Abstract
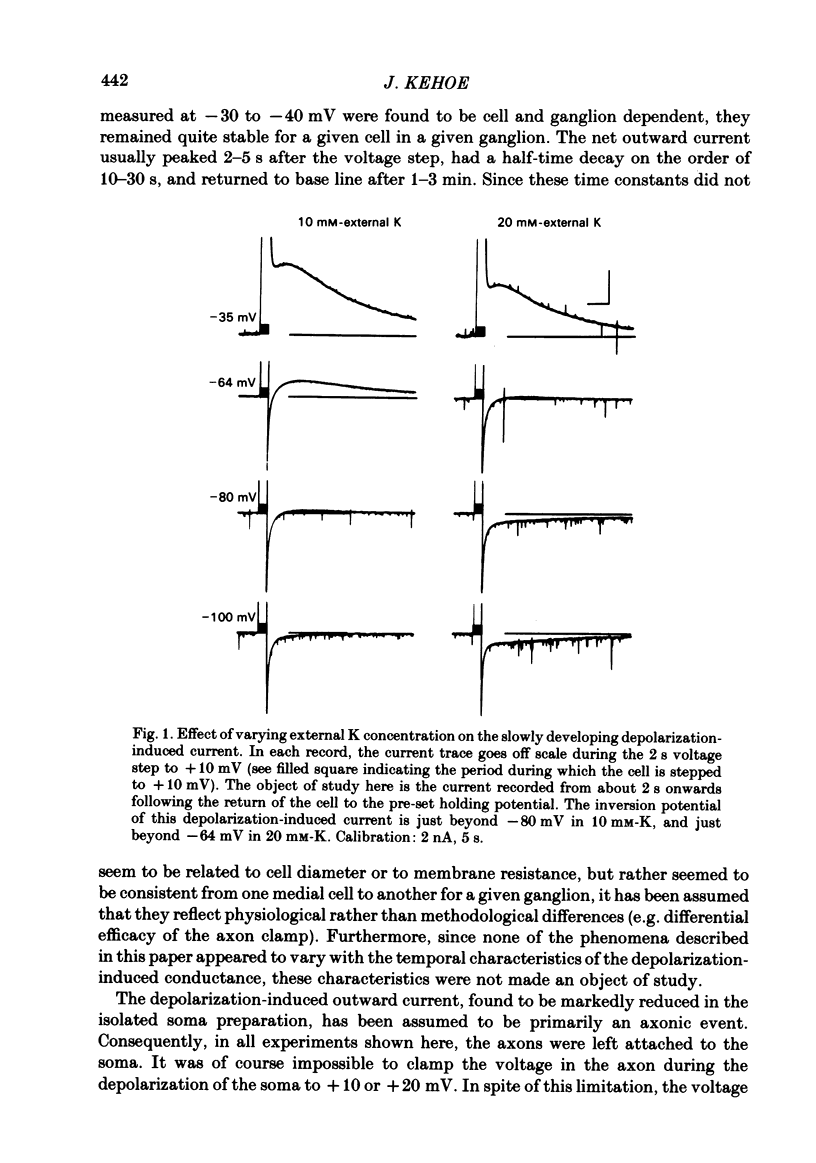
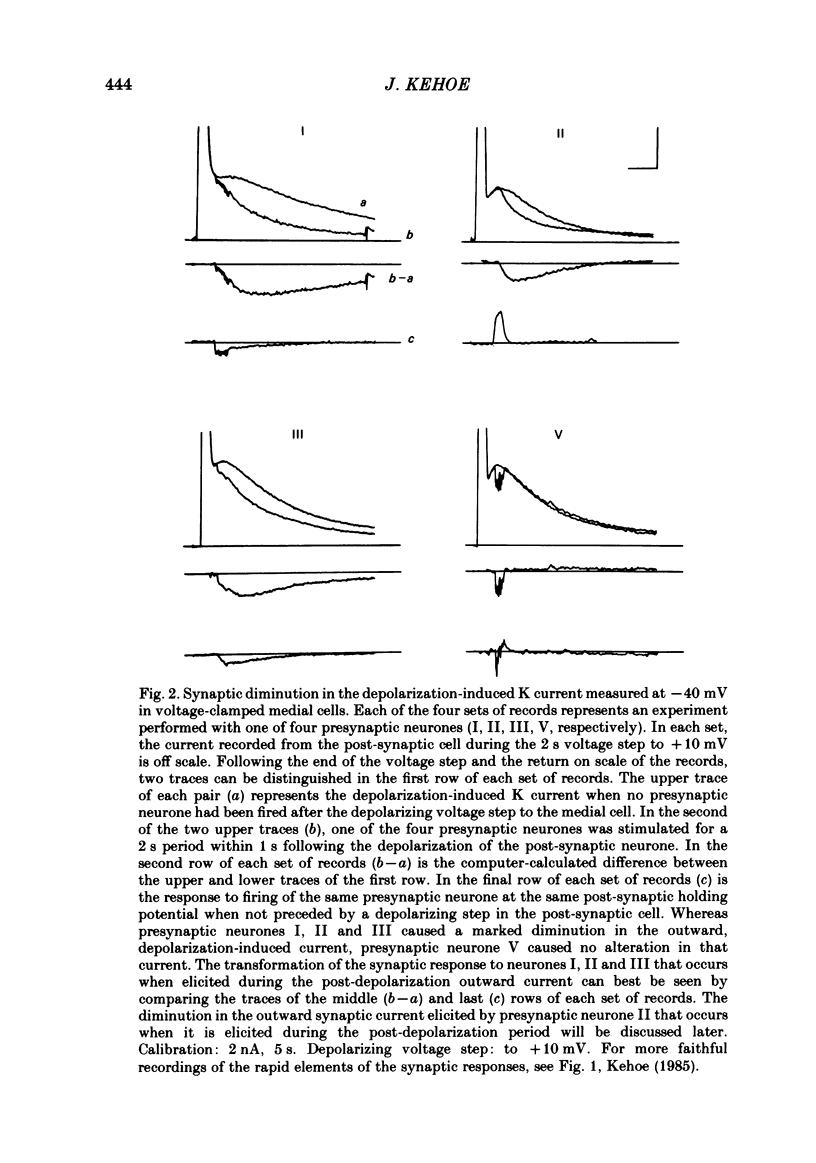
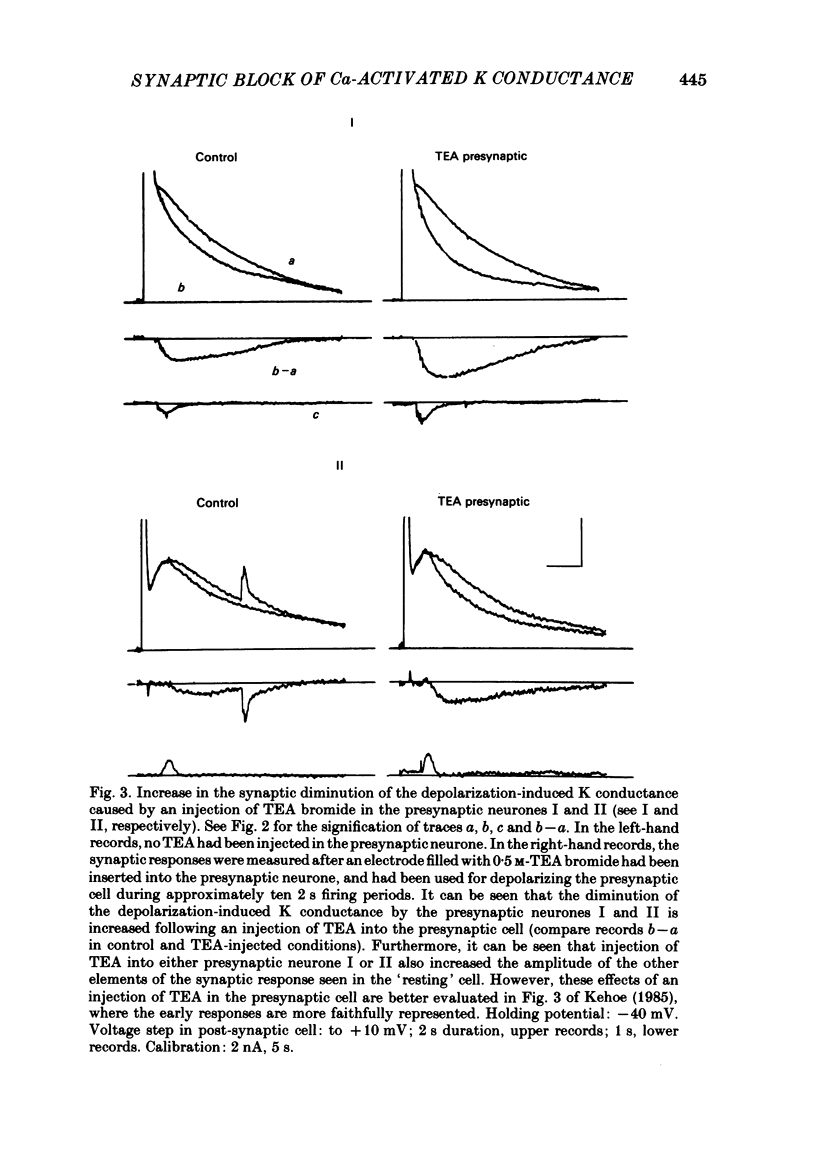
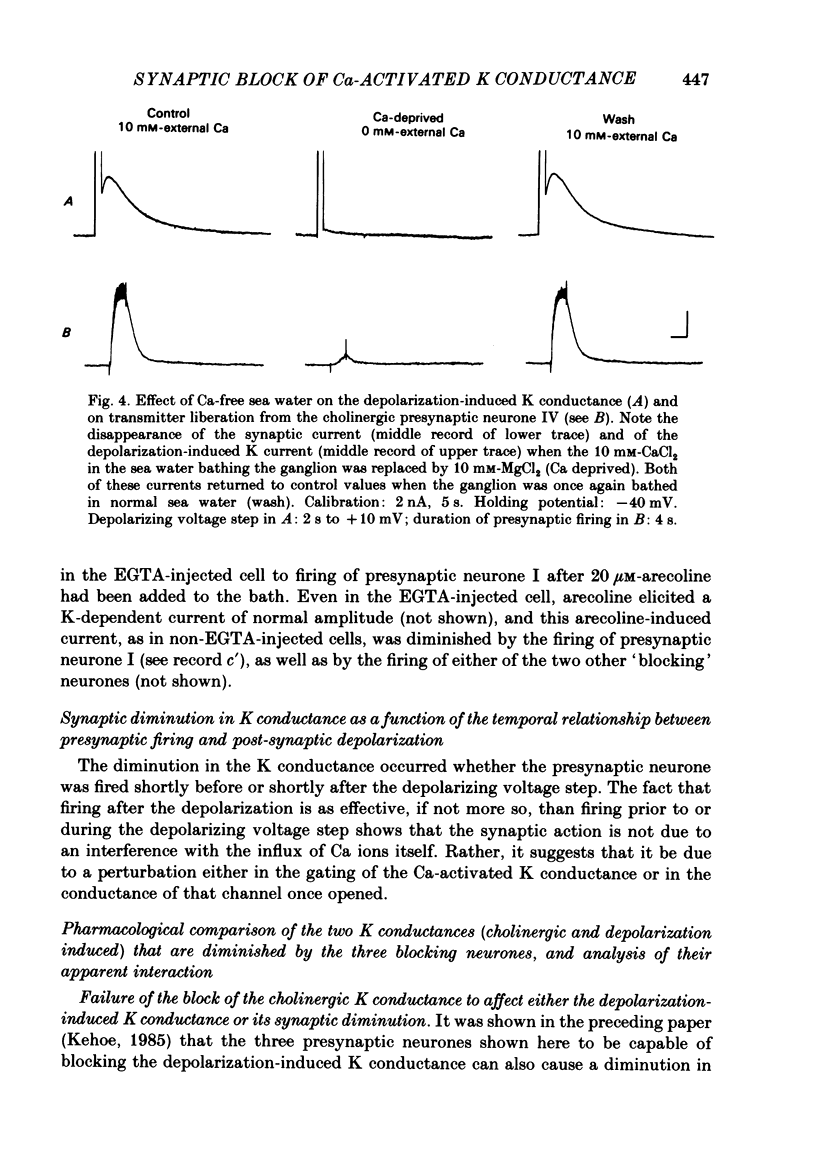
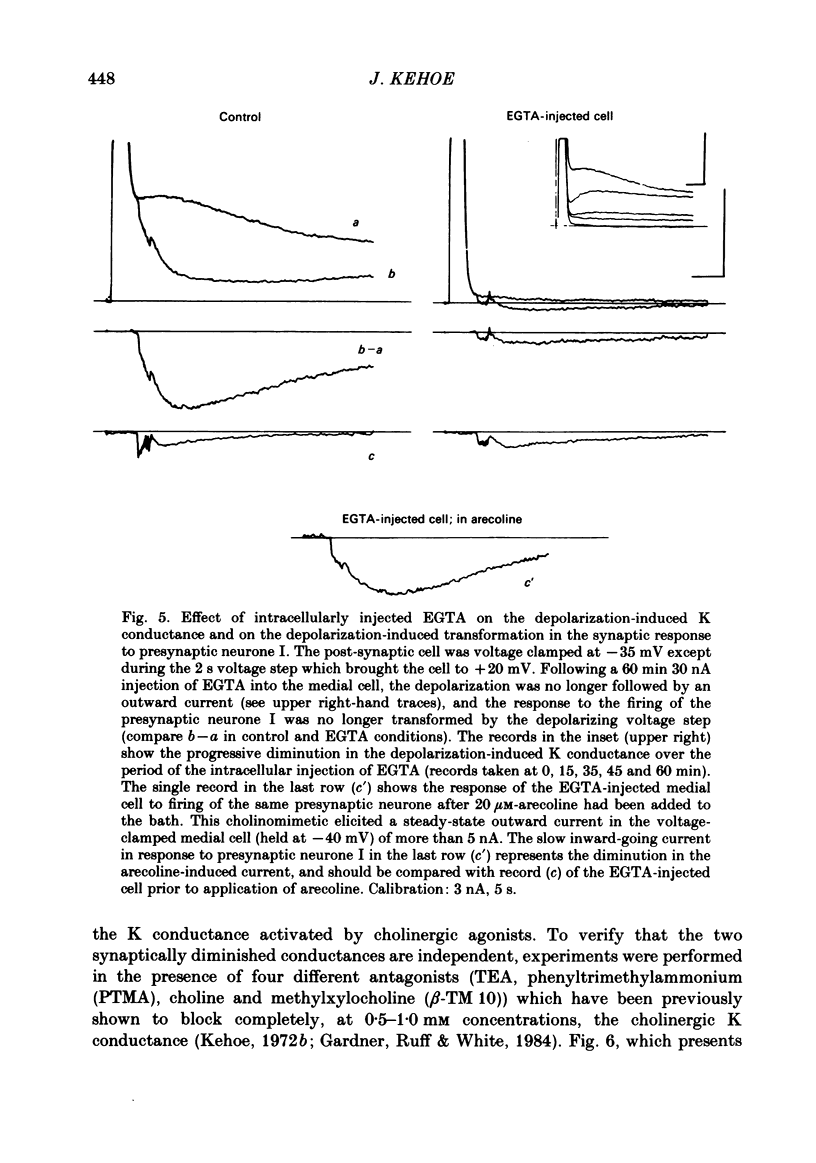
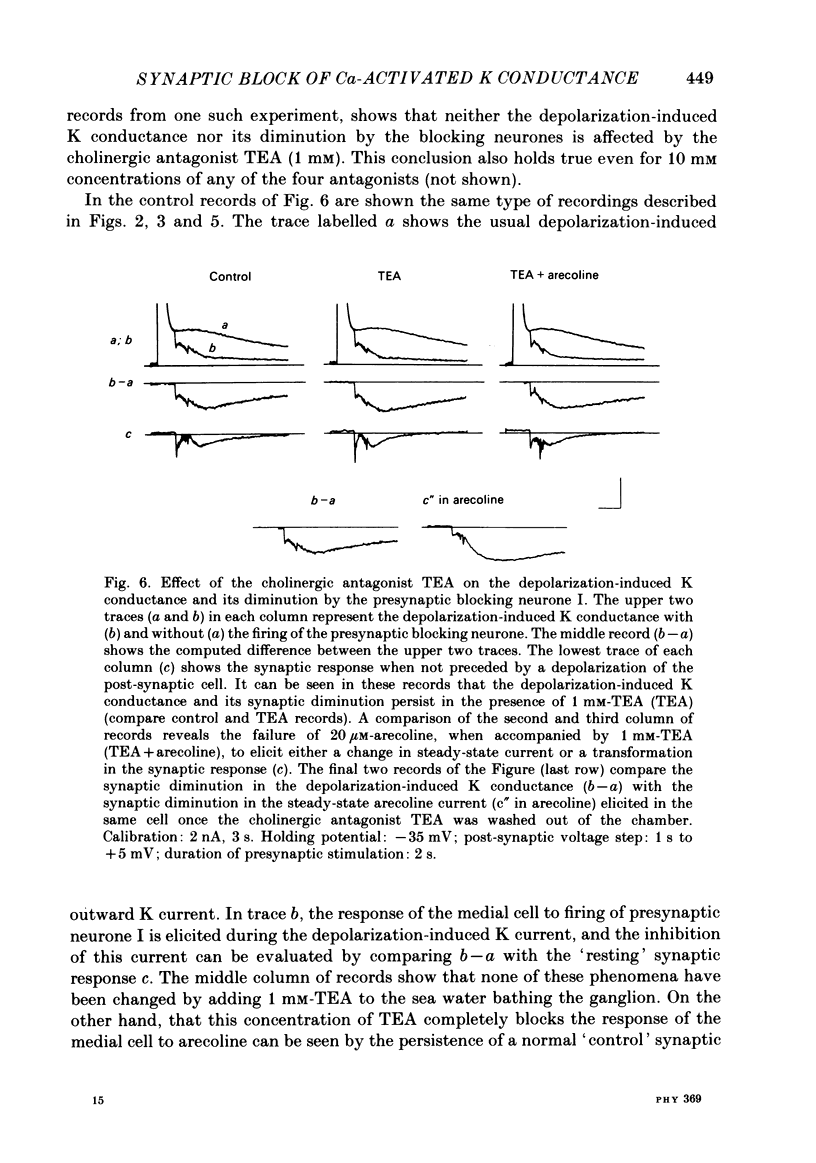
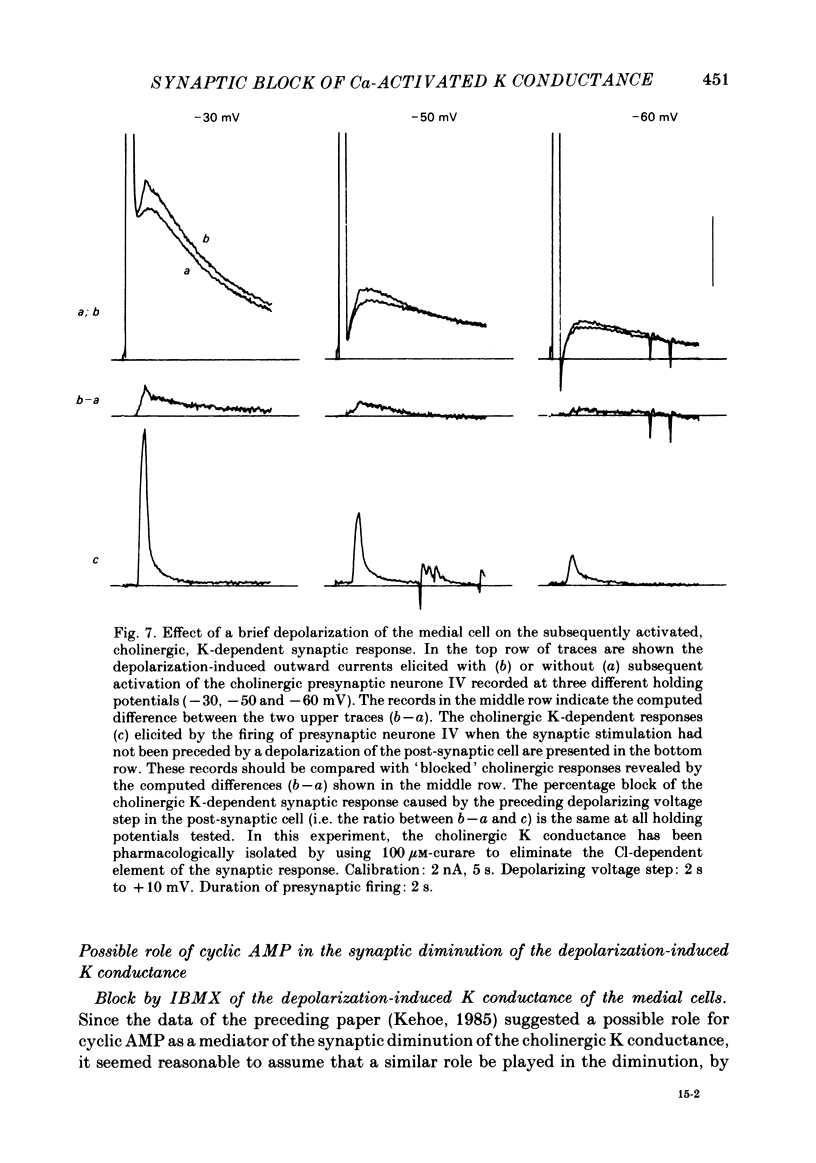
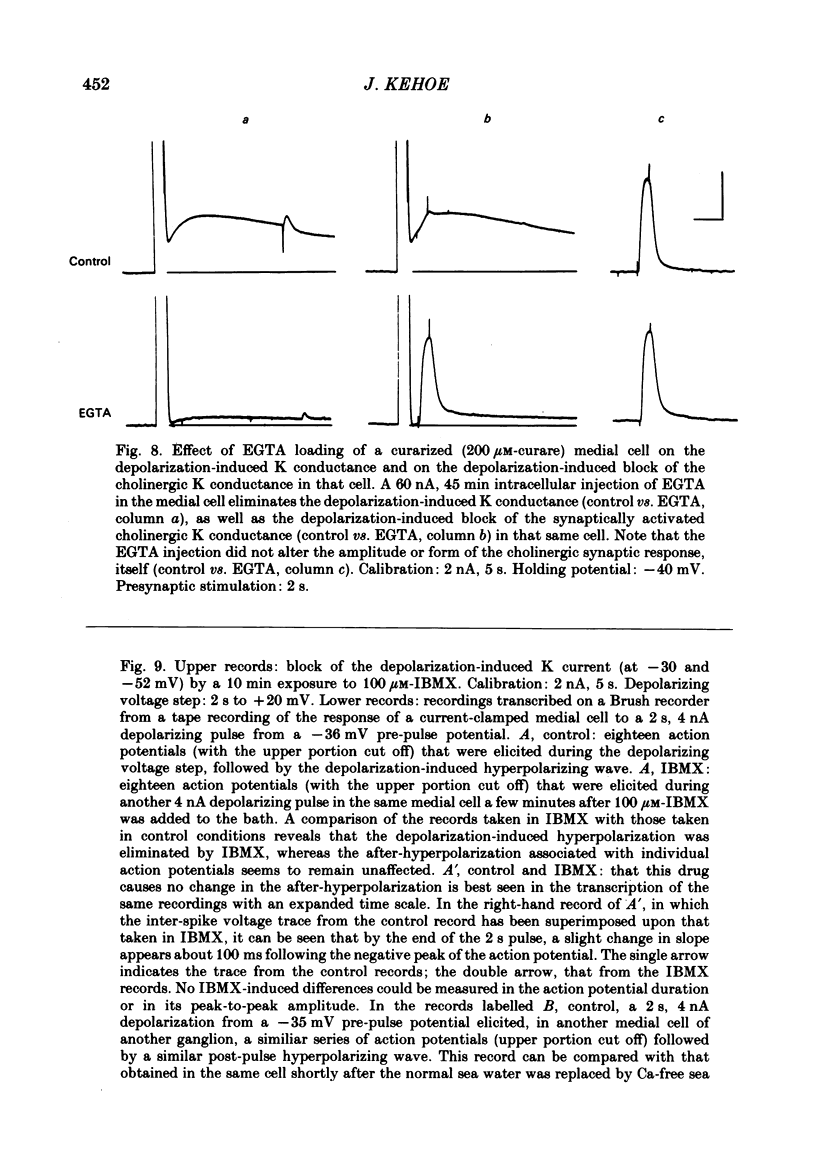
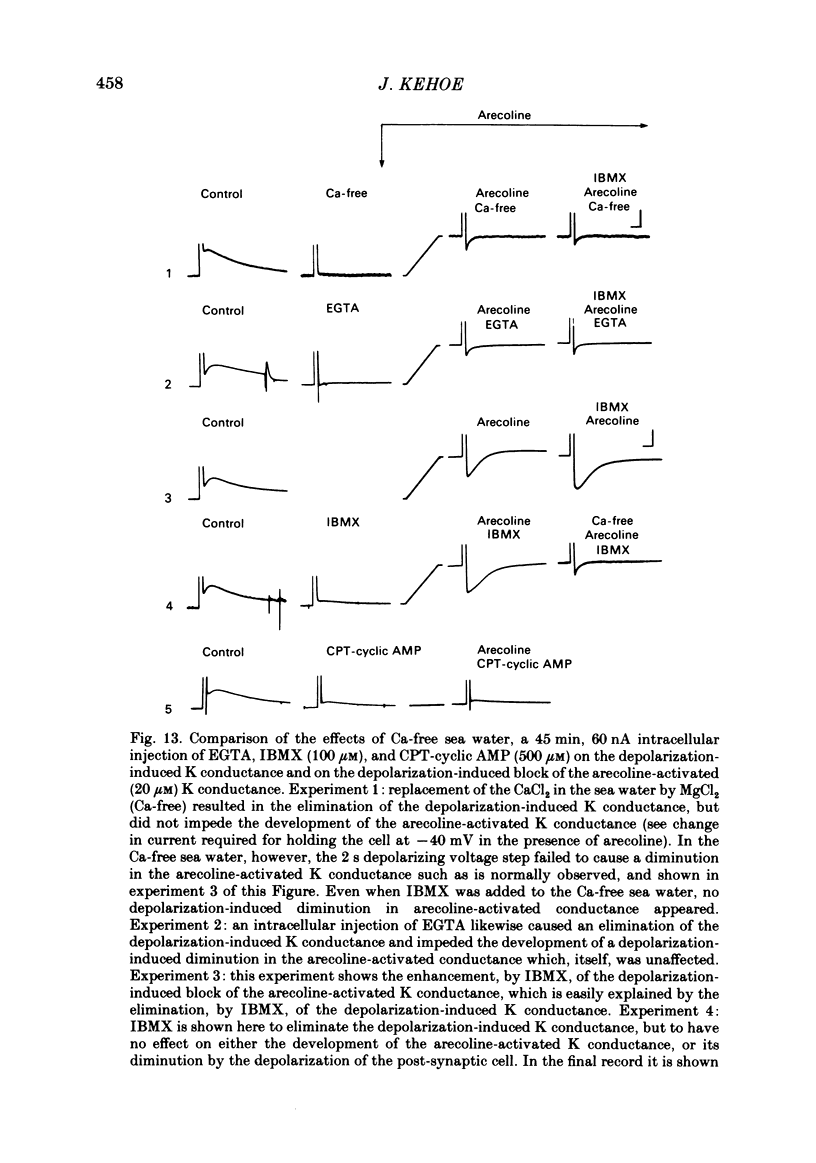
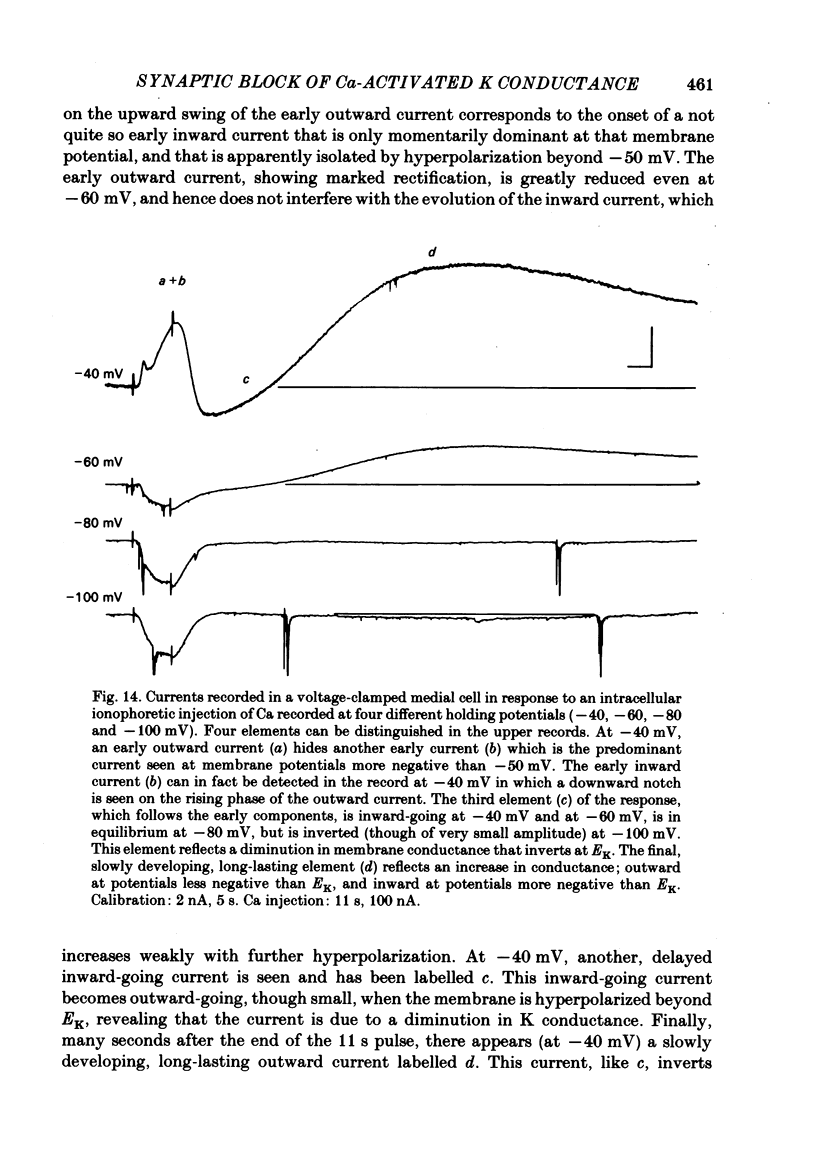
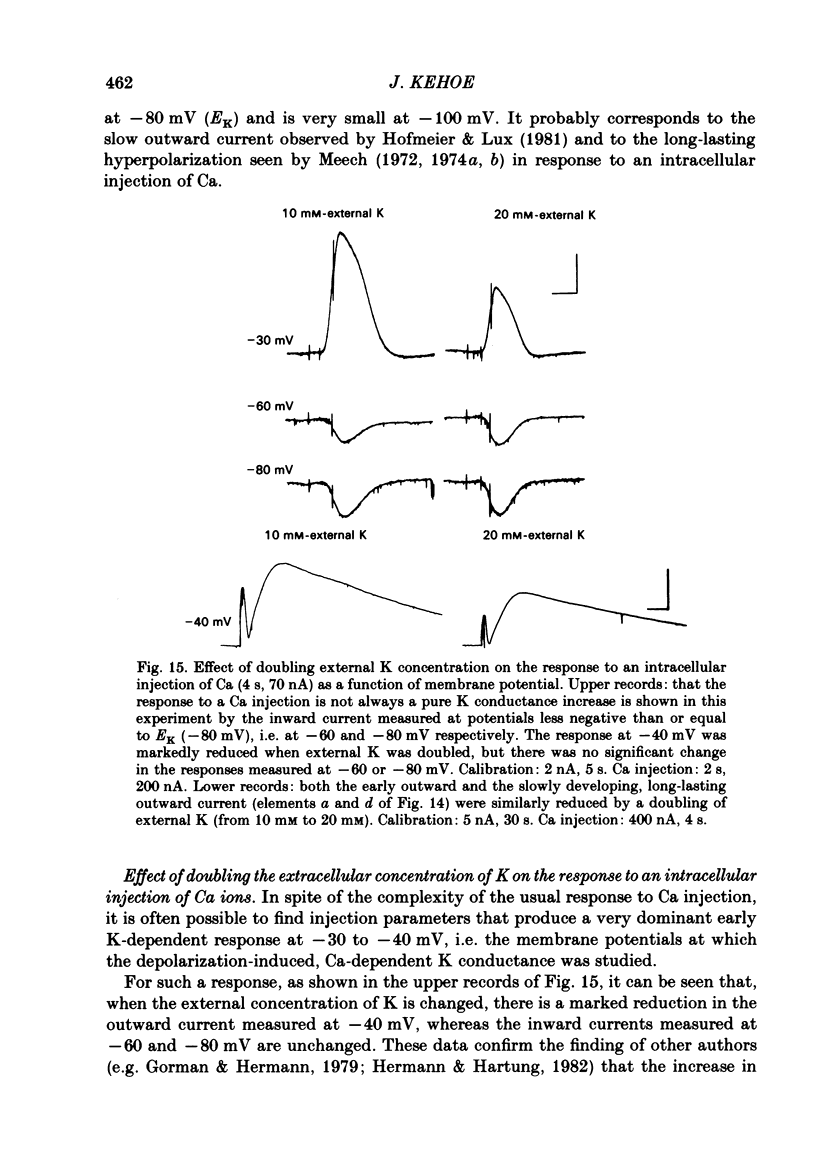
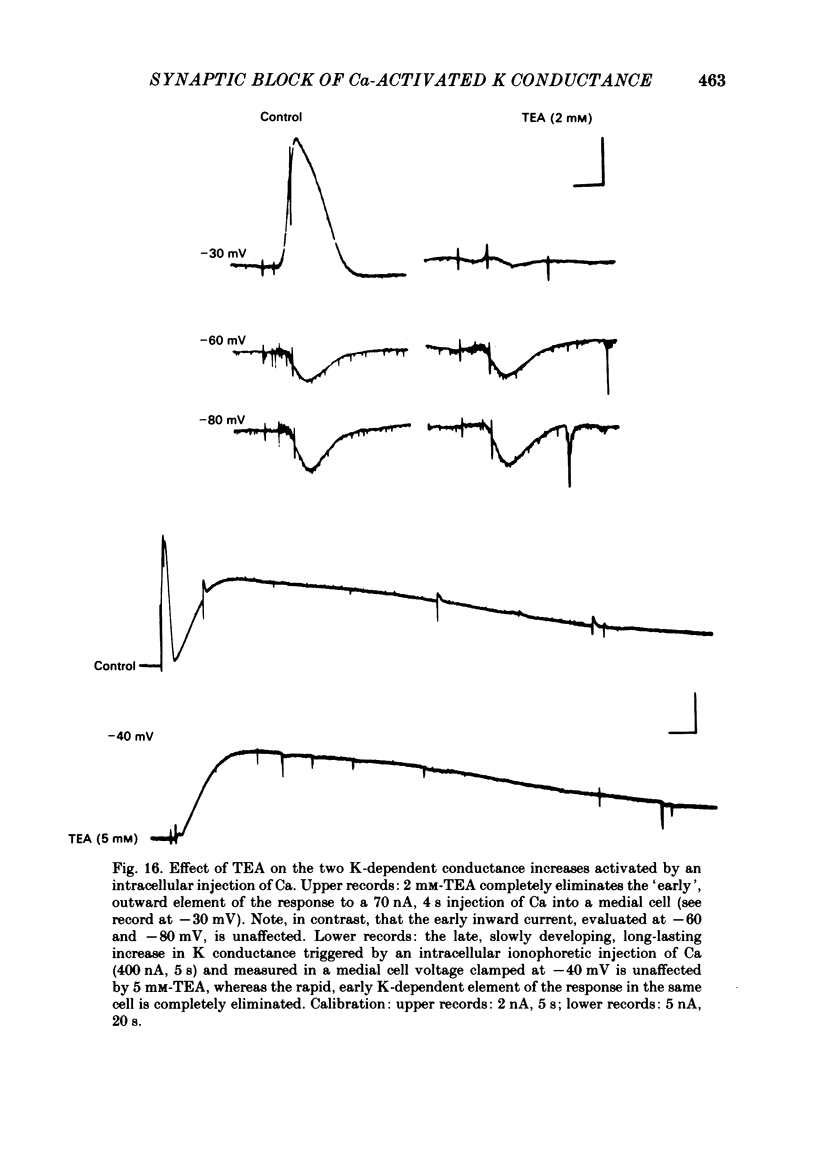
In the preceding paper (Kehoe, 1985) it was shown that the firing of any one of three neurones (I, II, III) presynaptic to the medial cells of the pleural ganglion of Aplysia californica causes a diminution of the cholinergically controlled K conductance in those cells. Firing of the same three presynaptic neurones was shown here to cause a similar diminution in a depolarization-induced K-dependent conductance in the same post-synaptic cells. The depolarization-induced K conductance was found to disappear when Ca ions were removed from the sea water bathing the ganglion or when the cell was injected with the Ca chelator ethyleneglycol-bis-(beta-aminoethylether)N,N'-tetra-acetic acid (EGTA). The diminution in this Ca-activated, K-dependent current occurred even when the presynaptic neurone was fired a few seconds after the end of the depolarizing voltage step to the post-synaptic neurone, showing that the diminution in K conductance was not an indirect effect of a transmitter-induced diminution in Ca influx during the depolarizing pulse. The two K conductances affected by the 'blocking neurones' could be selectively eliminated. The cholinergic conductance could be blocked by receptor-specific cholinergic antagonists (e.g. 1 mM concentrations of phenyltrimethylammonium (PTMA), choline and tetraethylammonium (TEA]. Even at 10 mM concentrations, none of these compounds (including TEA, which is known to block certain Ca-activated K conductances) had an effect on the depolarization-induced, Ca-activated K conductance studied here. This latter conductance, on the other hand, was selectively blocked by an intracellular injection of EGTA. The three blocking neurones continued to diminish the K conductance (cholinergic or depolarization induced) that remained intact under these different experimental conditions. The depolarization-induced influx of Ca was shown to block the cholinergically controlled K conductance, but Ca was excluded as the possible mediator of the diminution in K conductance caused by the three blocking neurones. An intracellular injection of Ca ions into the medial cells was shown to activate a variety of changes in membrane conductance; in particular, two K-conductance increases: an early, TEA-sensitive one, and a slowly developing, TEA-insensitive one. Both the permeant cyclic AMP analogue p-chlorophenylthioadenosine 3',5'-monophosphate (CPT-cyclic AMP) and the phosphodiesterase inhibitors amino-phylline and isobutyl-1-methylxanthine (IBMX) were shown to block the depolarization-induced K conductance, and to reduce, though not eliminate, the slowly developing K conductance activated by an intracellular injection of Ca.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF








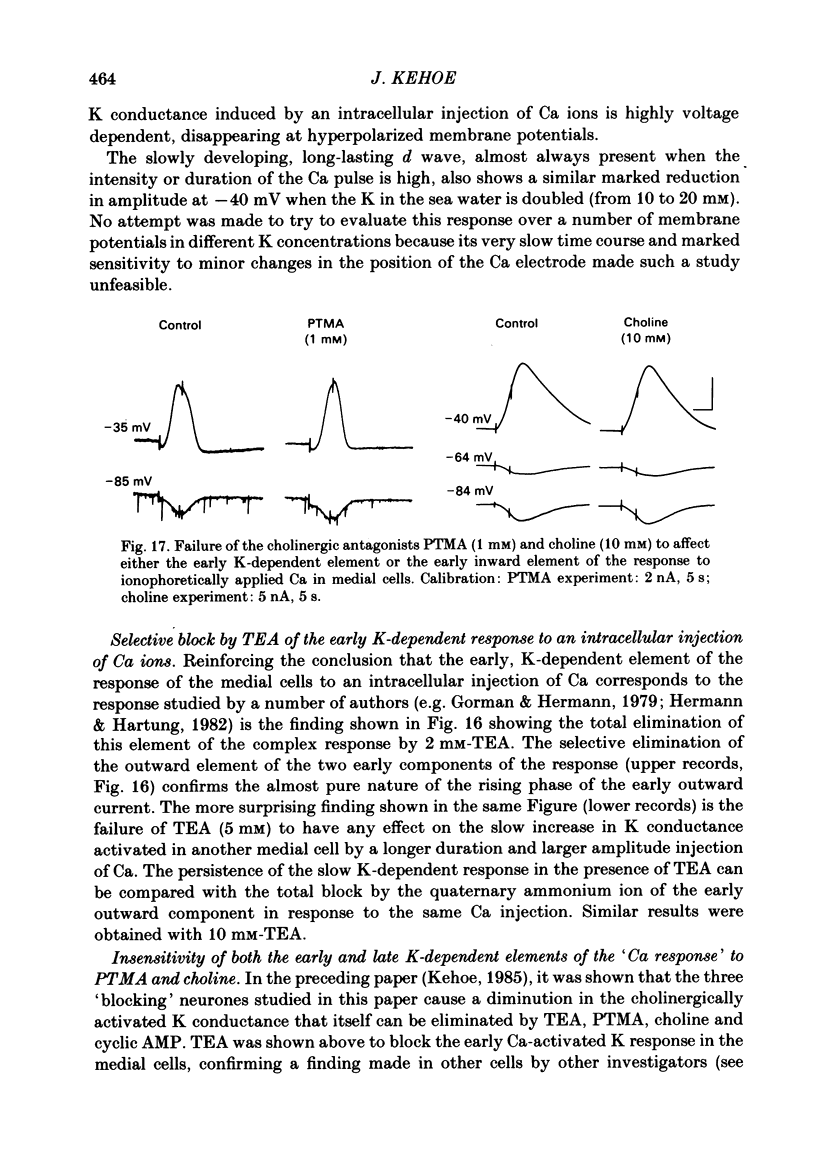
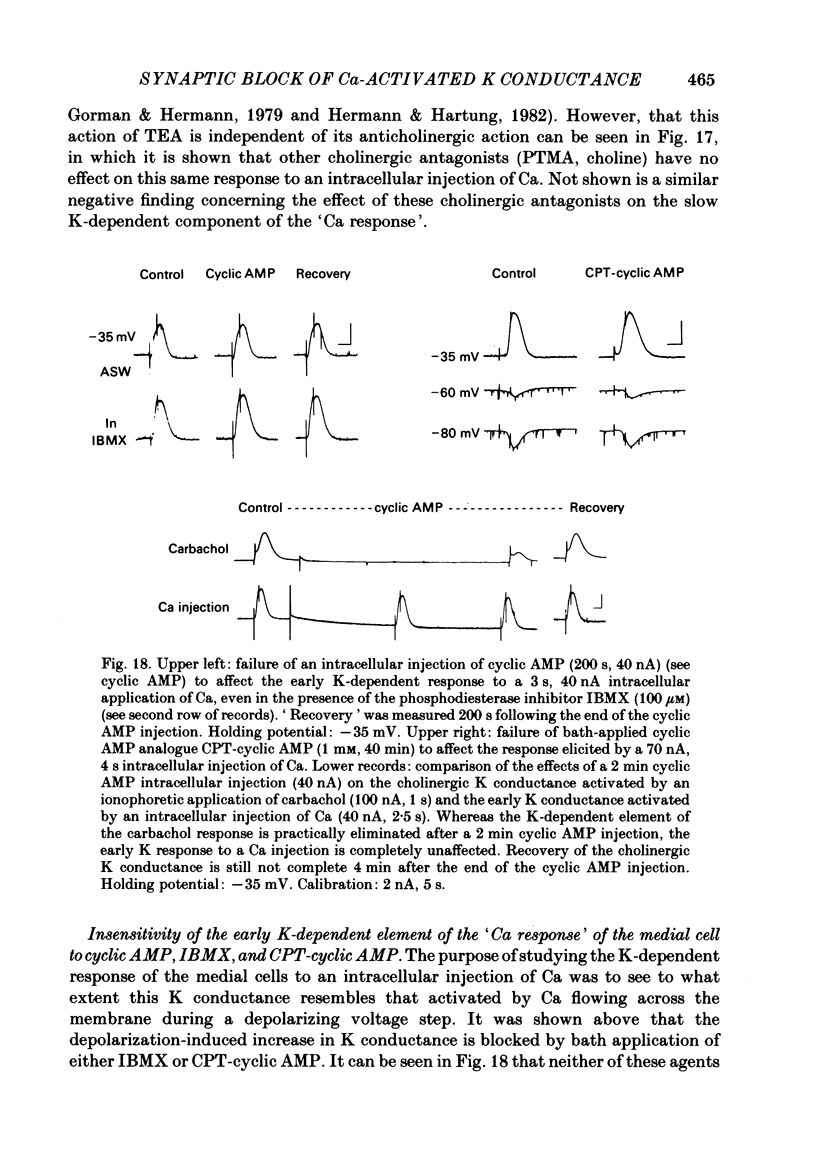





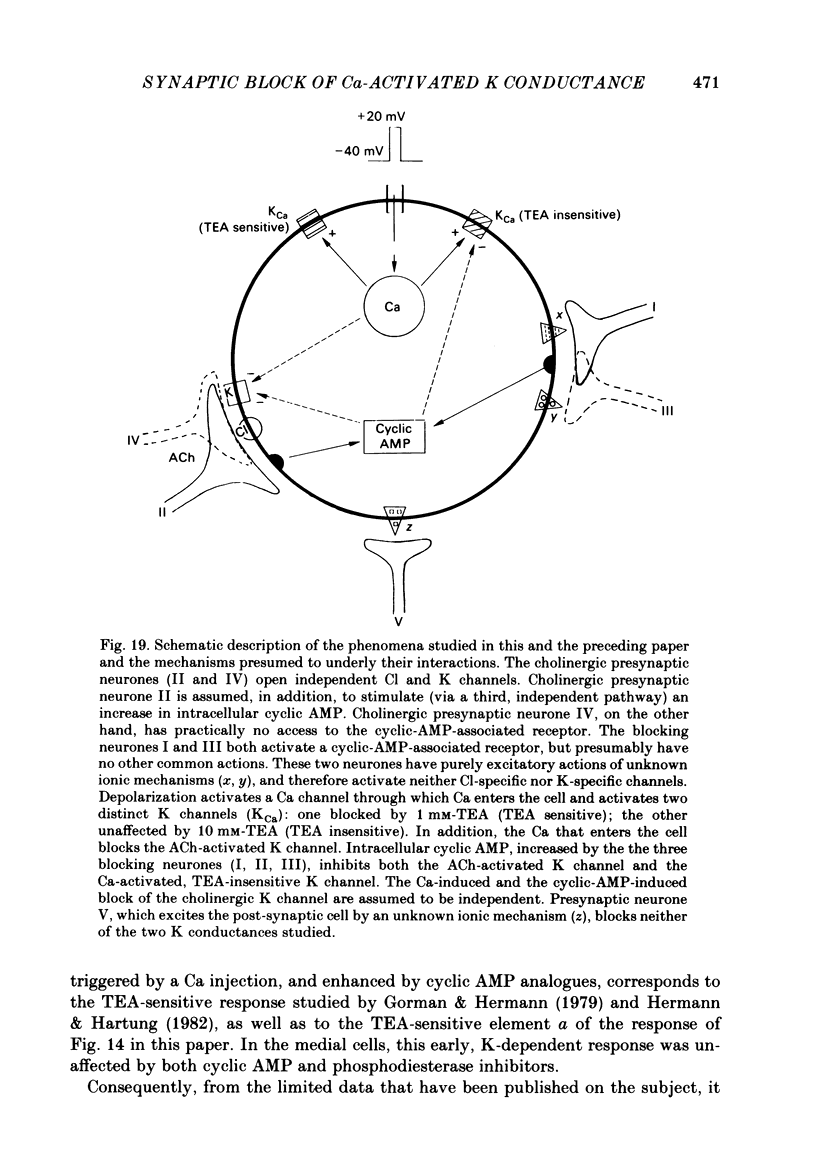



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A., Constanti A. M-currents and other potassium currents in bullfrog sympathetic neurones. J Physiol. 1982 Sep;330:537–572. doi: 10.1113/jphysiol.1982.sp014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldenhoff J. B., Gruol D. L., Rivier J., Vale W., Siggins G. R. Corticotropin releasing factor decreases postburst hyperpolarizations and excites hippocampal neurons. Science. 1983 Aug 26;221(4613):875–877. doi: 10.1126/science.6603658. [DOI] [PubMed] [Google Scholar]
- Aldrich R. W., Jr, Getting P. A., Thompson S. H. Inactivation of delayed outward current in molluscan neurone somata. J Physiol. 1979 Jun;291:507–530. doi: 10.1113/jphysiol.1979.sp012828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Chesnoy-Marchais D. Interactions between three slow potassium responses controlled by three distinct receptors in Aplysia neurones. J Physiol. 1982 Mar;324:67–92. doi: 10.1113/jphysiol.1982.sp014101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barish M. E., Thompson S. H. Calcium buffering and slow recovery kinetics of calcium-dependent outward current in molluscan neurones. J Physiol. 1983 Apr;337:201–219. doi: 10.1113/jphysiol.1983.sp014620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Barret J. N. Separation of two voltage-sensitive potassium currents, and demonstration of a tetrodotoxin-resistant calcium current in frog motoneurones. J Physiol. 1976 Mar;255(3):737–774. doi: 10.1113/jphysiol.1976.sp011306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benardo L. S., Prince D. A. Ionic mechanisms of cholinergic excitation in mammalian hippocampal pyramidal cells. Brain Res. 1982 Oct 14;249(2):333–344. doi: 10.1016/0006-8993(82)90067-1. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Selyanko A. A. Two components of muscarine-sensitive membrane current in rat sympathetic neurones. J Physiol. 1985 Jan;358:335–363. doi: 10.1113/jphysiol.1985.sp015554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci V. F., Kandel E. R., Schwartz J. H., Wilson F. D., Nairn A. C., Greengard P. Intracellular injection of t he catalytic subunit of cyclic AMP-dependent protein kinase simulates facilitation of transmitter release underlying behavioral sensitization in Aplysia. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7492–7496. doi: 10.1073/pnas.77.12.7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnoy-Marchais D., Ascher P. Effects of various cations on the slow K+ conductance increases induced by carbachol, histamine and dopamine in Aplysia neurones. Brain Res. 1983 Jan 17;259(1):57–67. doi: 10.1016/0006-8993(83)91066-1. [DOI] [PubMed] [Google Scholar]
- Cole A. E., Nicoll R. A. Acetylcholine mediates a slow synaptic potential in hippocampal pyramidal cells. Science. 1983 Sep 23;221(4617):1299–1301. doi: 10.1126/science.6612345. [DOI] [PubMed] [Google Scholar]
- Cottrell G. A., Davies N. W., Green K. A. Multiple actions of a molluscan cardioexcitatory neuropeptide and related peptides on identified Helix neurones. J Physiol. 1984 Nov;356:315–333. doi: 10.1113/jphysiol.1984.sp015467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer J. W., Eckert R. Two components of Ca-dependent potassium current in identified neurons of Aplysia californica. Pflugers Arch. 1985 Apr;403(4):353–359. doi: 10.1007/BF00589246. [DOI] [PubMed] [Google Scholar]
- Deterre P., Paupardin-Tritsch D., Bockaert J., Gerschenfeld H. M. Role of cyclic AMP in a serotonin-evoked slow inward current in snail neurones. Nature. 1981 Apr 30;290(5809):783–785. doi: 10.1038/290783a0. [DOI] [PubMed] [Google Scholar]
- Deterre P., Paupardin-Tritsch D., Bockaert J., Gerschenfeld H. M. cAMP-mediated decrease in K+ conductance evoked by serotonin and dopamine in the same neuron: a biochemical and physiological single-cell study. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7934–7938. doi: 10.1073/pnas.79.24.7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald D. A., Williams A., Levitan I. B. Modulation of single Ca2+-dependent K+-channel activity by protein phosphorylation. Nature. 1985 Jun 6;315(6019):503–506. doi: 10.1038/315503a0. [DOI] [PubMed] [Google Scholar]
- Ewald D., Eckert R. Cyclic AMP enhances calcium-dependent potassium current in Aplysia neurons. Cell Mol Neurobiol. 1983 Dec;3(4):345–353. doi: 10.1007/BF00734715. [DOI] [PubMed] [Google Scholar]
- Gardner D., Ruff R. L., White R. L. Choline acts as agonist and blocker for Aplysia cholinergic synapses. J Neurophysiol. 1984 Jan;51(1):1–15. doi: 10.1152/jn.1984.51.1.1. [DOI] [PubMed] [Google Scholar]
- Gorman A. L., Hermann A. Internal effects of divalent cations on potassium permeability in molluscan neurones. J Physiol. 1979 Nov;296:393–410. doi: 10.1113/jphysiol.1979.sp013012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafe P., Mayer C. J., Wood J. D. Synaptic modulation of calcium-dependent potassium conductance in myenteric neurones in the guinea-pig. J Physiol. 1980 Aug;305:235–248. doi: 10.1113/jphysiol.1980.sp013360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas H. L., Konnerth A. Histamine and noradrenaline decrease calcium-activated potassium conductance in hippocampal pyramidal cells. 1983 Mar 31-Apr 6Nature. 302(5907):432–434. doi: 10.1038/302432a0. [DOI] [PubMed] [Google Scholar]
- Halliwell J. V., Adams P. R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982 Oct 28;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Hermann A., Gorman A. L. Effects of tetraethylammonium on potassium currents in a molluscan neurons. J Gen Physiol. 1981 Jul;78(1):87–110. doi: 10.1085/jgp.78.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann A., Hartung K. Properties of a Ca2+ activated K+ conductance in Helix neurones investigated by intracellular Ca2+ ionophoresis. Pflugers Arch. 1982 May;393(3):248–253. doi: 10.1007/BF00584078. [DOI] [PubMed] [Google Scholar]
- Hofmeier G., Lux H. D. The time courses of intracellular free calcium and related electrical effects after injection of CaCl2 into neurons of the snail, Helix pomatia. Pflugers Arch. 1981 Sep;391(3):242–251. doi: 10.1007/BF00596178. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Peptidergic transmission in sympathetic ganglia of the frog. J Physiol. 1982 Jun;327:219–246. doi: 10.1113/jphysiol.1982.sp014228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek L. K., Strumwasser F. A voltage-clamp analysis of currents underlying cyclic AMP-induced membrane modulation in isolated peptidergic neurons of Aplysia. J Neurophysiol. 1984 Aug;52(2):340–349. doi: 10.1152/jn.1984.52.2.340. [DOI] [PubMed] [Google Scholar]
- Katayama Y., Nishi S. Voltage-clamp analysis of peptidergic slow depolarizations in bullfrog sympathetic ganglion cells. J Physiol. 1982 Dec;333:305–313. doi: 10.1113/jphysiol.1982.sp014455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. Ionic mechanisms of a two-component cholinergic inhibition in Aplysia neurones. J Physiol. 1972 Aug;225(1):85–114. doi: 10.1113/jphysiol.1972.sp009930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. Synaptic block of a transmitter-induced potassium conductance in Aplysia neurones. J Physiol. 1985 Dec;369:399–437. doi: 10.1113/jphysiol.1985.sp015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. Three acetylcholine receptors in Aplysia neurones. J Physiol. 1972 Aug;225(1):115–146. doi: 10.1113/jphysiol.1972.sp009931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P. G., Doroshenko P. A., Tsyndrenko A. Y. Calcium-dependent potassium conductance studied on internally dialysed nerve cells. Neuroscience. 1980;5(12):2187–2192. doi: 10.1016/0306-4522(80)90135-9. [DOI] [PubMed] [Google Scholar]
- Lux H. D., Neher E., Marty A. Single channel activity associated with the calcium dependent outward current in Helix pomatia. Pflugers Arch. 1981 Mar;389(3):293–295. doi: 10.1007/BF00584792. [DOI] [PubMed] [Google Scholar]
- Madison D. V., Nicoll R. A. Noradrenaline blocks accommodation of pyramidal cell discharge in the hippocampus. Nature. 1982 Oct 14;299(5884):636–638. doi: 10.1038/299636a0. [DOI] [PubMed] [Google Scholar]
- Meech R. W. Intracellular calcium injection causes increased potassium conductance in Aplysia nerve cells. Comp Biochem Physiol A Comp Physiol. 1972 Jun 1;42(2):493–499. doi: 10.1016/0300-9629(72)90128-4. [DOI] [PubMed] [Google Scholar]
- Meech R. W. The sensitivity of Helix aspersa neurones to injected calcium ions. J Physiol. 1974 Mar;237(2):259–277. doi: 10.1113/jphysiol.1974.sp010481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar W. H., Spector I. The calcium current and the activation of a slow potassium conductance in voltage-clamped mouse neuroblastoma cells. J Physiol. 1979 Jul;292:307–323. doi: 10.1113/jphysiol.1979.sp012852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K., North R. A., Tokimasa T. Muscarinic agonists inactivate potassium conductance of guinea-pig myenteric neurones. J Physiol. 1982 Dec;333:125–139. doi: 10.1113/jphysiol.1982.sp014443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. A., Tokimasa T. Depression of calcium-dependent potassium conductance of guinea-pig myenteric neurones by muscarinic agonists. J Physiol. 1983 Sep;342:253–266. doi: 10.1113/jphysiol.1983.sp014849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. A., Tokimasa T. Muscarinic synaptic potentials in guinea-pig myenteric plexus neurones. J Physiol. 1982 Dec;333:151–156. doi: 10.1113/jphysiol.1982.sp014445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennefather P., Lancaster B., Adams P. R., Nicoll R. A. Two distinct Ca-dependent K currents in bullfrog sympathetic ganglion cells. Proc Natl Acad Sci U S A. 1985 May;82(9):3040–3044. doi: 10.1073/pnas.82.9.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelbaum S. A., Camardo J. S., Kandel E. R. Serotonin and cyclic AMP close single K+ channels in Aplysia sensory neurones. Nature. 1982 Sep 30;299(5882):413–417. doi: 10.1038/299413a0. [DOI] [PubMed] [Google Scholar]
- Smith S. J., Zucker R. S. Aequorin response facilitation and intracellular calcium accumulation in molluscan neurones. J Physiol. 1980 Mar;300:167–196. doi: 10.1113/jphysiol.1980.sp013157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong J. A. Modulation of potassium current kinetics in bag cell neurons of Aplysia by an activator of adenylate cyclase. J Neurosci. 1984 Nov;4(11):2772–2783. doi: 10.1523/JNEUROSCI.04-11-02772.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. H. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977 Feb;265(2):465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weight F. F., Votava J. Slow synaptic excitation in sympathetic ganglion cells: evidence for synaptic inactivation of potassium conductance. Science. 1970 Nov 13;170(3959):755–758. doi: 10.1126/science.170.3959.755. [DOI] [PubMed] [Google Scholar]
- Wood J. D., Mayer C. J. Serotonergic activation of tonic-type enteric neurons in guinea pig small bowel. J Neurophysiol. 1979 Mar;42(2):582–593. doi: 10.1152/jn.1979.42.2.582. [DOI] [PubMed] [Google Scholar]
- de Peyer J. E., Cachelin A. B., Levitan I. B., Reuter H. Ca2+ -activated K+ conductance in internally perfused snail neurons is enhanced by protein phosphorylation. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4207–4211. doi: 10.1073/pnas.79.13.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]


