Abstract
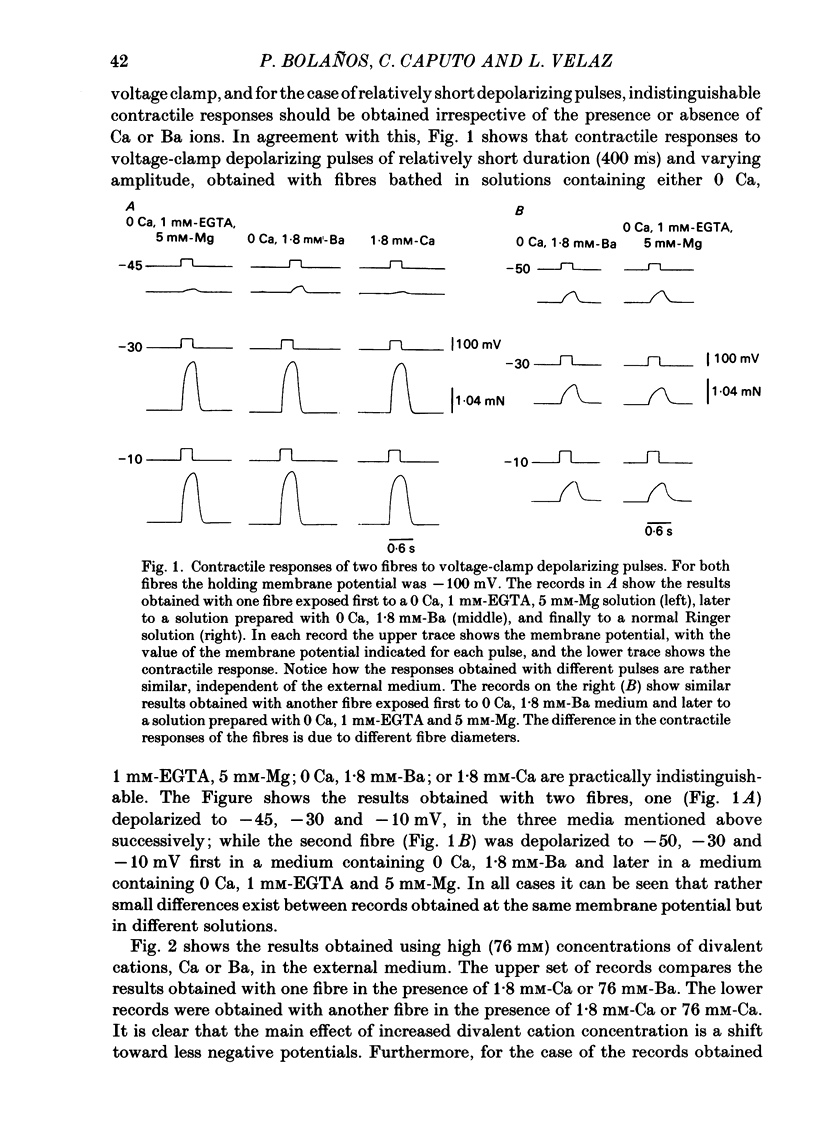
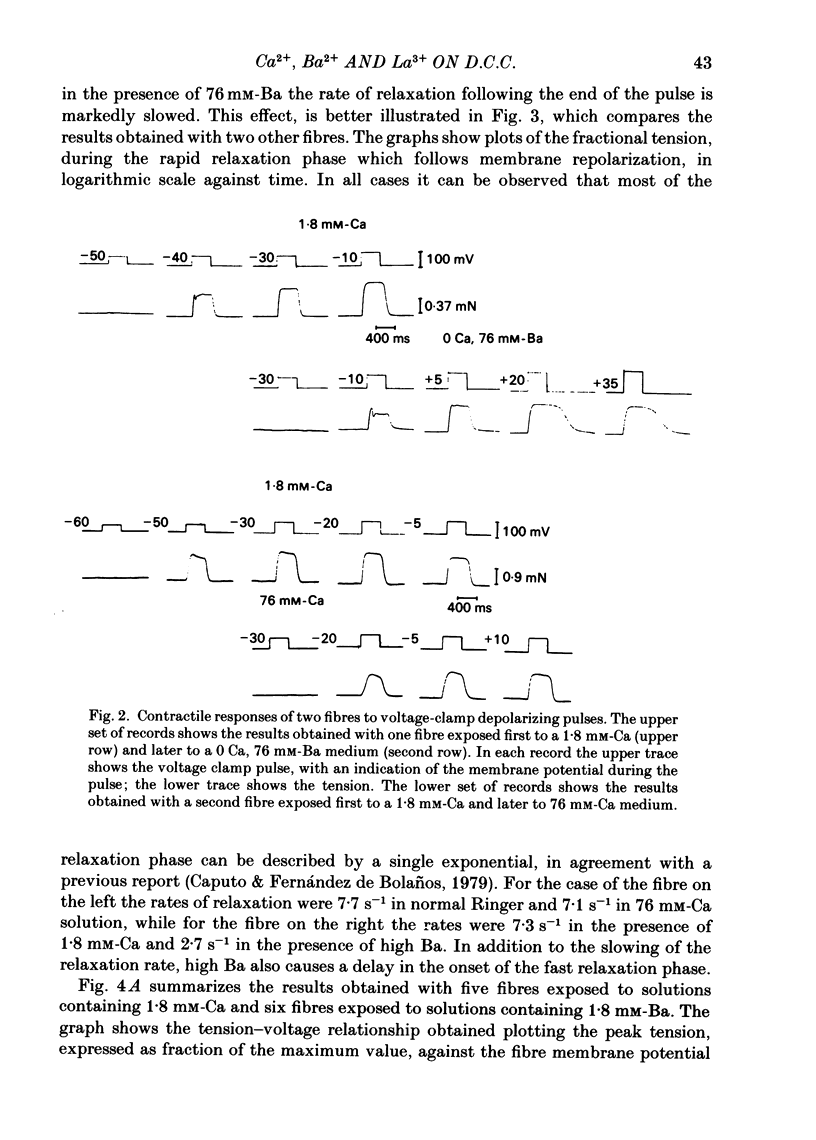
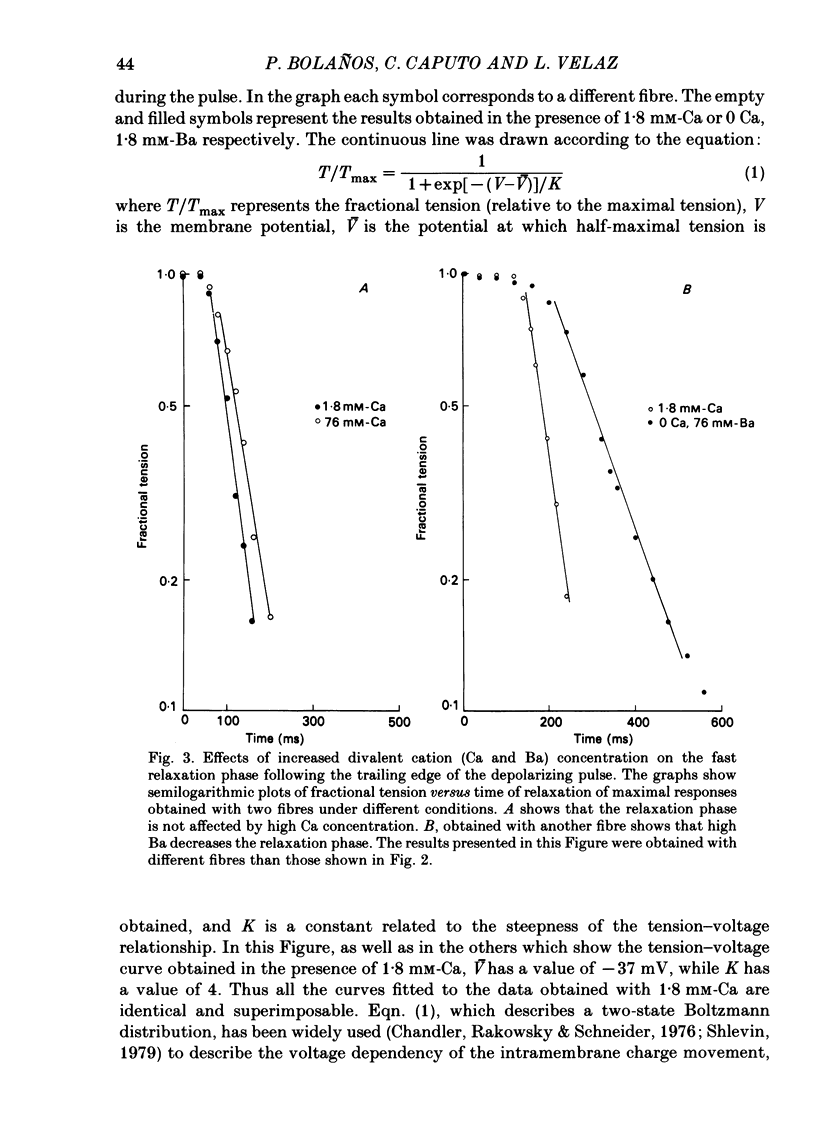
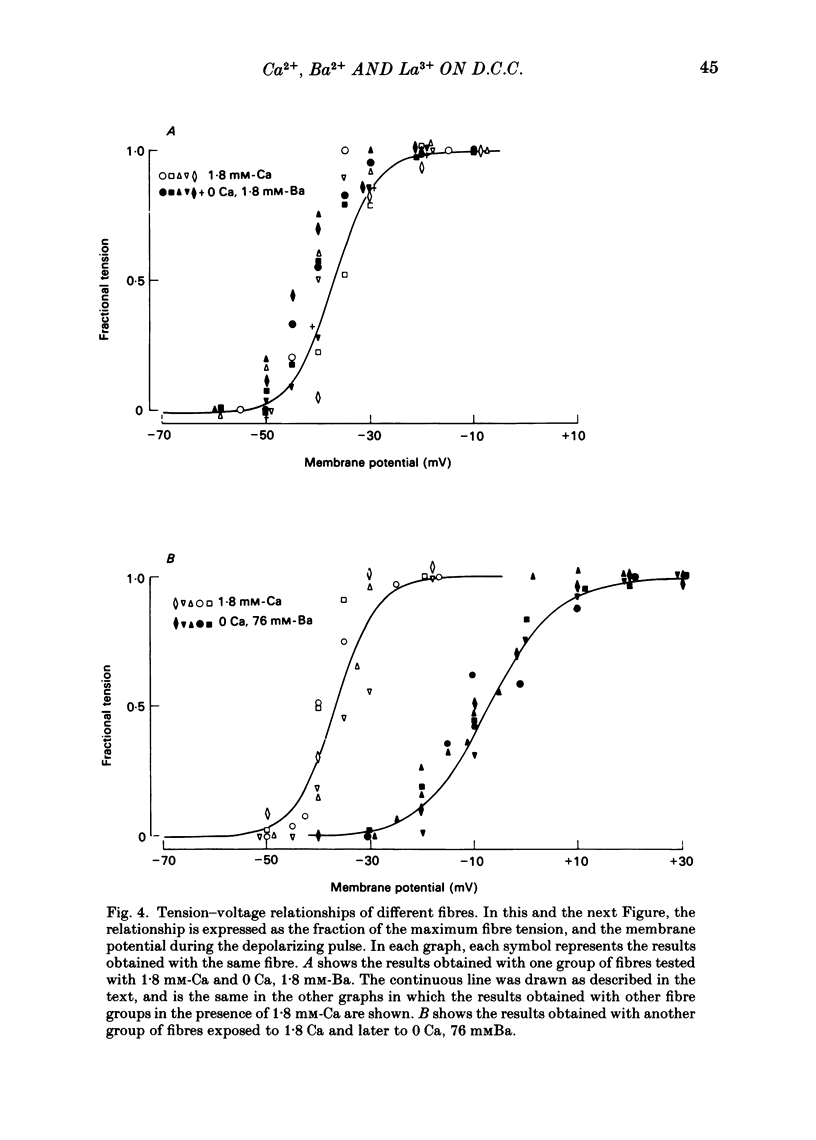
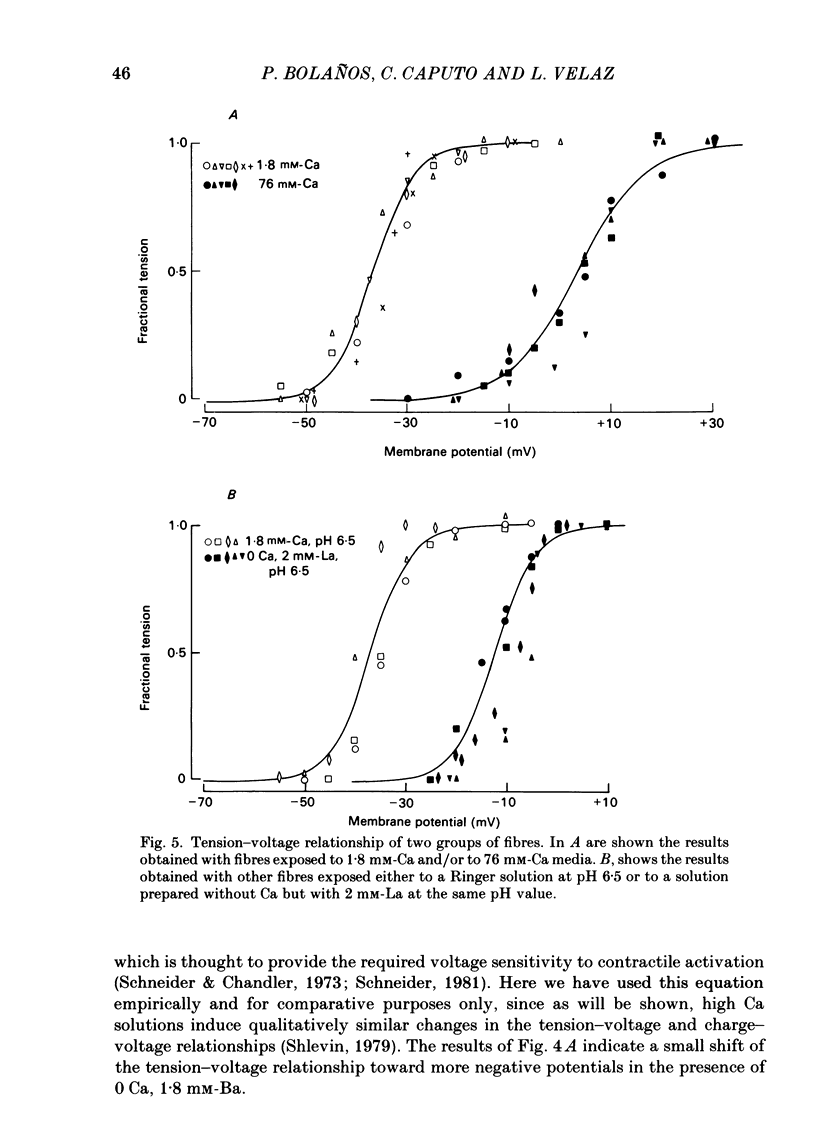
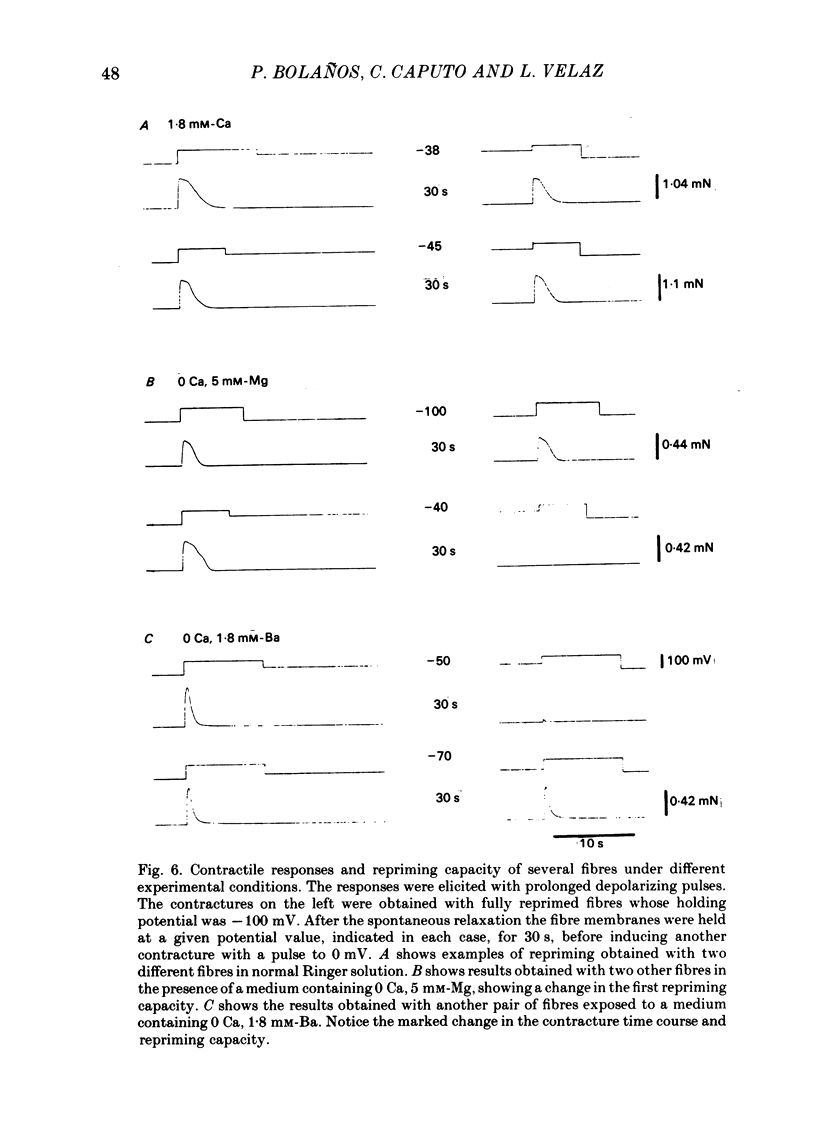
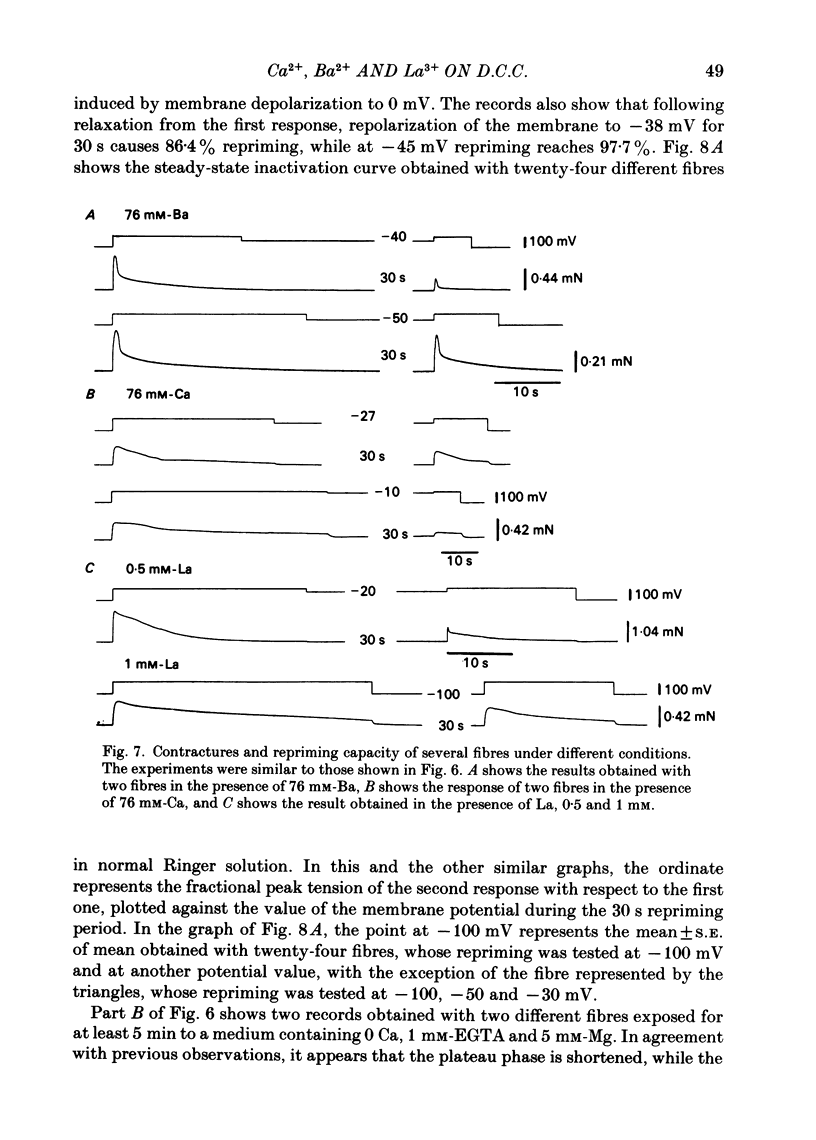
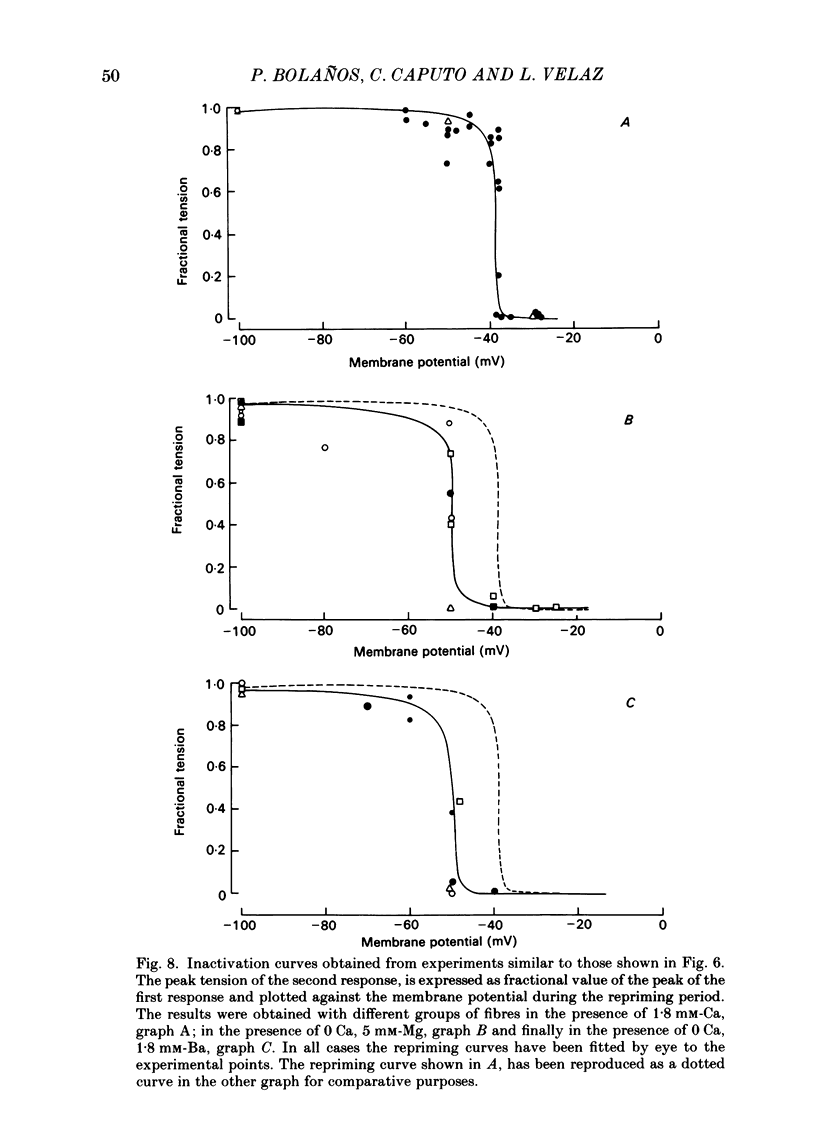
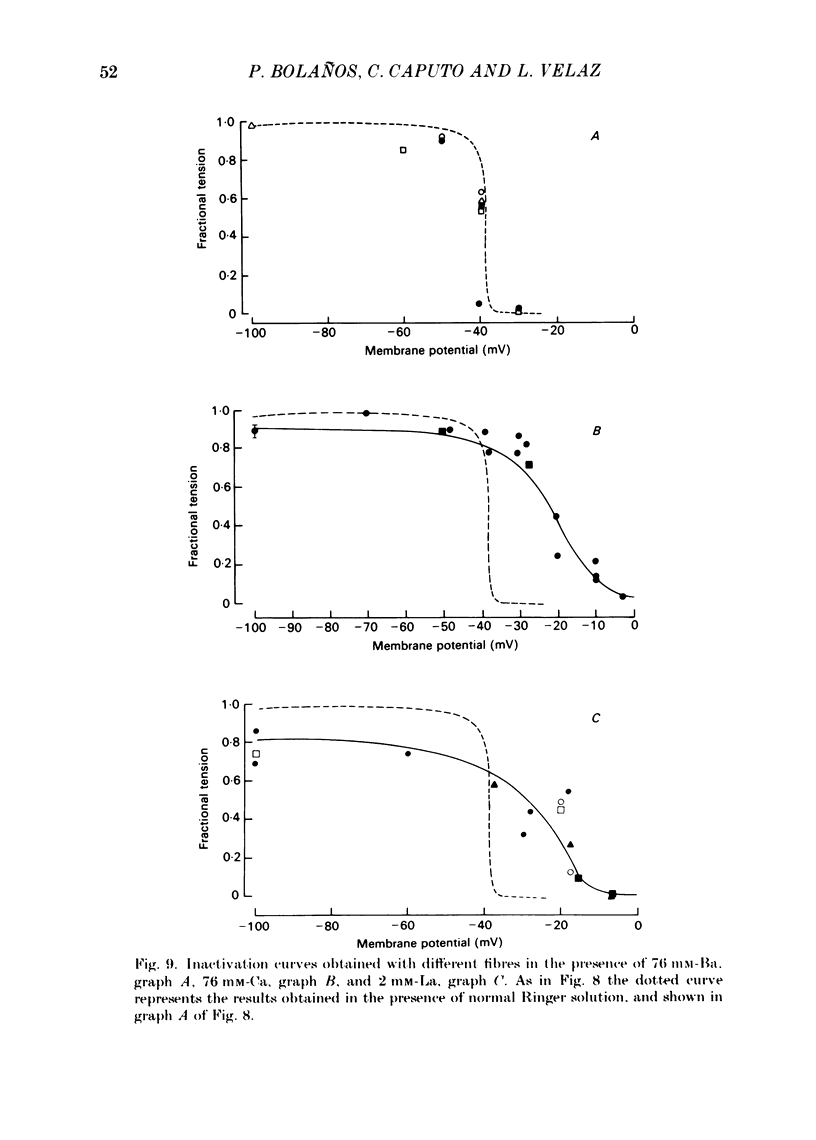
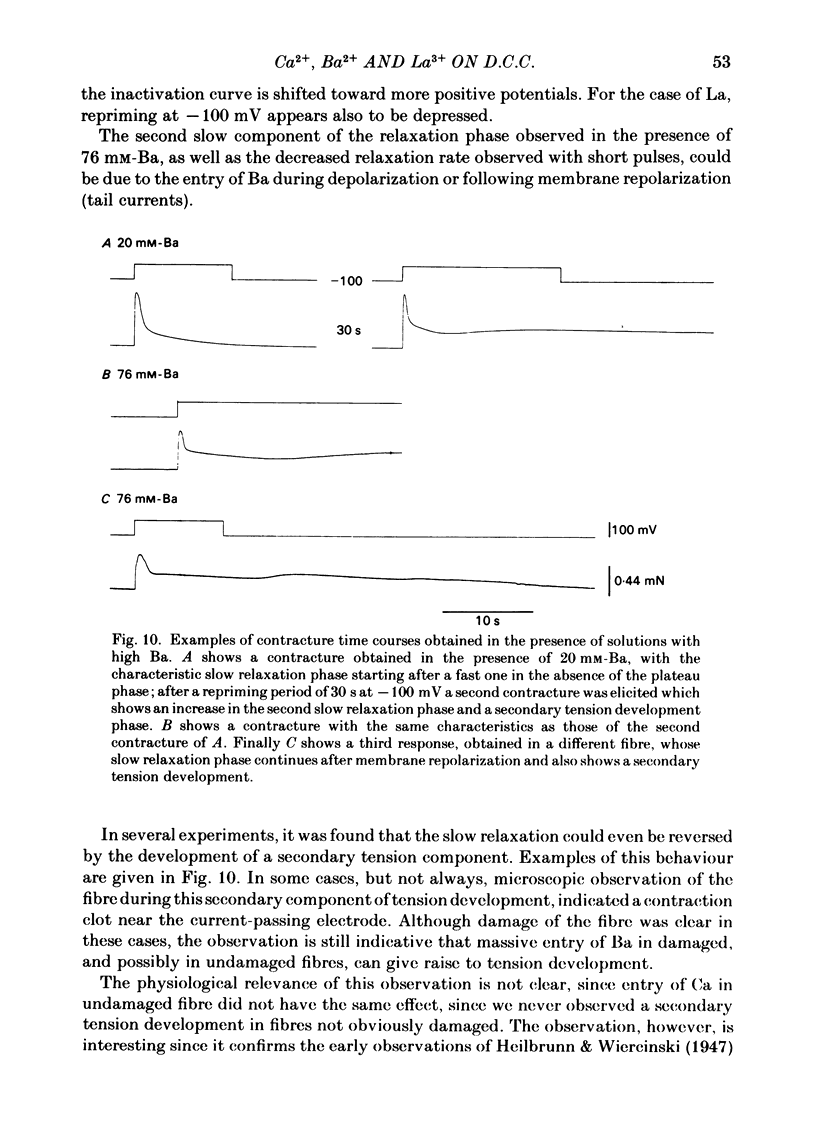
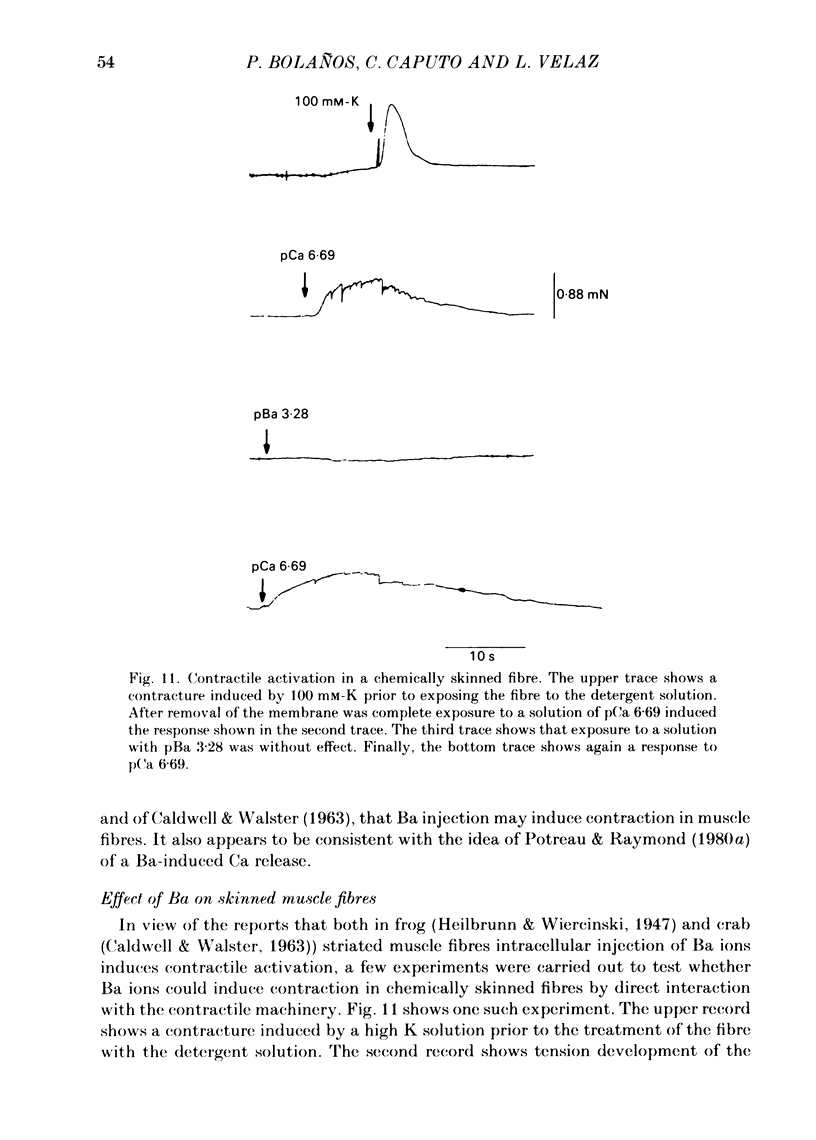
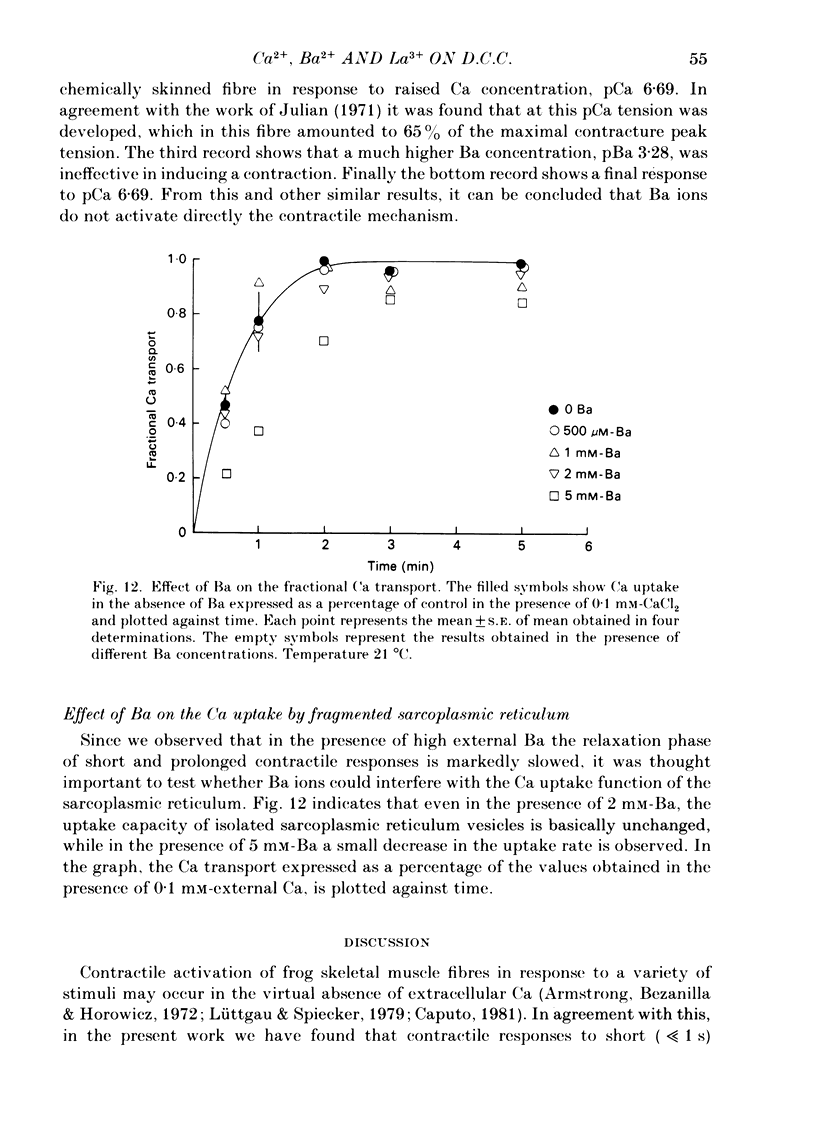
Voltage-clamp experiments were carried out to study the effect of Ca, Ba and La on the contractile responses of short (approximately 1.5 mm) muscle fibres. In the absence of external Ca or when 1.8 mM-Ca was substituted by 1.8 mM-Ba the contractile responses to short depolarizing pulses (400 ms) were not modified and only a minor shift in the tension-voltage relationship was observed. At high concentration (76 mM) of Ba or Ca, or in the presence of 2 mM-La, shifts in the tension-voltage relationship of 30, 41 and 25 mV respectively were observed. In addition the steepness of the activation curve was decreased in high Ba and Ca solutions. In the presence of 76 mM-Ba, the rate of the relaxation phase which follows membrane repolarization after a short pulse was diminished, possibly due to an intracellular action of Ba. In the absence of Ca, or when Ca was substituted by 1.8 mM-Ba the area under the prolonged contractile responses (contractures) was reduced considerably. In the presence of 76 mM-Ba, the normal contracture time course was altered, showing a late slower relaxation phase, occasionally with a secondary tension development. In the presence of high Ca or La, the time course of the contractures was greatly prolonged. The steady-state inactivation curve was shifted toward more negative potentials, in the absence of external Ca or when 1.8 mM-Ca was substituted by 1.8 mM-Ba. In the presence of 76 mM-Ba the steady-state inactivation curve was not affected. In the presence of 76 mM-Ca or 2 mM-La the curve was shifted toward less negative potential, with a marked decrease in its steepness. Ba at a concentration as high as 1 or 2 mM did not activate tension development in chemically skinned muscle fibres. Ba at 1 or 2 mM did not appreciably alter the Ca uptake capacity of isolated sarcoplasmic reticulum vesicles. At 5 mM a decrease in the Ca uptake capacity was observed. The results indicate that Ba at low (1.8 mM) concentration is not effective in substituting for Ca. The effects of divalent cations at high concentration, and of La, on the tension-voltage relationship and on the steady-state inactivation curve are most probably mediated by interaction with the external surface of the fibre membrane.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Palade P. T. Slow calcium and potassium currents across frog muscle membrane: measurements with a vaseline-gap technique. J Physiol. 1981 Mar;312:159–176. doi: 10.1113/jphysiol.1981.sp013622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson K. E., Edman K. A. Effects of lanthanum on potassium contractures of isolated twitch muscle fibres of the frog. Acta Physiol Scand. 1974 Jan;90(1):124–131. doi: 10.1111/j.1748-1716.1974.tb05570.x. [DOI] [PubMed] [Google Scholar]
- Andersson K. E., Edman K. A. Effects of lanthanum on the coupling between membrane excitation and contraction of isolated frog muscle fibres. Acta Physiol Scand. 1974 Jan;90(1):113–123. doi: 10.1111/j.1748-1716.1974.tb05569.x. [DOI] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F. M., Horowicz P. Twitches in the presence of ethylene glycol bis( -aminoethyl ether)-N,N'-tetracetic acid. Biochim Biophys Acta. 1972 Jun 23;267(3):605–608. doi: 10.1016/0005-2728(72)90194-6. [DOI] [PubMed] [Google Scholar]
- CALDWELL P. C., WALSTER G. STUDIES ON THE MICRO-INJECTION OF VARIOUS SUBSTANCES INTO CRAB MUSCLE FIBRES. J Physiol. 1963 Nov;169:353–372. doi: 10.1113/jphysiol.1963.sp007261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C., Bezanilla F., Horowicz P. Depolarization-contraction coupling in short frog muscle fibers. A voltage clamp study. J Gen Physiol. 1984 Jul;84(1):133–154. doi: 10.1085/jgp.84.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C., Bolaños P., Gonzalez G. F. Effect of membrane polarization on contractile threshold and time course of prolonged contractile responses in skeletal muscle fibers. J Gen Physiol. 1984 Dec;84(6):927–943. doi: 10.1085/jgp.84.6.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C., Fernandez de Bolaños P. Membrane potential, contractile activation and relaxation rates in voltage clamped short muscle fibres of the frog. J Physiol. 1979 Apr;289:175–189. doi: 10.1113/jphysiol.1979.sp012731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C., Gimenez M. Effects of external calcium deprivation on single muscle fibers. J Gen Physiol. 1967 Oct;50(9):2177–2195. doi: 10.1085/jgp.50.9.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C. Nickel substitution for calcium and the time course of potassium contractures of single muscle fibres. J Muscle Res Cell Motil. 1981 Jun;2(2):167–182. doi: 10.1007/BF00711867. [DOI] [PubMed] [Google Scholar]
- Chandler W. K., Rakowski R. F., Schneider M. F. A non-linear voltage dependent charge movement in frog skeletal muscle. J Physiol. 1976 Jan;254(2):245–283. doi: 10.1113/jphysiol.1976.sp011232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarandini D. J., Stefani E. Effects of manganese on the electrical and mechanical properties of frog skeletal muscle fibres. J Physiol. 1973 Jul;232(1):129–147. doi: 10.1113/jphysiol.1973.sp010260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota G., Stefani E. Effects of external calcium reduction on the kinetics of potassium contractures in frog twitch muscle fibres. J Physiol. 1981 Aug;317:303–316. doi: 10.1113/jphysiol.1981.sp013826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C., Lorković H., Weber A. The effect of the replacement of calcium by strontium on excitation-contraction coupling in frog skeletal muscle. J Physiol. 1966 Oct;186(2):295–306. doi: 10.1113/jphysiol.1966.sp008035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. S., McCarthy R. T., Milton R. L. Paralysis of frog skeletal muscle fibres by the calcium antagonist D-600. J Physiol. 1983 Aug;341:495–505. doi: 10.1113/jphysiol.1983.sp014819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Fink R., Grocki K., Lüttgau H. C. The effect of energy deprivation and hyperosmolarity upon tubular structures and electrophysiological parameters of muscle fibres. Eur J Cell Biol. 1980 Apr;21(1):101–108. [PubMed] [Google Scholar]
- Frank G. B. Blockade of Ca2+ channels inhibits K+ contractures but not twitches in skeletal muscle. Can J Physiol Pharmacol. 1984 Apr;62(4):374–378. doi: 10.1139/y84-059. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Serratos H., Valle-Aguilera R., Lathrop D. A., Garcia M. C. Slow inward calcium currents have no obvious role in muscle excitation-contraction coupling. Nature. 1982 Jul 15;298(5871):292–294. doi: 10.1038/298292a0. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Horowicz P., Schneider M. F. Membrane charge moved at contraction thresholds in skeletal muscle fibres. J Physiol. 1981 May;314:595–633. doi: 10.1113/jphysiol.1981.sp013726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto N., Sreter F. A., Gergely J. Structural features of the surface of the vesicles of FSR--lack of functional role in Ca 2+ uptake and ATPase activity. Arch Biochem Biophys. 1971 Dec;147(2):571–582. doi: 10.1016/0003-9861(71)90415-2. [DOI] [PubMed] [Google Scholar]
- Julian F. J. The effect of calcium on the force-velocity relation of briefly glycerinated frog muscle fibres. J Physiol. 1971 Oct;218(1):117–145. doi: 10.1113/jphysiol.1971.sp009607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUETTGAU H. C. THE ACTION OF CALCIUM IONS ON POTASSIUM CONTRACTURES OF SINGLE MUSCLE FIBRES. J Physiol. 1963 Oct;168:679–697. doi: 10.1113/jphysiol.1963.sp007215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorković H. Effects of some divalent cations on frog twitch muscles. Am J Physiol. 1967 Mar;212(3):623–628. doi: 10.1152/ajplegacy.1967.212.3.623. [DOI] [PubMed] [Google Scholar]
- Lorković H., Rüdel R. Influence of divalent cations on potassium contracture duration in frog muscle fibres. Pflugers Arch. 1983 Jul;398(2):114–119. doi: 10.1007/BF00581057. [DOI] [PubMed] [Google Scholar]
- Lüttgau H. C., Spiecker W. The effects of calcium deprivation upon mechanical and electrophysiological parameters in skeletal muscle fibres of the frog. J Physiol. 1979 Nov;296:411–429. doi: 10.1113/jphysiol.1979.sp013013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTONOSI A., FERETOS R. SARCOPLASMIC RETICULUM. I. THE UPTAKE OF CA++ BY SARCOPLASMIC RETICULUM FRAGMENTS. J Biol Chem. 1964 Feb;239:648–658. [PubMed] [Google Scholar]
- Potreau D., Raymond G. Calcium-dependent electrical activity and contraction of voltage-clamped frog single muscle fibres. J Physiol. 1980 Oct;307:9–22. doi: 10.1113/jphysiol.1980.sp013420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potreau D., Raymond G. Slow inward barium current and contraction on frog single muscle fibres. J Physiol. 1980 Jun;303:91–109. doi: 10.1113/jphysiol.1980.sp013273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez J. A., Stefani E. Inward calcium current in twitch muscle fibres of the frog. J Physiol. 1978 Oct;283:197–209. doi: 10.1113/jphysiol.1978.sp012496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973 Mar 23;242(5395):244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- Schneider M. F. Membrane charge movement and depolarization-contraction coupling. Annu Rev Physiol. 1981;43:507–517. doi: 10.1146/annurev.ph.43.030181.002451. [DOI] [PubMed] [Google Scholar]
- Shlevin H. H. Effects of external calcium concentration and pH on charge movement in frog skeletal muscle. J Physiol. 1979 Mar;288:129–158. [PMC free article] [PubMed] [Google Scholar]


