharges in most afferent fibres. A phasic reflex response was evoked in the triceps surae muscle by brief or ramp-and-hold stretch of the muscle in the new-born rat. The threshold stretch required for evoking the reflex response was close to that for eliciting the afferent discharge. A reflex response in the triceps surae muscle was also evoked by electrical stimulation of the triceps surae muscle nerve or the sciatic nerve in the new-born rat. Excitatory post-synaptic potentials (e.p.s.p.s) in the triceps surae motoneurones were evoked by stimulation of the muscle nerve in the new-born rat. The amplitude of the e.p.s.p.s was large enough to generate spike potentials. Homonymous e.p.s.p.s were significantly larger than heteronymous e.p.s.p.s. The amplitudes of the e.p.s.p.s were very susceptible to the rate of stimulus repetition. At a stimulus frequency of 10 Hz they were depressed to less than 10% of the control value. Presynaptic impulses evoked by stimulation of afferents in the muscle nerve appear in the motor nucleus less than 1.0 ms before the onset of synaptically evoked field potentials. The interval between the arrival of impulses evoked by dorsal root stimulation and the onset of e.p.s.p.s in motoneurones was 0.56 +/- 0.16 ms, indicating monosynaptic transmission from the primary afferents to the motoneurones. In the fetus, a reflex response in the triceps surae muscle was observed following a small stretch of the muscle (or electrical stimulation of the sciatic nerve) in all preparations at embryonic day 20.5 and in about half of those examined at embryonic day 19.5. Neither stimulation evoked a reflex response at embryonic day 18.5. Latencies of the reflex responses evoked by muscle stretch or by nerve stimulation were similar to those in the new-born rat. It is concluded that the monosynaptically evoked stretch reflex response in the triceps surae muscle first appear at embryonic day 19.5. Natural and electrical stimulation of the plantar skin evoked a reflex response with long latencies in flexor muscles. Such a cutaneous reflex was first present at embryonic day 17.5, two days earlier than the onset of the stretch reflex.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burke R. E. Group Ia synaptic input to fast and slow twitch motor units of cat triceps surae. J Physiol. 1968 Jun;196(3):605–630. doi: 10.1113/jphysiol.1968.sp008526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., ECCLES J. C., FATT P. Excitatory synaptic action in motoneurones. J Physiol. 1955 Nov 28;130(2):374–395. doi: 10.1113/jphysiol.1955.sp005413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS D. R., ECCLES J. C. Synaptic action during and after repetitive stimulation. J Physiol. 1960 Feb;150:374–398. doi: 10.1113/jphysiol.1960.sp006393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol. 1957 Jun 18;137(1):22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., SHEALY C. N., WILLIS W. D. Experiments utilizing monosynaptic excitatory action on motoneurons for testing hypotheses relating to specificity of neuronal connections. J Neurophysiol. 1962 Jul;25:559–580. doi: 10.1152/jn.1962.25.4.559. [DOI] [PubMed] [Google Scholar]
- ECCLES R. M., WILLIS W. D. THE EFFECT OF REPETITIVE STIMULATION UPON MONOSYNAPTIC TRANSMISSION IN KITTENS. J Physiol. 1965 Jan;176:311–321. doi: 10.1113/jphysiol.1965.sp007552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles R. M., Shealy C. N., Willis W. D. Patterns of innervation of kitten motoneurones. J Physiol. 1963 Mar;165(3):392–402. doi: 10.1113/jphysiol.1963.sp007065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide A. L., Jansen J. K., Ribchester R. R. The effect of lesions in the neural crest on the formation of synaptic connexions in the embryonic chick spinal cord. J Physiol. 1982 Mar;324:453–478. doi: 10.1113/jphysiol.1982.sp014124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E., Westerfield M. Development of sensory-motor synapses in the spinal cord of the frog. J Physiol. 1983 Oct;343:593–610. doi: 10.1113/jphysiol.1983.sp014912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo T., Lundberg A., Phillips C. G., Thompson R. F. The pattern of monosynaptic Ia-connections to hindlimb motor nuclei in the baboon: a comparison with the cat. Proc R Soc Lond B Biol Sci. 1984 May 22;221(1224):261–289. doi: 10.1098/rspb.1984.0034. [DOI] [PubMed] [Google Scholar]
- Kellerth J. O., Mellström A., Skoglund S. Postnatal excitability changes of kitten motoneurones. Acta Physiol Scand. 1971 Sep;83(1):31–41. doi: 10.1111/j.1748-1716.1971.tb05048.x. [DOI] [PubMed] [Google Scholar]
- Landon D. N. The fine structure of the equatorial regions of developing muscle spindles in the rat. J Neurocytol. 1972 Sep;1(2):189–210. doi: 10.1007/BF01099184. [DOI] [PubMed] [Google Scholar]
- Matthews B. H. Nerve endings in mammalian muscle. J Physiol. 1933 Apr 13;78(1):1–53. doi: 10.1113/jphysiol.1933.sp002984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May M. K., Biscoe T. J. An investigation of the foetal rat spinal cord. I. Ultrastructural observations on the onset of synaptogenesis. Cell Tissue Res. 1975;158(2):241–249. doi: 10.1007/BF00219963. [DOI] [PubMed] [Google Scholar]
- Milburn A. The early development of muscle spindles in the rat. J Cell Sci. 1973 Jan;12(1):175–195. doi: 10.1242/jcs.12.1.175. [DOI] [PubMed] [Google Scholar]
- NAKA K. I. ELECTROPHYSIOLOGY OF THE FETAL SPINAL CORD. II. INTERACTION AMONG PERIPHERAL INPUTS AND RECURRENT INHIBITION. J Gen Physiol. 1964 May;47:1023–1038. doi: 10.1085/jgp.47.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan C. H., Fox M. W., Hamburger V. Prenatal development of spontaneous and evoked activity in the rat (Rattus norvegicus albinus). Behaviour. 1971;40(1):100–134. doi: 10.1163/156853971x00357. [DOI] [PubMed] [Google Scholar]
- Nicolopoulos-Stournaras S., Iles J. F. Motor neuron columns in the lumbar spinal cord of the rat. J Comp Neurol. 1983 Jun 10;217(1):75–85. doi: 10.1002/cne.902170107. [DOI] [PubMed] [Google Scholar]
- Saito K. Development of spinal reflexes in the rat fetus studied in vitro. J Physiol. 1979 Sep;294:581–594. doi: 10.1113/jphysiol.1979.sp012947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. L. The development and postnatal organization of primary afferent projections to the rat thoracic spinal cord. J Comp Neurol. 1983 Oct 10;220(1):29–43. doi: 10.1002/cne.902200105. [DOI] [PubMed] [Google Scholar]
- WILSON V. J. Reflex transmission in the kitten. J Neurophysiol. 1962 Mar;25:263–276. doi: 10.1152/jn.1962.25.2.263. [DOI] [PubMed] [Google Scholar]


