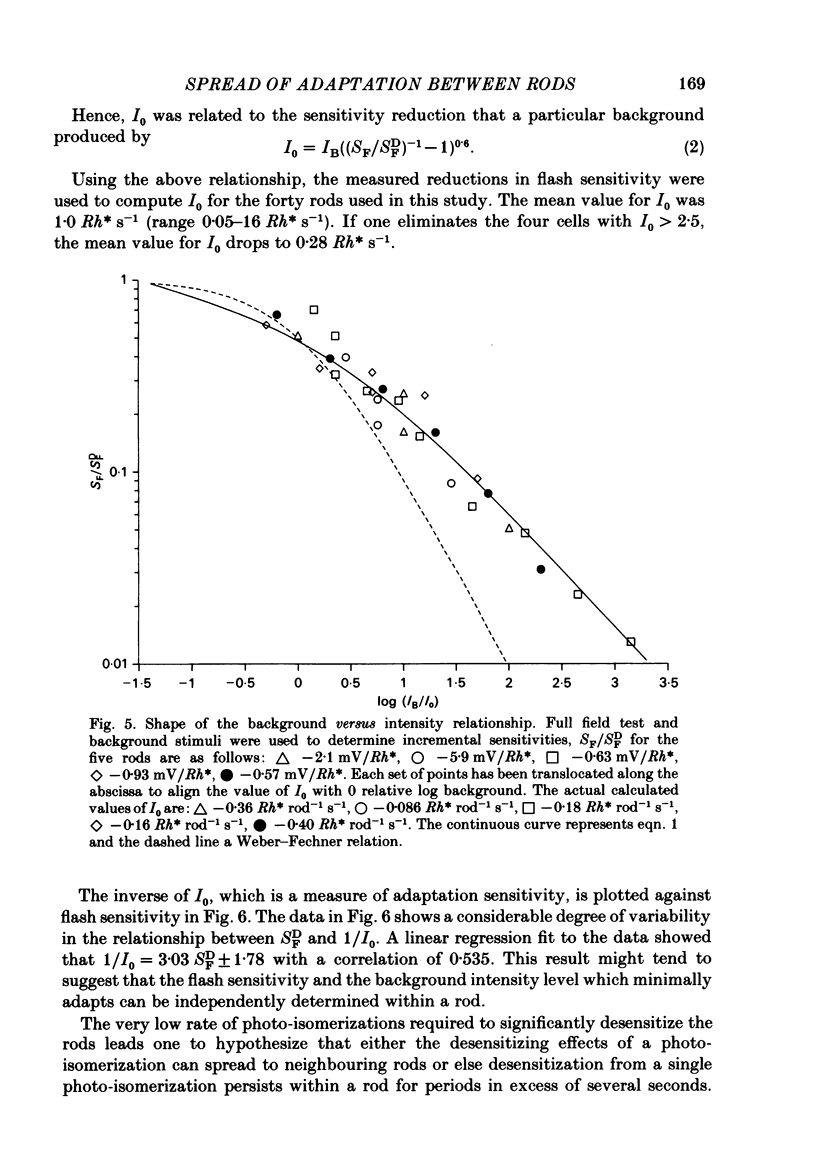
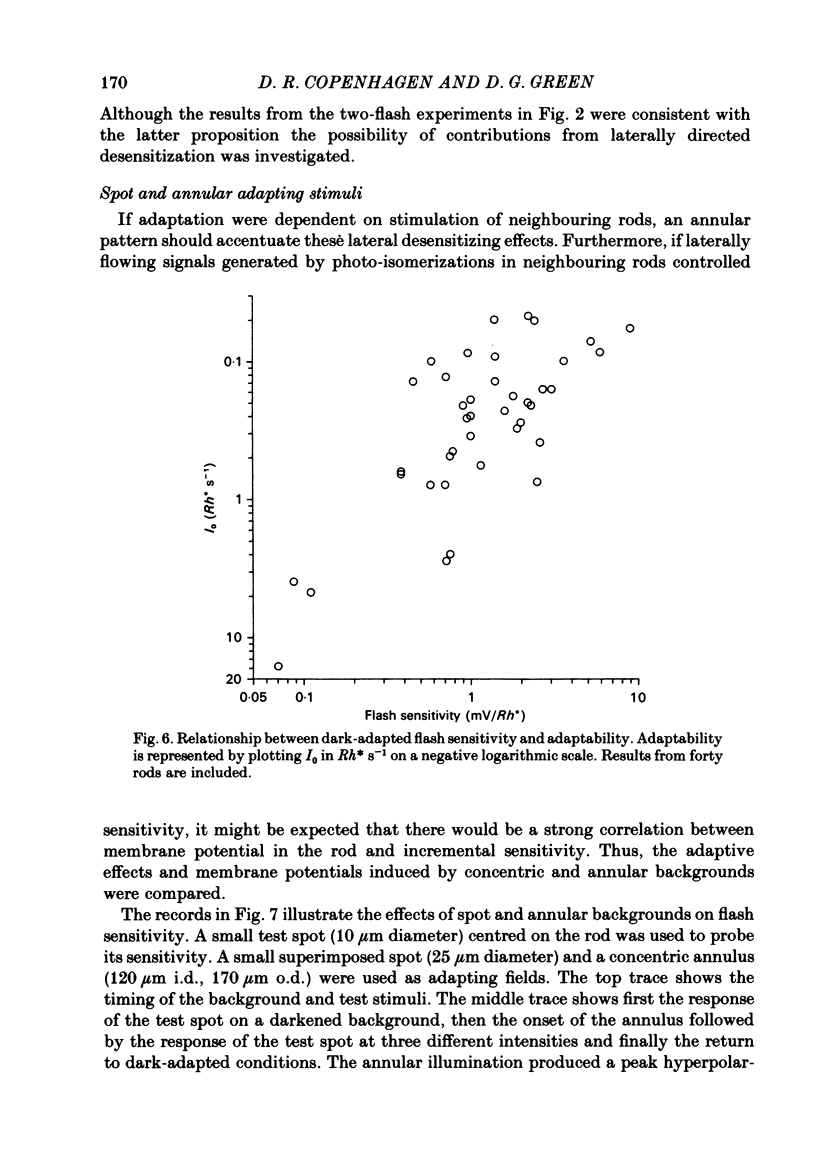
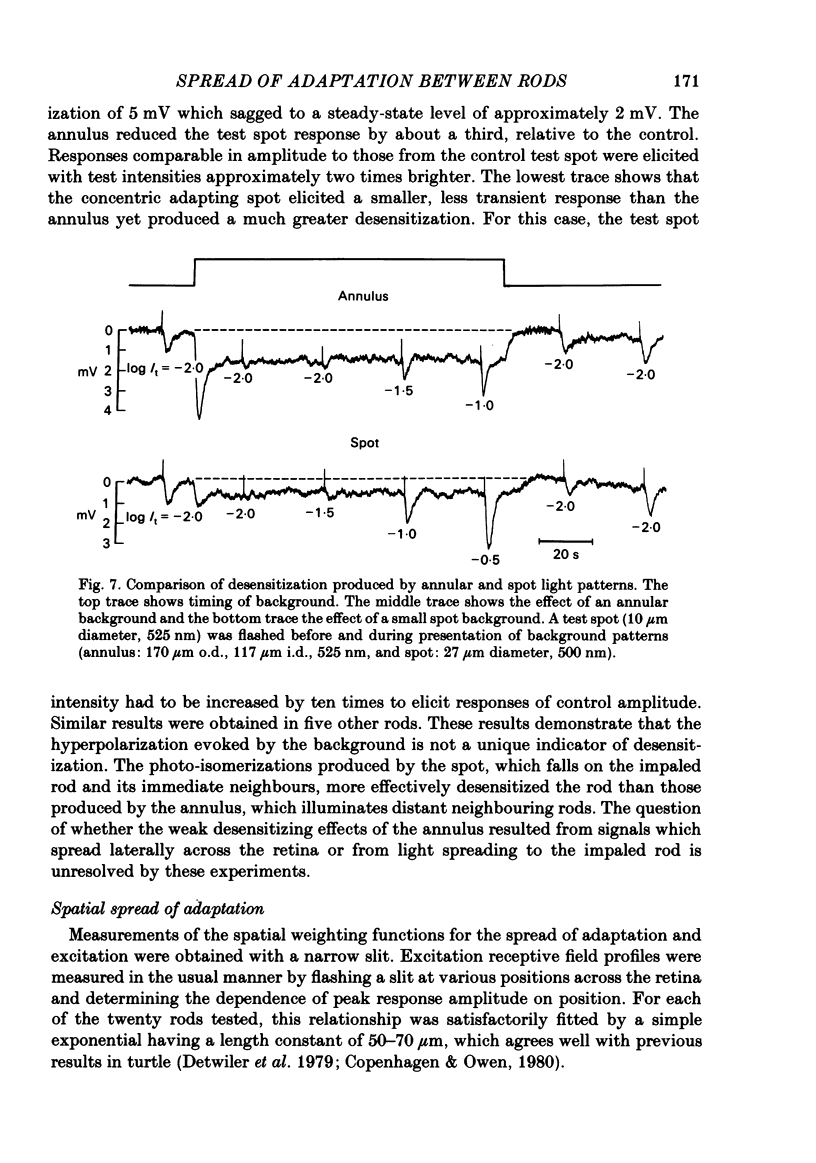
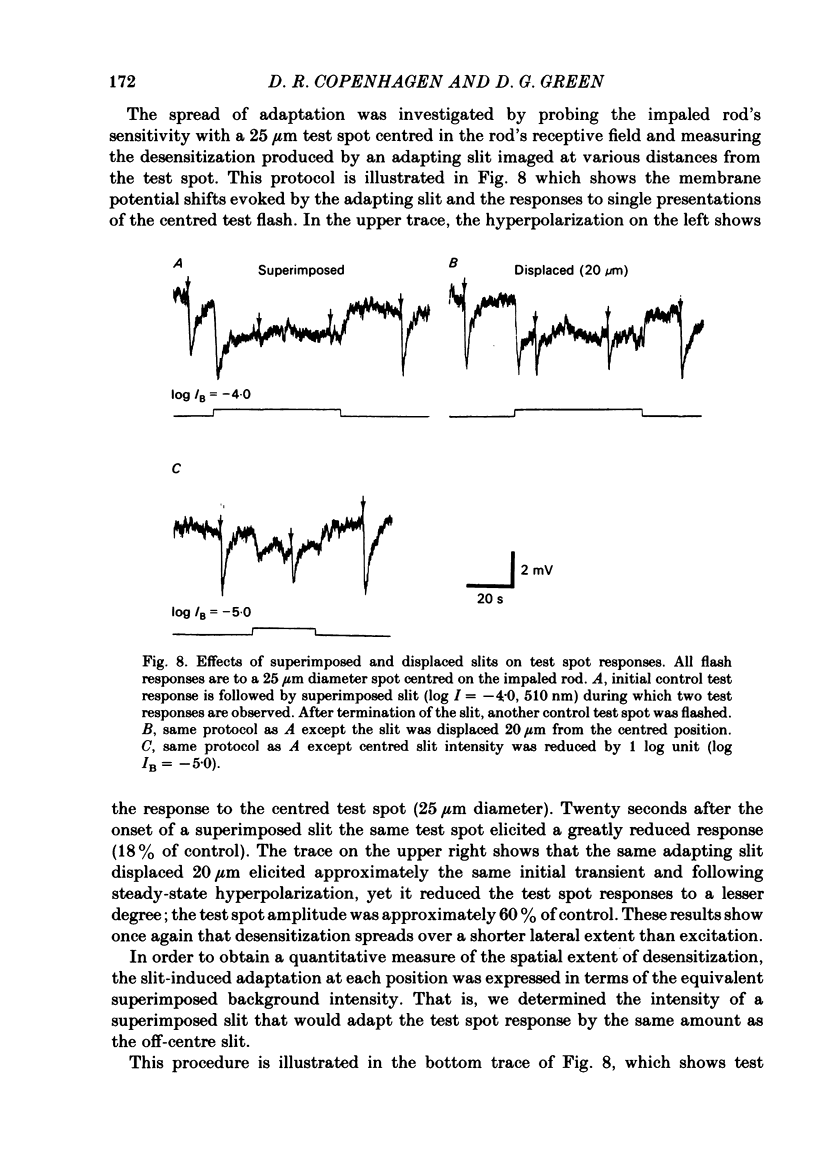
Abstract
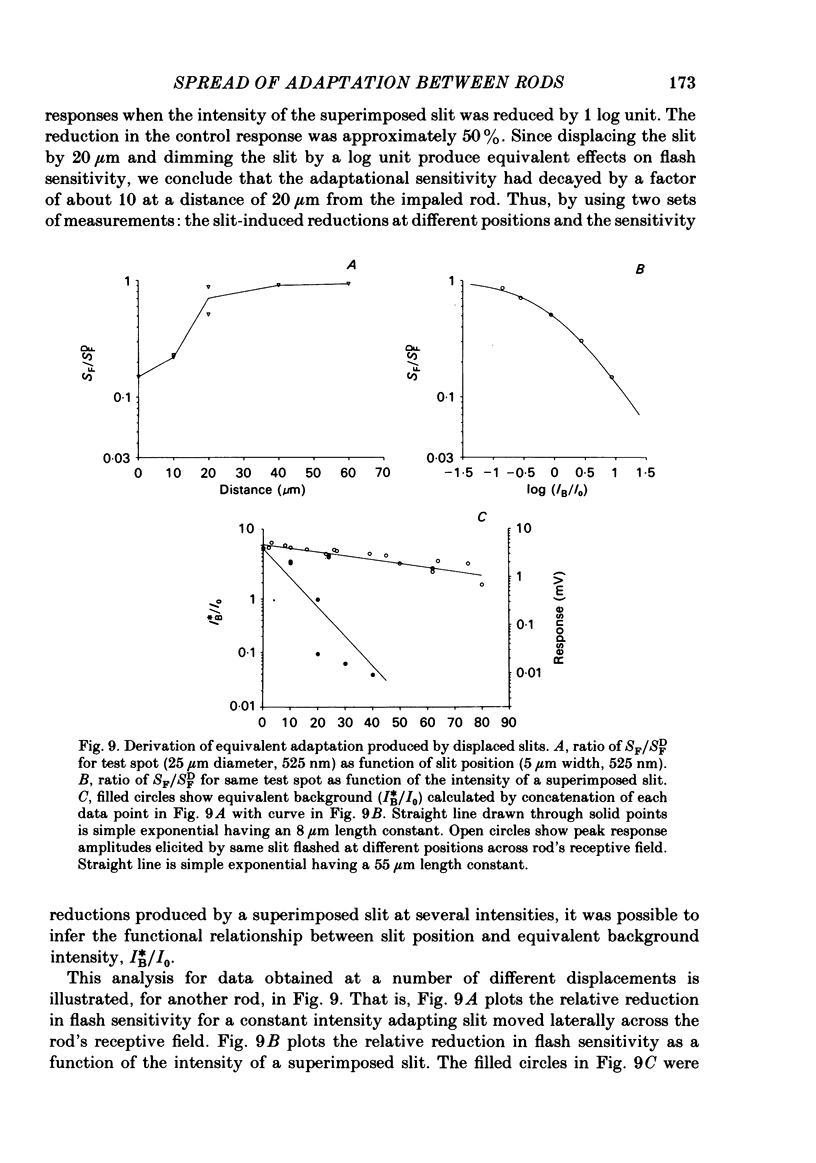
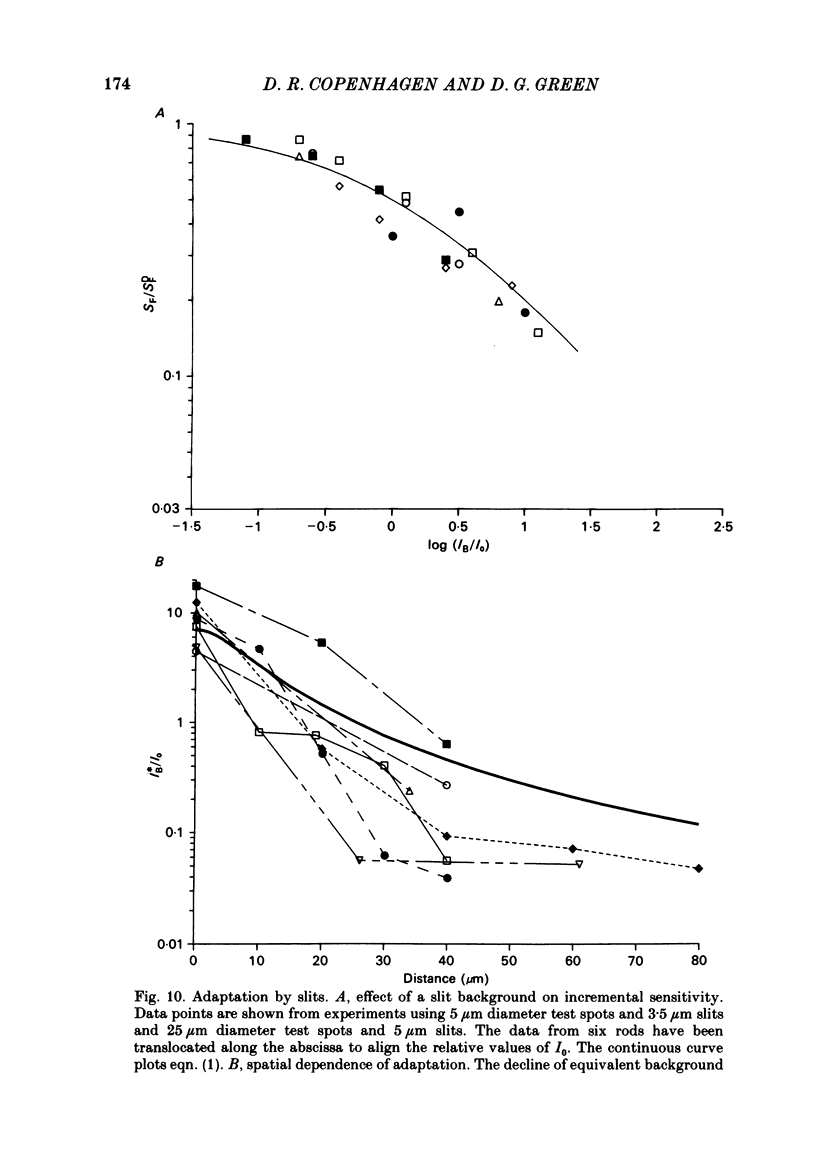
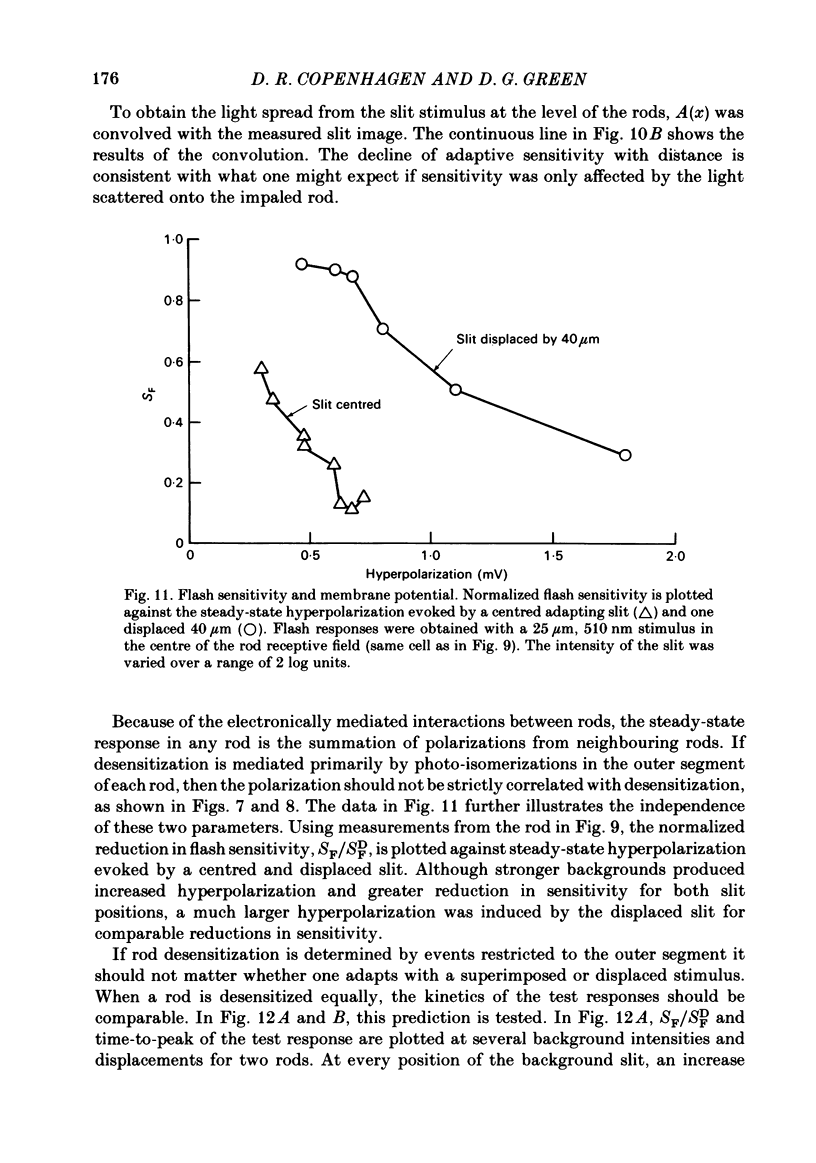
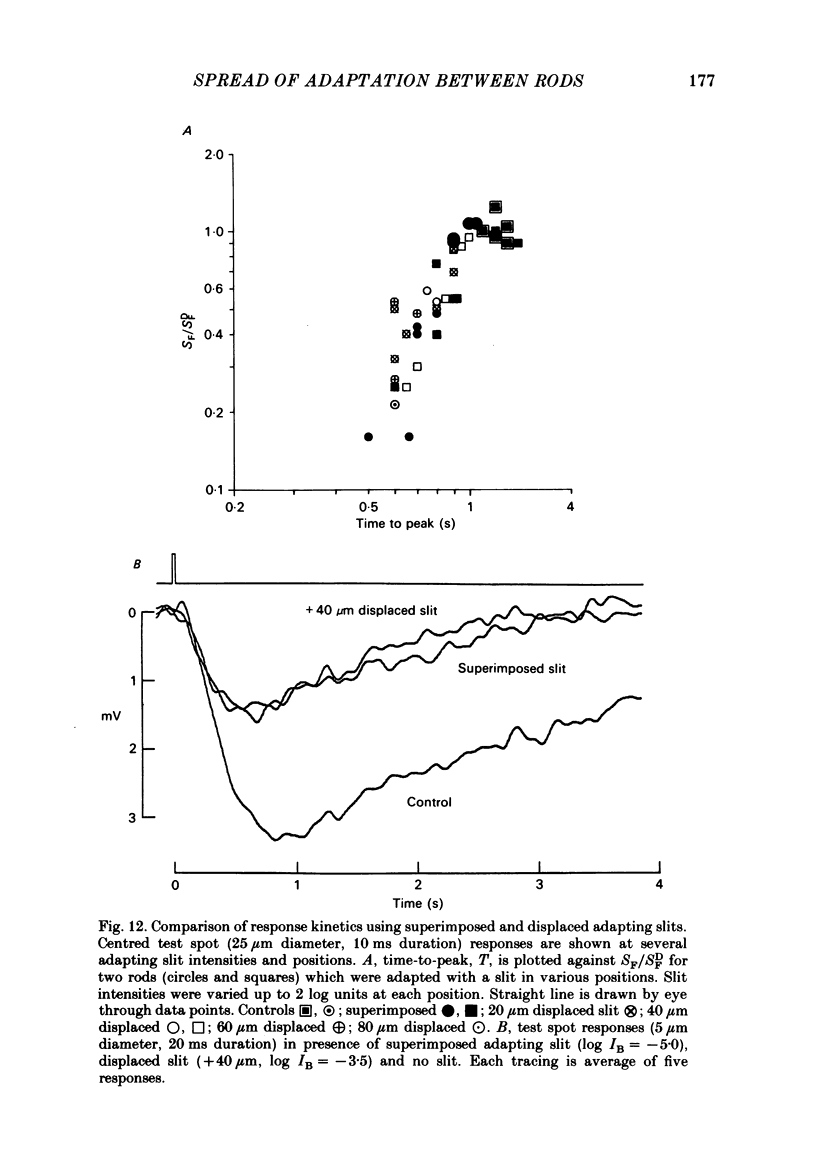
Adaptation by weak backgrounds and the spatial spread of desensitization between rods was studied in the snapping turtle retina, Chelydra serpentina. Intracellular membrane potentials were recorded from these photoreceptors in an eyecup preparation. The kinetics and sensitivity of rod responses were changed significantly by large, very dim backgrounds. For the twenty-five most sensitive rods where the dark-adapted flash sensitivity, SDF, was greater than 1.0 mV/Rh*, Rh* being the number of effective photo-isomerizations per rod, the background intensity required to halve the amplitude of the linear range response averaged 0.21 Rh* s-1. The time-to-peak of the test responses was reduced up to 50% by these dim backgrounds. The desensitizing effects of full field backgrounds of various intensities on the responses to large test spots were measured. The dependence of incremental flash sensitivity, SF, on background intensity, IB, followed the form (FORMULA: SEE TEXT) where I0 is the background intensity which halved SDF. The same intensity dependence held for slit-shaped background fields that desensitized responses to small test spots. The desensitizing effects of large, very dim flashed and continuous backgrounds took several seconds to appear and decay to dark levels. This in conjunction with the sparsity of photons suggests, that the desensitization from a single photoisomerization can persist for several seconds. A comparison of the desensitizing effects of spot and annular backgrounds revealed that small spot backgrounds superimposed on the centered test spots desensitized rods more effectively than annular fields. This finding held true even when annular patterns produced a greater maintained hyperpolarization in the rods. Thus, there was no unique relationship between desensitization and the steady maintained hyperpolarization evoked by a background field. The dependence of adaptation on distance from the impaled rod was determined with slit-shaped background fields placed at different positions across the rod's receptive field. The desensitizing effect of displaced slit stimuli was found to decline much more rapidly with distance than excitation. Displacing the slit by 20 micron from the centre reduced its desensitizing effect by more than 1 log unit. In contrast, excitation fell to about 80% at the same distance (lambda ranging from 50 to 70 micron). The fall off of desensitization with distance matched the calculated fall off with distance of light scatter from a slit. No difference was noted in the kinetics of test responses in the presence of equally desensitizing, superimposed and displaced slits.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow H. B., Andrews D. P. Sensitivity of receptors and receptor "pools". J Opt Soc Am. 1967 Jun;57(6):837–838. doi: 10.1364/josa.57.000837. [DOI] [PubMed] [Google Scholar]
- Bastian B. L., Fain G. L. Light adaptation in toad rods: requirement for an internal messenger which is not calcium. J Physiol. 1979 Dec;297(0):493–520. doi: 10.1113/jphysiol.1979.sp013053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Changes in time scale and sensitivity in turtle photoreceptors. J Physiol. 1974 Nov;242(3):729–758. doi: 10.1113/jphysiol.1974.sp010732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Matthews G., Yau K. W. Two components of electrical dark noise in toad retinal rod outer segments. J Physiol. 1980 Dec;309:591–621. doi: 10.1113/jphysiol.1980.sp013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capovilla M., Cervetto L., Torre V. The effect of phosphodiesterase inhibitors on the electrical activity of toad rods. J Physiol. 1983 Oct;343:277–294. doi: 10.1113/jphysiol.1983.sp014892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicerone C. M., Green D. G. Light adaptation within the receptive field centre of rat retinal ganglion cells. J Physiol. 1980 Apr;301:517–534. doi: 10.1113/jphysiol.1980.sp013221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clack J. W., Oakley B., 2nd, Pepperberg D. R. Light-dependent effects of a hydrolysis-resistant analog of GTP on rod photoresponses in the toad retina. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2690–2694. doi: 10.1073/pnas.79.8.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Enroth-Cugell C. Quantitative aspects of gain and latency in the cat retina. J Physiol. 1970 Jan;206(1):73–91. doi: 10.1113/jphysiol.1970.sp008998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copenhagen D. R., Owen W. G. Coupling between rod photoreceptors in a vertebrate retina. Nature. 1976 Mar 4;260(5546):57–59. doi: 10.1038/260057a0. [DOI] [PubMed] [Google Scholar]
- Copenhagen D. R., Owen W. G. Current-voltage relations in the rod photoreceptor network of the turtle retina. J Physiol. 1980 Nov;308:159–184. doi: 10.1113/jphysiol.1980.sp013466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copenhagen D. R., Owen W. G. Functional characteristics of lateral interactions between rods in the retina of the snapping turtle. J Physiol. 1976 Jul;259(2):251–282. doi: 10.1113/jphysiol.1976.sp011465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detwiler P. B., Hodgkin A. L., McNaughton P. A. Temporal and spatial characteristics of the voltage response of rods in the retina of the snapping turtle. J Physiol. 1980 Mar;300:213–250. doi: 10.1113/jphysiol.1980.sp013159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner K. O., Hemilä S. Excitation and adaptation in the vertebrate rod photoreceptor. Med Biol. 1978 Apr;56(2):52–63. [PubMed] [Google Scholar]
- Easter S. S., Jr Adaptation in the goldfish retina. J Physiol. 1968 Mar;195(2):273–281. doi: 10.1113/jphysiol.1968.sp008458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Shapley R. M. Flux, not retinal illumination, is what cat retinal ganglion cells really care about. J Physiol. 1973 Sep;233(2):311–326. doi: 10.1113/jphysiol.1973.sp010309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L. Sensitivity of toad rods: Dependence on wave-length and background illumination. J Physiol. 1976 Sep;261(1):71–101. doi: 10.1113/jphysiol.1976.sp011549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. G., Tong L., Cicerone C. M. Lateral spread of light adaptation in the rat retina. Vision Res. 1977;17(3):479–486. doi: 10.1016/0042-6989(77)90042-6. [DOI] [PubMed] [Google Scholar]
- Hemilä S. Background adaptation in the rods of the frog's retina. J Physiol. 1977 Mar;265(3):721–741. doi: 10.1113/jphysiol.1977.sp011740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIPETZ L. E. A mechanism of light adaptation. Science. 1961 Mar 3;133(3453):639–640. doi: 10.1126/science.133.3453.639. [DOI] [PubMed] [Google Scholar]
- Lamb T. D., McNaughton P. A., Yau K. W. Spatial spread of activation and background desensitization in toad rod outer segments. J Physiol. 1981;319:463–496. doi: 10.1113/jphysiol.1981.sp013921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D. The involvement of rod photoreceptors in dark adaptation. Vision Res. 1981;21(12):1773–1782. doi: 10.1016/0042-6989(81)90211-x. [DOI] [PubMed] [Google Scholar]
- McNaughton P. A., Yau K. W., Lamb T. D. Spread of activation and desensitisation in rod outer segments. Nature. 1980 Jan 3;283(5742):85–87. doi: 10.1038/283085a0. [DOI] [PubMed] [Google Scholar]
- O'BRIEN B. Vision and resolution in the central retina. J Opt Soc Am. 1951 Dec;41(12):882–894. doi: 10.1364/josa.41.000882. [DOI] [PubMed] [Google Scholar]
- Pepperberg D. R. Rhodopsin and visual adaptation: analysis of photoreceptor thresholds in the isolated skate retina. Vision Res. 1984;24(4):357–366. doi: 10.1016/0042-6989(84)90061-0. [DOI] [PubMed] [Google Scholar]
- Rushton W. A. Bleached rhodopson and visual adaptation. J Physiol. 1965 Dec;181(3):645–655. doi: 10.1113/jphysiol.1965.sp007789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Rod-rod interaction in the retina of the turtle. J Physiol. 1975 Apr;246(3):617–638. doi: 10.1113/jphysiol.1975.sp010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong L., Green D. G. Adaptation pools and excitation receptive fields of rat retinal ganglion cells. Vision Res. 1977;17(10):1233–1236. doi: 10.1016/0042-6989(77)90160-2. [DOI] [PubMed] [Google Scholar]
- Westheimer G. Spatial interaction in the human retina during scotopic vision. J Physiol. 1965 Dec;181(4):881–894. doi: 10.1113/jphysiol.1965.sp007803. [DOI] [PMC free article] [PubMed] [Google Scholar]


