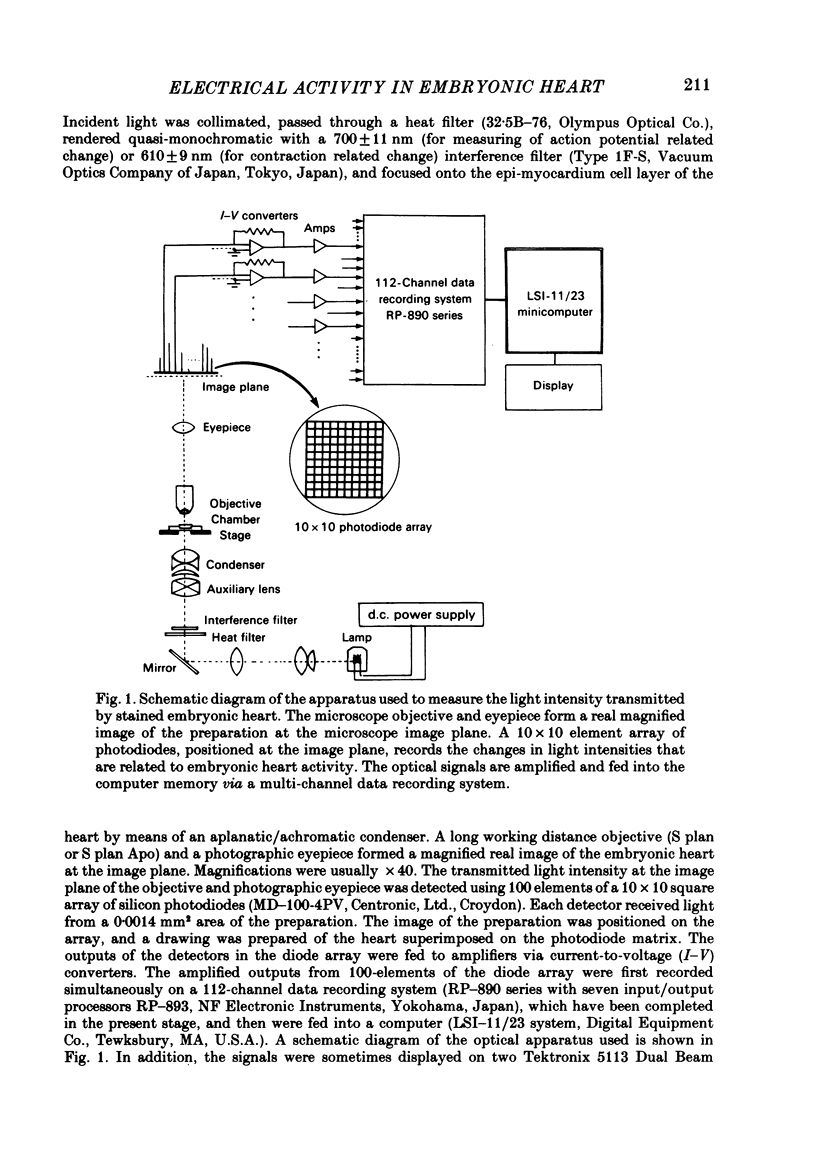
Abstract
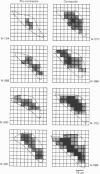
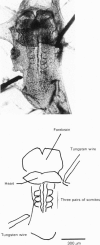
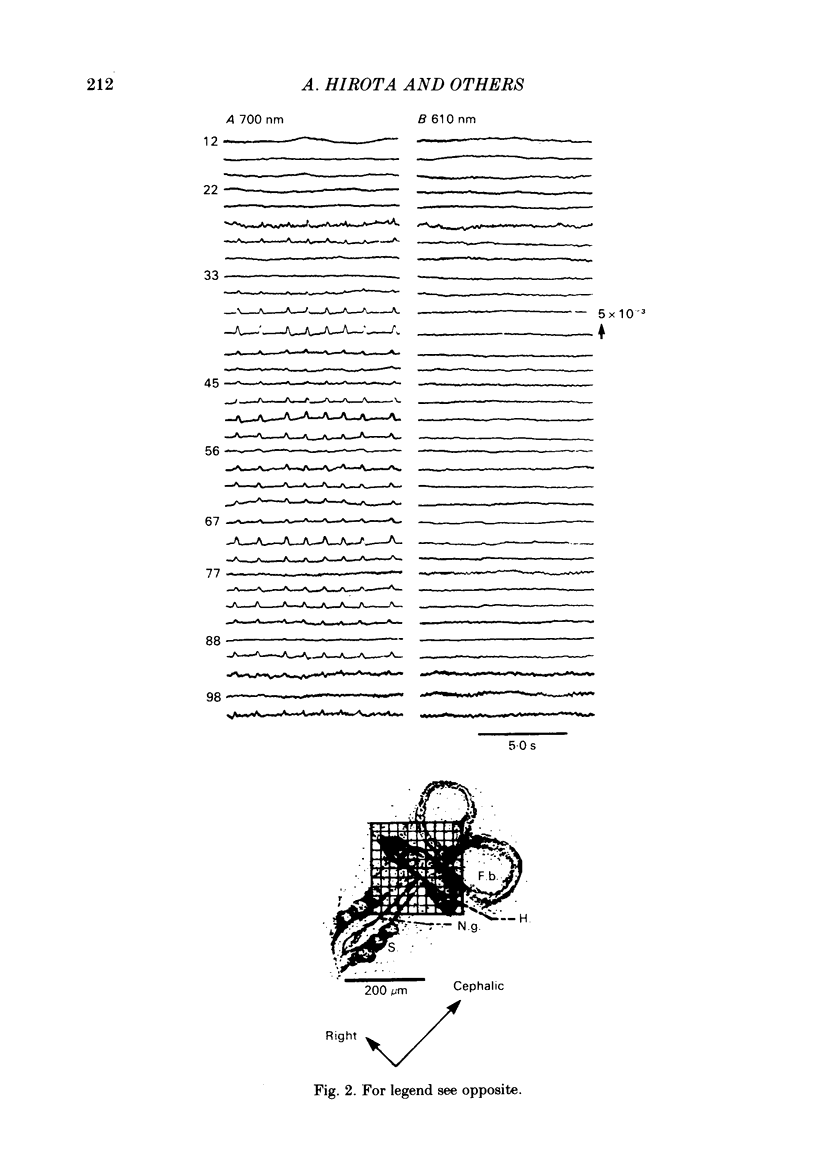
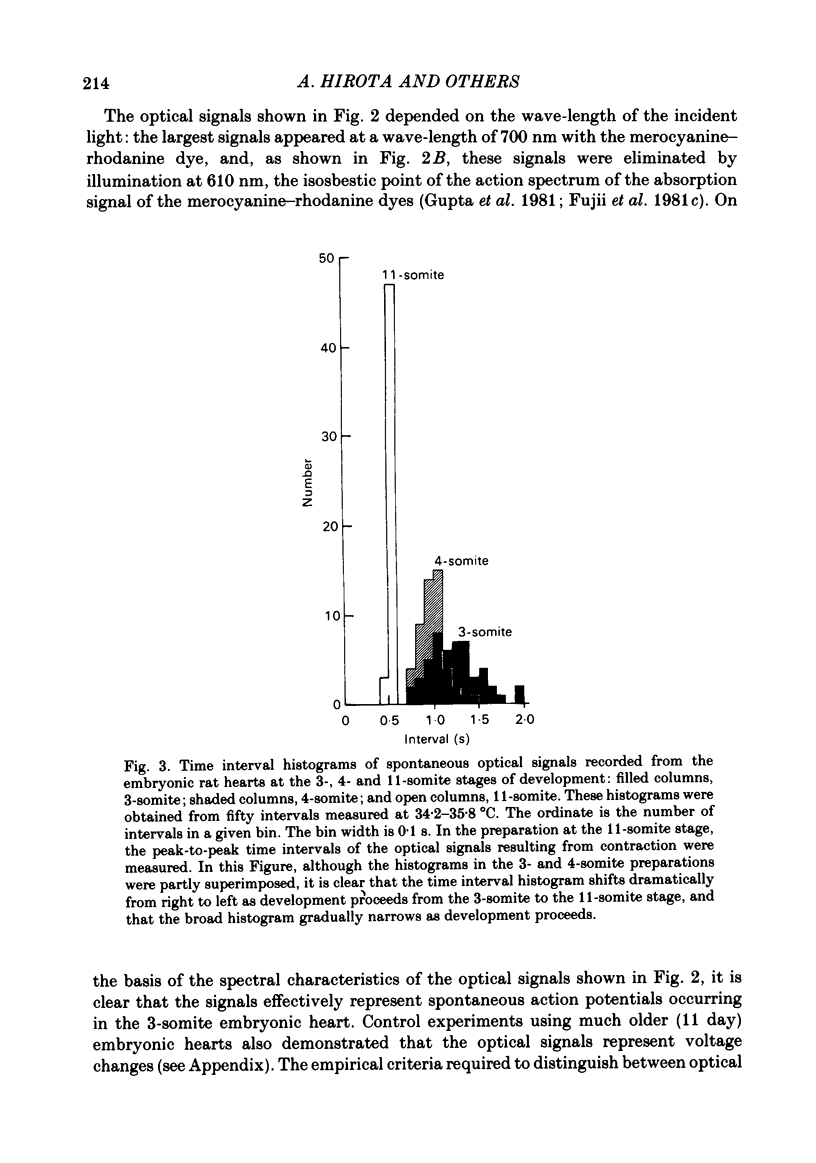
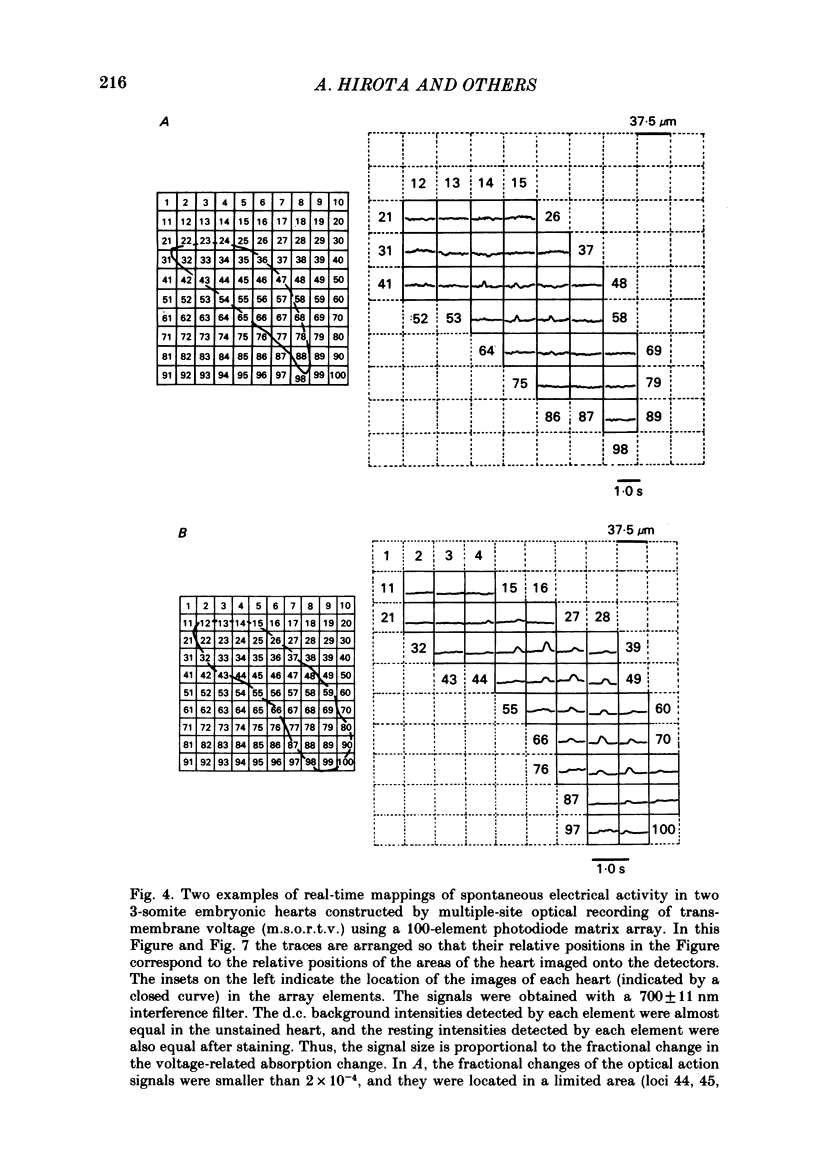
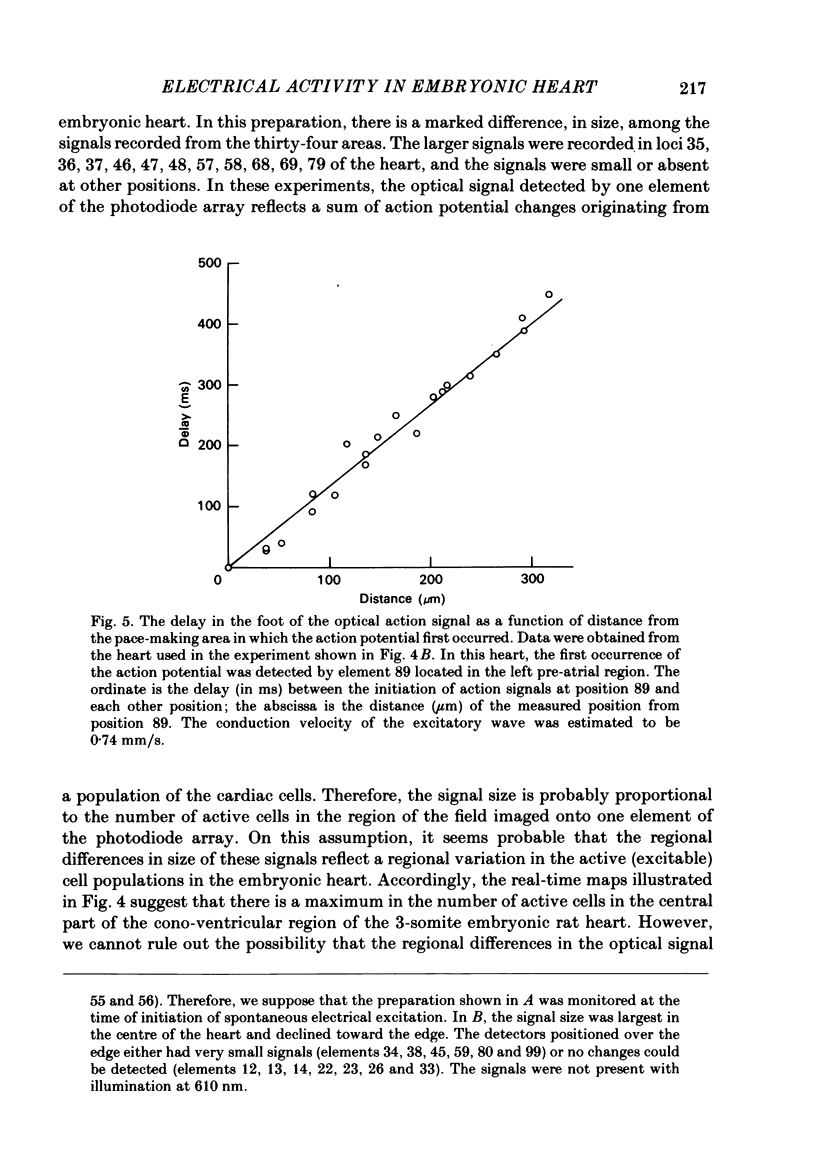
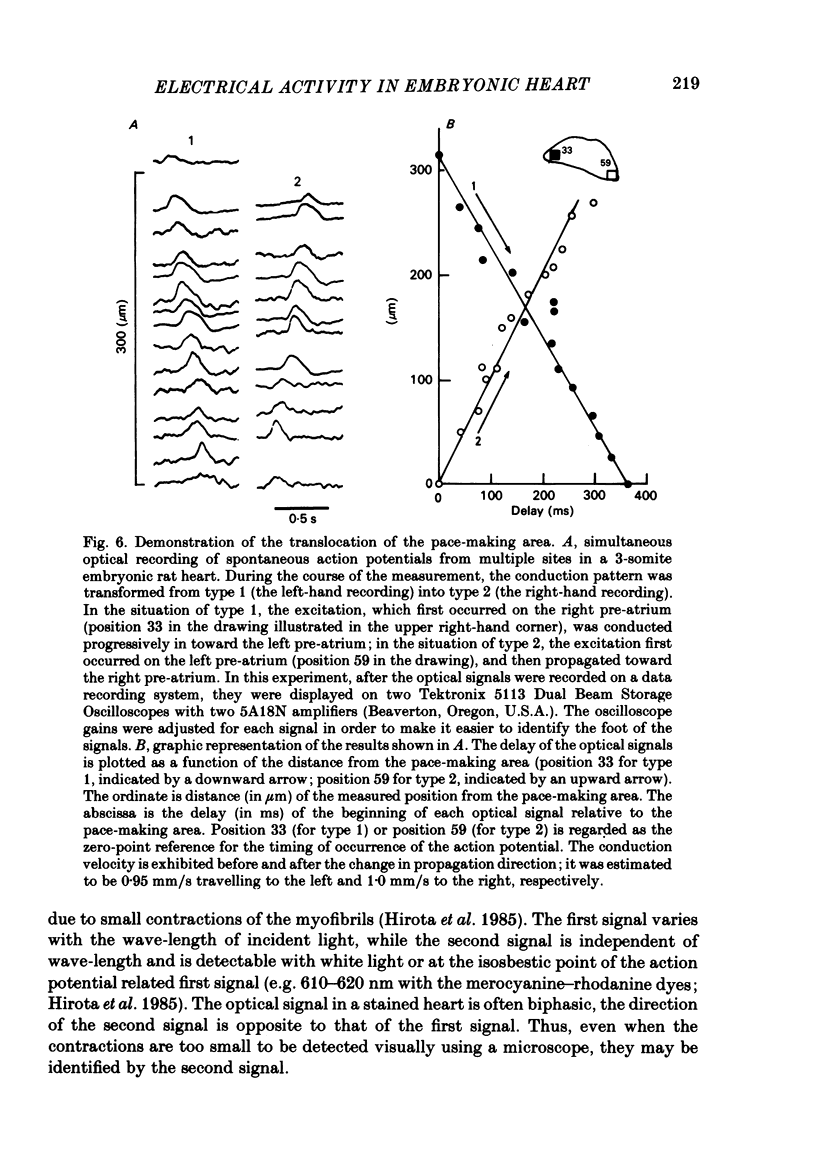
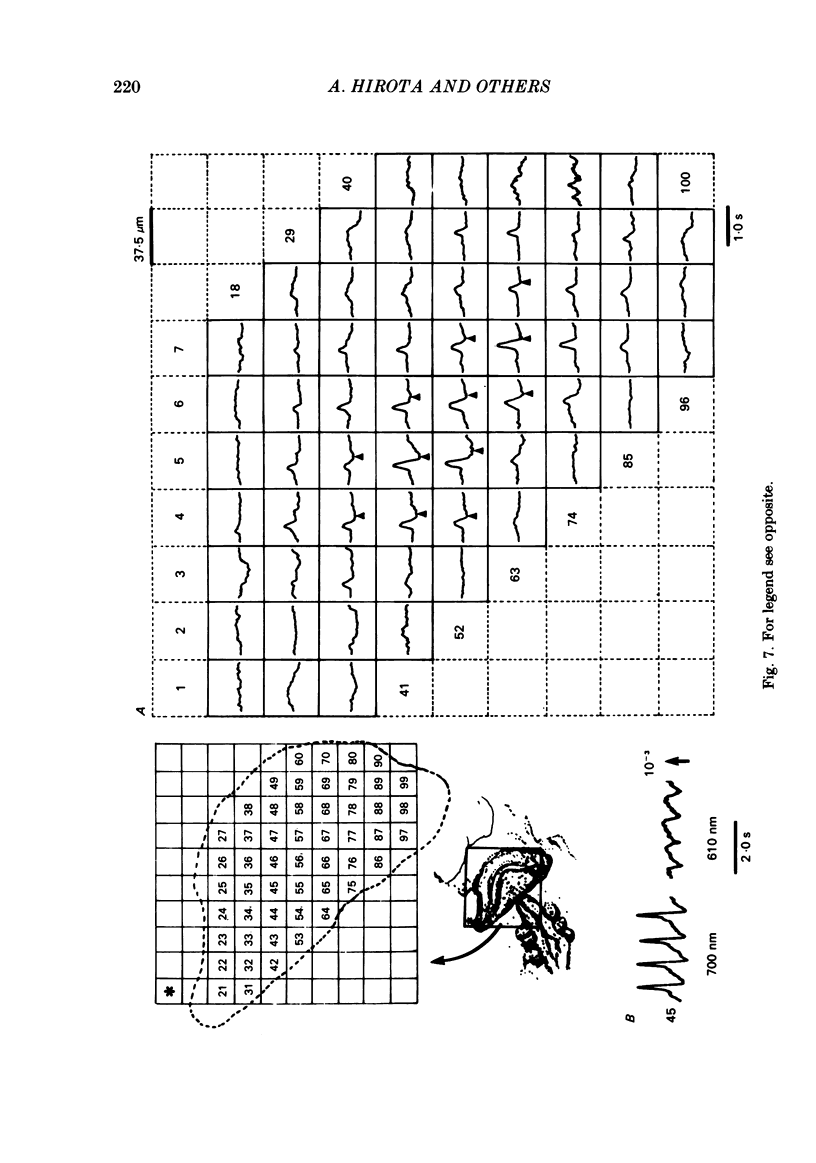
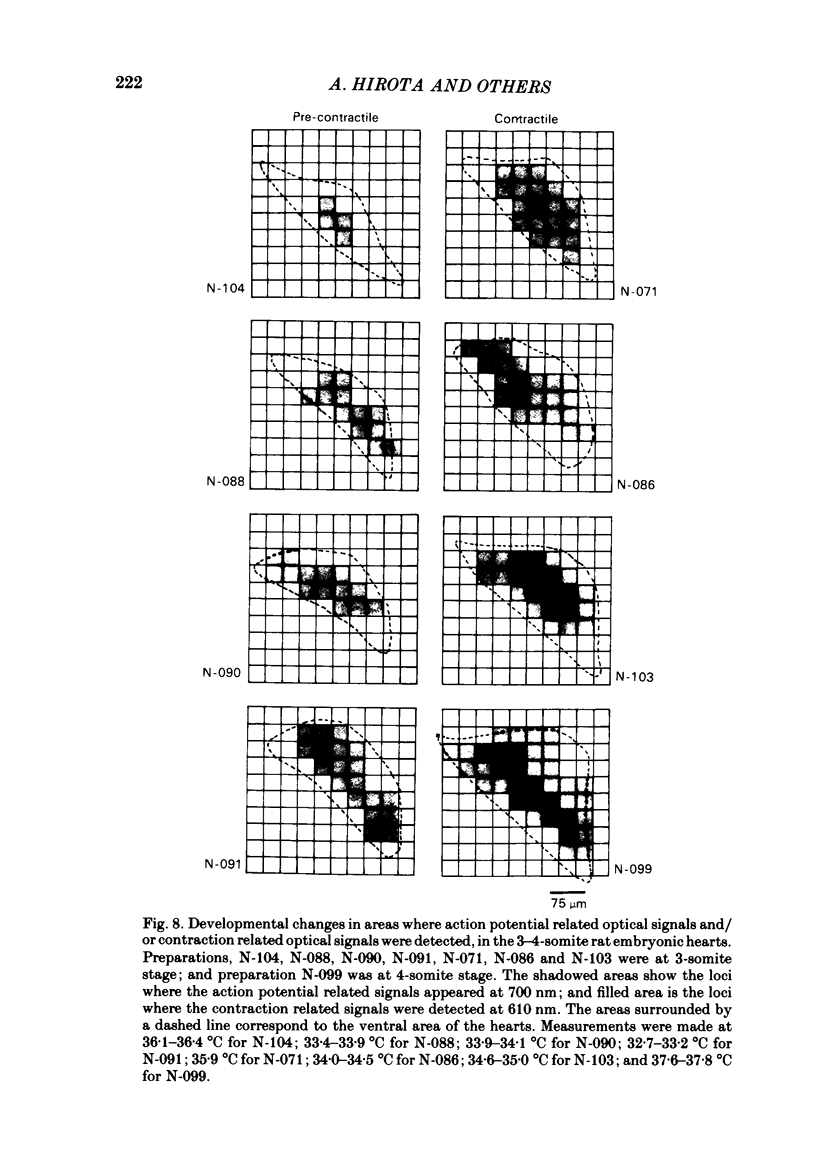
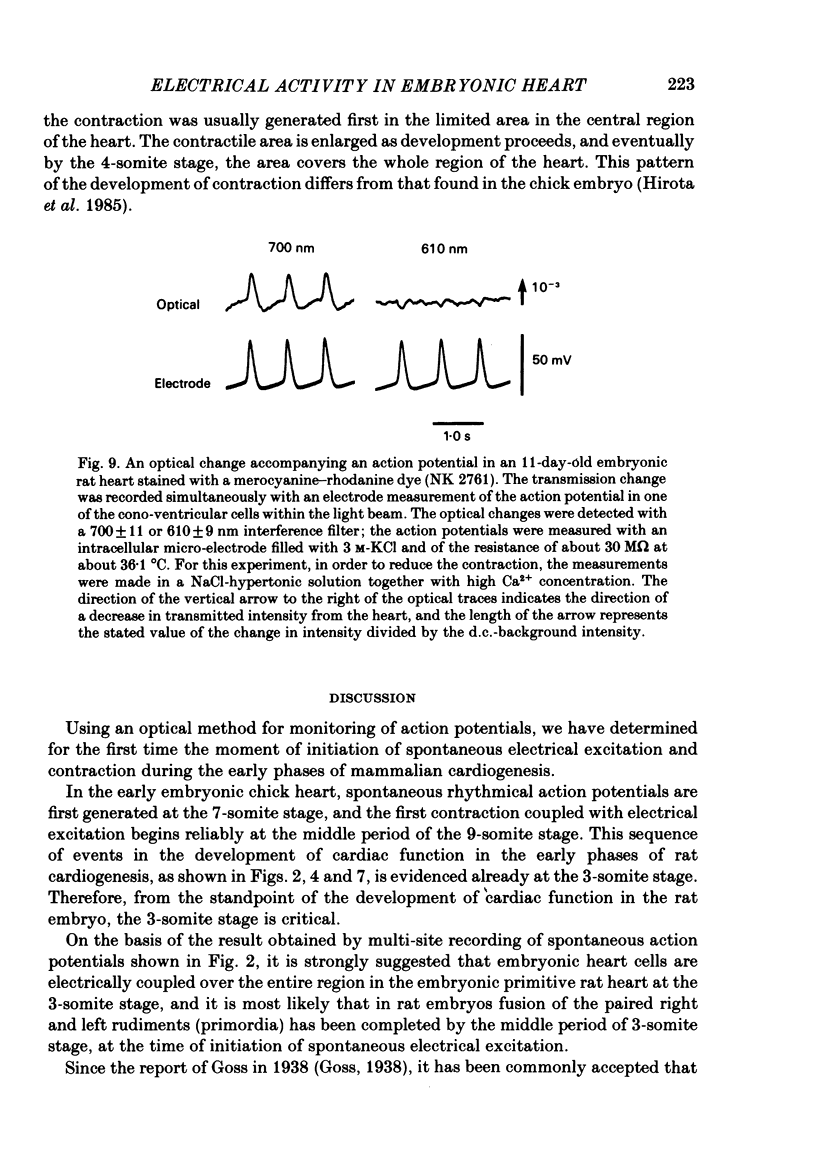
Spontaneous action potential and contraction in the early embryonic heart of the rat have been monitored optically using a voltage-sensitive merocyanine-rhodanine dye together with a multiple-element photodiode matrix array, and the onset of rhythmical action-potential activity in the early phases of rat cardiogenesis was conclusively determined for the first time. Spontaneous rhythmical action potentials were first generated in the central part of the embryonic heart at the middle period of the 3-somite stage of development, at 91/2 days after copulation. Subsequently, contractions coupled with the action potential also appeared at the end of the 3-somite stage. Usually, at the 3-somite stage, spontaneous action signals were synchronized among the different areas in the heart. From this result, it is evident that the paired right and left cardiac primordia are fused completely at the time of initiation of spontaneous electrical activity. In the 3-somite embryonic heart, excitatory waves were conducted radially over the heart, at a uniform rate (0.4-0.8 mm/s), from the pace-making area. However, the regional priority of pace-making activity is not rigid but is flexible.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen L. B., Salzberg B. M. Optical measurement of membrane potential. Rev Physiol Biochem Pharmacol. 1978;83:35–88. doi: 10.1007/3-540-08907-1_2. [DOI] [PubMed] [Google Scholar]
- DeHaan R. L., Sachs H. G. Cell coupling in developing systems: the heart-cell paradigm. Curr Top Dev Biol. 1972;7:193–228. doi: 10.1016/s0070-2153(08)60072-1. [DOI] [PubMed] [Google Scholar]
- Freedman J. C., Laris P. C. Electrophysiology of cells and organelles: studies with optical potentiometric indicators. Int Rev Cytol Suppl. 1981;12:177–246. doi: 10.1016/b978-0-12-364373-5.50015-9. [DOI] [PubMed] [Google Scholar]
- Fujii S., Hirota A., Kamino K. Action potential synchrony in embryonic precontractile chick heart: optical monitoring with potentiometric dyes. J Physiol. 1981;319:529–541. doi: 10.1113/jphysiol.1981.sp013924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S., Hirota A., Kamino K. Optical indications of pace-maker potential and rhythm generation in early embryonic chick heart. J Physiol. 1981 Mar;312:253–263. doi: 10.1113/jphysiol.1981.sp013627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S., Hirota A., Kamino K. Optical recording of development of electrical activity in embryonic chick heart during early phases of cardiogenesis. J Physiol. 1981 Feb;311:147–160. doi: 10.1113/jphysiol.1981.sp013578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S., Hirota A., Kamino K. Optical signals from early embryonic chick heart stained with potential sensitive dyes: evidence for electrical activity. J Physiol. 1980 Jul;304:503–518. doi: 10.1113/jphysiol.1980.sp013339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOSS C. M. Development of the median coordinated ventricle from the lateral hearts in rat embryos with three to six somites. Anat Rec. 1952 Apr;112(4):761–796. doi: 10.1002/ar.1091120405. [DOI] [PubMed] [Google Scholar]
- Grinvald A., Anglister L., Freeman J. A., Hildesheim R., Manker A. Real-time optical imaging of naturally evoked electrical activity in intact frog brain. 1984 Apr 26-May 2Nature. 308(5962):848–850. doi: 10.1038/308848a0. [DOI] [PubMed] [Google Scholar]
- Grinvald A., Cohen L. B., Lesher S., Boyle M. B. Simultaneous optical monitoring of activity of many neurons in invertebrate ganglia using a 124-element photodiode array. J Neurophysiol. 1981 May;45(5):829–840. doi: 10.1152/jn.1981.45.5.829. [DOI] [PubMed] [Google Scholar]
- Gupta R. K., Salzberg B. M., Grinvald A., Cohen L. B., Kamino K., Lesher S., Boyle M. B., Waggoner A. S., Wang C. H. Improvements in optical methods for measuring rapid changes in membrane potential. J Membr Biol. 1981 Feb 15;58(2):123–137. doi: 10.1007/BF01870975. [DOI] [PubMed] [Google Scholar]
- Heppner D. B., Plonsey R. Simulation of electrical interaction of cardiac cells. Biophys J. 1970 Nov;10(11):1057–1075. doi: 10.1016/S0006-3495(70)86352-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill B. C., Courtney K. R. Voltage-sensitive dyes. Discerning contraction and electrical signals in myocardium. Biophys J. 1982 Dec;40(3):255–257. doi: 10.1016/S0006-3495(82)84481-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota A., Fujii S., Sakai T., Kamino K. Temperature dependence of spontaneous electrical activity in early embryonic heart monitored optically with a potential-sensitive dye. Jpn J Physiol. 1983;33(1):85–100. doi: 10.2170/jjphysiol.33.85. [DOI] [PubMed] [Google Scholar]
- Hirota A., Kamino K., Komuro H., Sakai T., Yada T. Optical studies of excitation-contraction coupling in the early embryonic chick heart. J Physiol. 1985 Sep;366:89–106. doi: 10.1113/jphysiol.1985.sp015786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota A., Sakai T., Fujii S., Kamino K. Initial development of conduction pattern of spontaneous action potential in early embryonic precontractile chick heart. Dev Biol. 1983 Oct;99(2):517–523. doi: 10.1016/0012-1606(83)90301-9. [DOI] [PubMed] [Google Scholar]
- Kamino K., Hirota A., Fujii S. Localization of pacemaking activity in early embryonic heart monitored using voltage-sensitive dye. Nature. 1981 Apr 16;290(5807):595–597. doi: 10.1038/290595a0. [DOI] [PubMed] [Google Scholar]
- Komuro H., Hirota A., Yada T., Sakai T., Fujii S., Kamino K. Effects of calcium on electrical propagation in early embryonic precontractile heart as revealed by multiple-site optical recording of action potentials. J Gen Physiol. 1985 Mar;85(3):365–382. doi: 10.1085/jgp.85.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manasek F. J. Embryonic development of the heart. I. A light and electron microscopic study of myocardial development in the early chick embryo. J Morphol. 1968 Jul;125(3):329–365. doi: 10.1002/jmor.1051250306. [DOI] [PubMed] [Google Scholar]
- McNutt N. S. Ultrastructure of intercellular junctions in adult and developing cardiac muscle. Am J Cardiol. 1970 Feb;25(2):169–183. doi: 10.1016/0002-9149(70)90577-1. [DOI] [PubMed] [Google Scholar]
- Ross W. N., Salzberg B. M., Cohen L. B., Grinvald A., Davila H. V., Waggoner A. S., Wang C. H. Changes in absorption, fluorescence, dichroism, and Birefringence in stained giant axons: : optical measurement of membrane potential. J Membr Biol. 1977 May 6;33(1-2):141–183. doi: 10.1007/BF01869514. [DOI] [PubMed] [Google Scholar]
- Sakai T., Fujii S., Hirota A., Kamino K. Optical evidence for calcium-action potentials in early embryonic precontractile chick heart using a potential-sensitive dye. J Membr Biol. 1983;72(3):205–212. doi: 10.1007/BF01870587. [DOI] [PubMed] [Google Scholar]
- Sakai T., Hirota A., Fujii S., Kamino K. Flexibility of regional pacemaking priority in early embryonic heart monitored by simultaneous optical recording of action potentials from multiple sites. Jpn J Physiol. 1983;33(3):337–350. doi: 10.2170/jjphysiol.33.337. [DOI] [PubMed] [Google Scholar]
- Salzberg B. M., Grinvald A., Cohen L. B., Davila H. V., Ross W. N. Optical recording of neuronal activity in an invertebrate central nervous system: simultaneous monitoring of several neurons. J Neurophysiol. 1977 Nov;40(6):1281–1291. doi: 10.1152/jn.1977.40.6.1281. [DOI] [PubMed] [Google Scholar]
- Salzberg B. M., Obaid A. L., Senseman D. M., Gainer H. Optical recording of action potentials from vertebrate nerve terminals using potentiometric probes provides evidence for sodium and calcium components. Nature. 1983 Nov 3;306(5938):36–40. doi: 10.1038/306036a0. [DOI] [PubMed] [Google Scholar]
- Senseman D. M., Salzberg B. M. Electrical activity in an exocrine gland: optical recording with a potentiometric dye. Science. 1980 Jun 13;208(4449):1269–1271. doi: 10.1126/science.7375937. [DOI] [PubMed] [Google Scholar]