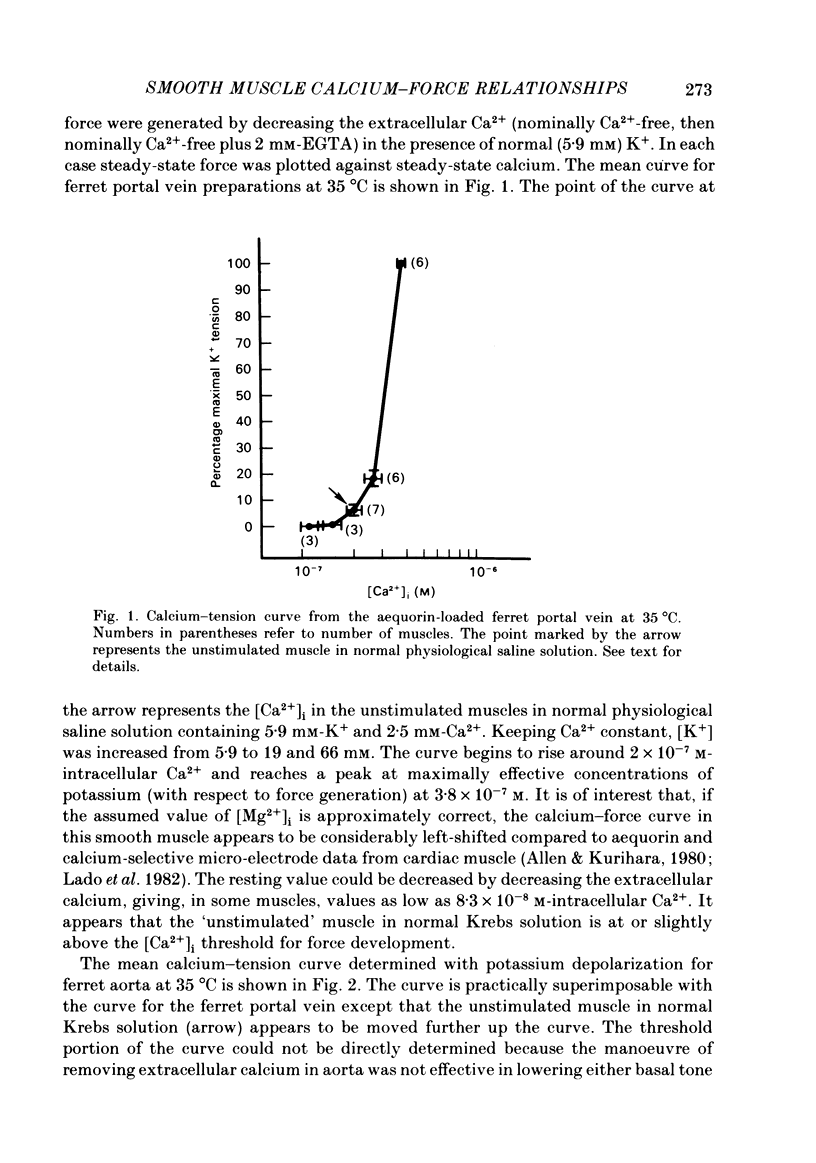
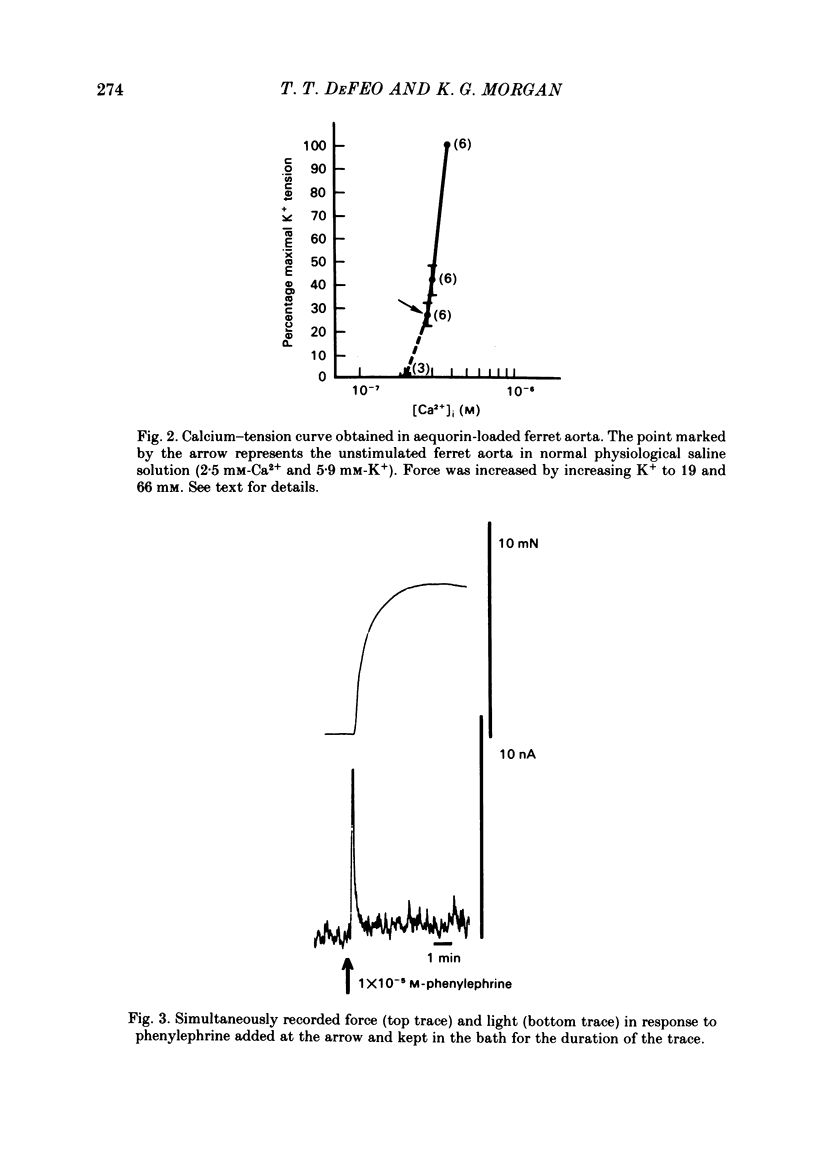
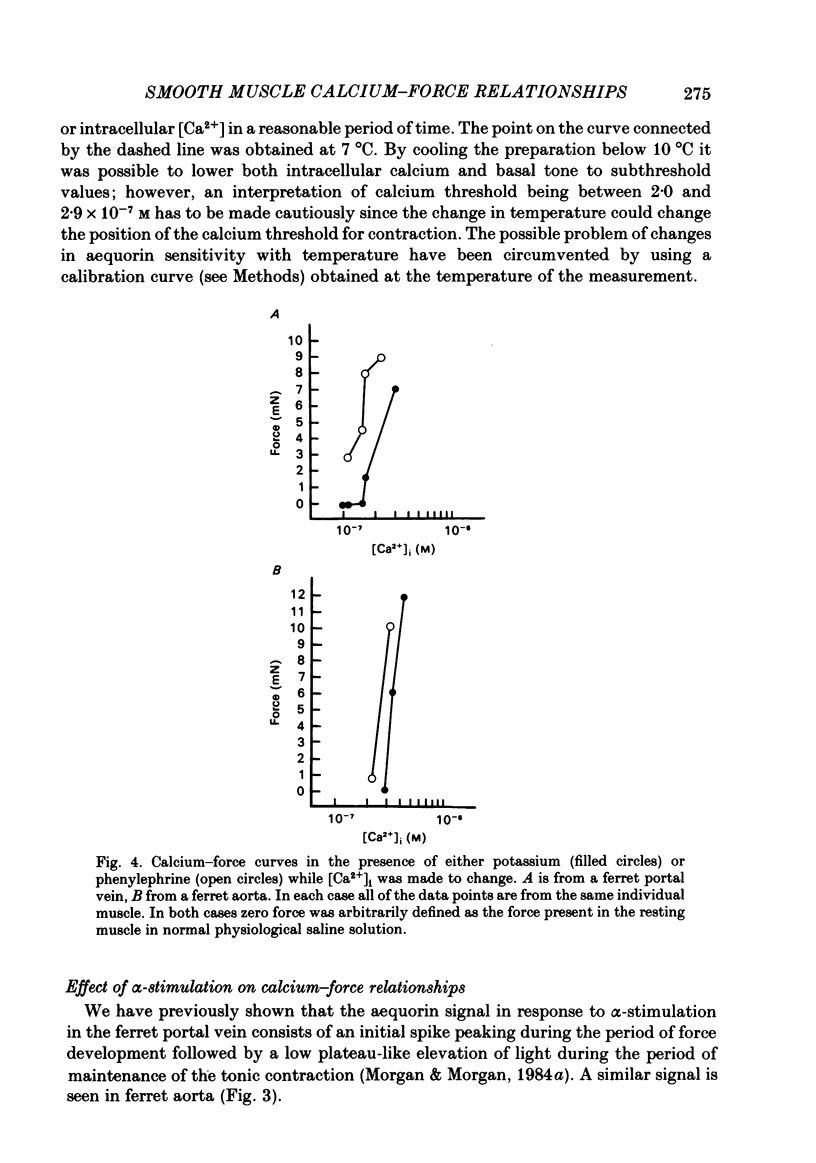
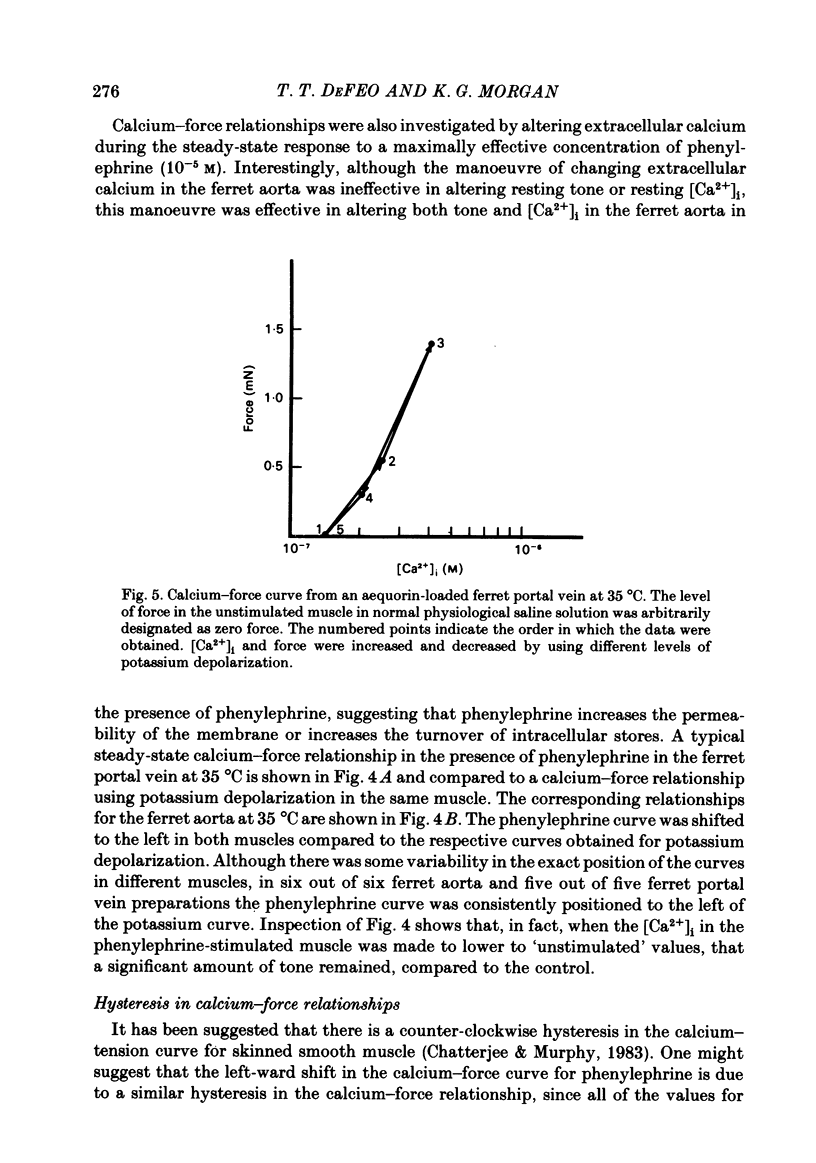
Abstract
The bioluminescent calcium indicator aequorin was chemically loaded into isolated strips of ferret portal vein and ferret aorta. Aequorin light emission (a function of [Ca2+]i) was recorded simultaneously with tension. Assuming an [Mg2+]i of 0.5 mM, [Ca2+]i was 1.8 X 10(-7) M in the unstimulated portal vein at 22 degrees C where there was negligible resting tone. In contrast, in the unstimulated aorta at 22 degrees C where there was significant basal tone, the [Ca2+]i was 2.7 X 10(-7) M. In both portal vein and aorta, potassium depolarization caused a monophasic rise in intracellular Ca2+ in parallel with the rise in tension, whereas phenylephrine caused an initial spike of light during the period of the force development which then fell to a much lower plateau level during the period of force maintenance. Calcium-force curves were generated by plotting calibrated aequorin light against force while intracellular [Ca2+] was made to change either by increasing degrees of potassium depolarization or decreasing extracellular [Ca2+]. The steady-state calcium-force curve in the presence of phenylephrine was shifted to the left of the curve in the presence of potassium depolarization in both the portal vein and aorta. In the aorta there was a counter-clockwise hysteresis in the calcium-force relationship. In contrast, in the portal vein there was no demonstrable hysteresis, indicating that the apparent change in calcium sensitivity of the contractile apparatus in the presence of phenylephrine must be caused by a second messenger other than calcium.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Kurihara S. Calcium transients in mammalian ventricular muscle. Eur Heart J. 1980;Suppl A:5–15. doi: 10.1093/eurheartj/1.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- Allen D. G., Orchard C. H. The effects of changes of pH on intracellular calcium transients in mammalian cardiac muscle. J Physiol. 1983 Feb;335:555–567. doi: 10.1113/jphysiol.1983.sp014550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Chatterjee M., Murphy R. A. Calcium-dependent stress maintenance without myosin phosphorylation in skinned smooth muscle. Science. 1983 Jul 29;221(4609):464–466. doi: 10.1126/science.6867722. [DOI] [PubMed] [Google Scholar]
- Cobbold P. H., Bourne P. K. Aequorin measurements of free calcium in single heart cells. 1984 Nov 29-Dec 5Nature. 312(5993):444–446. doi: 10.1038/312444a0. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Rapid ionic modifications during the aequorin-detected calcium transient in a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):189–246. doi: 10.1085/jgp.85.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P., Metzger P., Weingart R. Free magnesium in sheep, ferret and frog striated muscle at rest measured with ion-selective micro-electrodes. J Physiol. 1982 Dec;333:173–188. doi: 10.1113/jphysiol.1982.sp014447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kuriyama H., Ueno H. Mechanisms of the nitroglycerine-induced vasodilation in vascular smooth muscles of the rabbit and pig. J Physiol. 1983 Oct;343:233–252. doi: 10.1113/jphysiol.1983.sp014890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. C., Ware J. A., Cliveden P. B., Smith M., Dvorak A. M., Salzman E. W. Measurement of ionized calcium in blood platelets with the photoprotein aequorin. Comparison with Quin 2. J Biol Chem. 1985 Feb 25;260(4):2069–2076. [PubMed] [Google Scholar]
- Lado M. G., Sheu S. S., Fozzard H. A. Changes in intracellular Ca2+ activity with stimulation in sheep cardiac Purkinje strands. Am J Physiol. 1982 Jul;243(1):H133–H137. doi: 10.1152/ajpheart.1982.243.1.H133. [DOI] [PubMed] [Google Scholar]
- Lee C. O., Dagostino M. Effect of strophanthidin on intracellular Na ion activity and twitch tension of constantly driven canine cardiac Purkinje fibers. Biophys J. 1982 Dec;40(3):185–198. doi: 10.1016/S0006-3495(82)84474-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López J. R., Alamo L., Caputo C., DiPolo R., Vergara S. Determination of ionic calcium in frog skeletal muscle fibers. Biophys J. 1983 Jul;43(1):1–4. doi: 10.1016/S0006-3495(83)84316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. P., Morgan K. G. Alteration of cytoplasmic ionized calcium levels in smooth muscle by vasodilators in the ferret. J Physiol. 1984 Dec;357:539–551. doi: 10.1113/jphysiol.1984.sp015516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. P., Morgan K. G. Stimulus-specific patterns of intracellular calcium levels in smooth muscle of ferret portal vein. J Physiol. 1984 Jun;351:155–167. doi: 10.1113/jphysiol.1984.sp015239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. P., Morgan K. G. Vascular smooth muscle: the first recorded Ca2+ transients. Pflugers Arch. 1982 Oct;395(1):75–77. doi: 10.1007/BF00584972. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Peterson J. W., 3rd Rate-limiting steps in the tension development of freeze-glycerinated vascular smooth muscle. J Gen Physiol. 1982 Mar;79(3):437–452. doi: 10.1085/jgp.79.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H., Barrett P. Q. Calcium messenger system: an integrated view. Physiol Rev. 1984 Jul;64(3):938–984. doi: 10.1152/physrev.1984.64.3.938. [DOI] [PubMed] [Google Scholar]
- Rüegg J. C., Paul R. J. Vascular smooth muscle. Calmodulin and cyclic AMP-dependent protein kinase after calcium sensitivity in porcine carotid skinned fibers. Circ Res. 1982 Mar;50(3):394–399. doi: 10.1161/01.res.50.3.394. [DOI] [PubMed] [Google Scholar]
- Saida K., Nonomura Y. Characteristics of Ca2+- and Mg2+-induced tension development in chemically skinned smooth muscle fibers. J Gen Physiol. 1978 Jul;72(1):1–14. doi: 10.1085/jgp.72.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobieszek A. Ca-linked phosphorylation of a light chain of vertebrate smooth-muscle myosin. Eur J Biochem. 1977 Mar 1;73(2):477–483. doi: 10.1111/j.1432-1033.1977.tb11340.x. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V., Shuman H., Endo M. Calcium and monovalent ions in smooth muscle. Fed Proc. 1982 Oct;41(12):2883–2890. [PubMed] [Google Scholar]
- Sparrow M. P., Mrwa U., Hofmann F., Rüegg J. C. Calmodulin is essential for smooth muscle contraction. FEBS Lett. 1981 Mar 23;125(2):141–145. doi: 10.1016/0014-5793(81)80704-1. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J. Neutral carrier ion-selective microelectrodes for measurement of intracellular free calcium. Biochim Biophys Acta. 1980 Jul;599(2):623–638. doi: 10.1016/0005-2736(80)90205-9. [DOI] [PubMed] [Google Scholar]
- Weingart R., Hess P. Free calcium in sheep cardiac tissue and frog skeletal muscle measured with Ca2+-selective microelectrodes. Pflugers Arch. 1984 Sep;402(1):1–9. doi: 10.1007/BF00584824. [DOI] [PubMed] [Google Scholar]
- Wier W. G., Hess P. Excitation-contraction coupling in cardiac Purkinje fibers. Effects of cardiotonic steroids on the intracellular [Ca2+] transient, membrane potential, and contraction. J Gen Physiol. 1984 Mar;83(3):395–415. doi: 10.1085/jgp.83.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]


