Summary
The basal forebrain (BF) cholinergic system (BFCS) participates in functions that are global across the brain, such as sleep-wake cycles, but also participates in capacities that are more behaviorally and anatomically specific, including sensory perception. However, how it orchestrates all the diverse local and global functions remains to be understood. To uncover the underlying organization principles, we combined data from rat brains by tracing projections from the BF to cortical areas and analyzed spatial-numerical relations of neurons to their cortical targets. The combined dataset revealed algorithmically identified and hierarchically organized three principal networks: somatosensory-motor, auditory, and visual, as defined by the sensory modality most predominant within them. These clusters of cholinergic neurons could enable the BFCS to coordinate spatially selective signaling, including the parallel modulation of multiple functionally interconnected yet diverse groups of cortical areas. This previously unseen blueprint of the hierarchy of cholinergic clusters is ready for functional testing.
Subject areas: Neuroscience, Sensory neuroscience
Graphical abstract

Highlights
-
•
How the forebrain cholinergic system orchestrates diverse functions is unknown
-
•
Cholinergic neurons organized into spatially selective clusters in the basal forebrain
-
•
Cholinergic neurons organized in somatic sensory-motor, visual, and auditory networks
-
•
Functionally connected cortical regions are reflected by the cell cluster composition
Neuroscience; Sensory neuroscience
Introduction
The basal forebrain (BF) corticopetal projection system is the main source of acetylcholine (ACh) for all cortical areas, the hippocampus, and the amygdaloid body. Manipulating of ACh release in the cortex results in changes in perception,1 cognitive flexibility,2 executive function, experience-dependent and intrinsic homeostatic plasticity,3,4,5,6 information transfer, selective attention,7,8,9 sleep-wake control, and global ignition.10,11 Responses of cholinergic neurons are associated with reward collection and/or reward per se that modify behavior.12,13,14,15,16,17 During aging, in Alzheimer’s Disease (AD) and other neurodegenerative diseases, the cholinergic space (e.g., the region containing cholinergic projection neurons) shows volume reduction, which correlates with atrophy of their cortical targets and the decline in cognitive functions.18,19,20
Evidence from tracing studies and lesions has suggested that the cholinergic projection system is organized topographically. For example, lesions in posterior BF produce more damage in the cholinergic innervation of the auditory cortex than lesions in more anterior BF locations.21 Historically the projections of the BFCS were considered part of a “diffuse cortical projection system,22” and recently suggested as the “low road” for generalized arousal.23 Several factors contributed to characterize the BFCS as part of the diffuse cortical projection system, including the variable spatial distribution of cholinergic cells across the rostro-caudal extent of the BF, the observation that adjacent cholinergic neurons projecting to distant regions of the cortex appear to have overlapping dendritic fields,24 and that measurements of cortical ACh release using in vivo microdialysis techniques failed to indicate differences between cholinergic activities in different cortical regions during various behaviors.25 Additionally, certain functional data have contributed to the view that cholinergic signaling in the cortex is slow and non-specific, and likely acts through volume transmission.26,27,28
Recent anatomical studies paint a more complex picture wherein the cholinergic projection to the neocortex is not diffuse but instead is organized into cortical target-specific groups of cholinergic neurons that receive specific combinations of inputs.29,30 Moreover, cholinergic cells (Ch) that project to the superficial or deep layers of the medial prefrontal (mPFC) and somatosensory cortex in mice are largely separated in the BF.31,32 Also, new evidence from real-time amperometric recordings indicates cholinergic signaling in attentional contexts is rapid, phasic, transient, and probably synaptic.33
In a limited set of rat brains (n = 9), using distinct retrograde tracers deposited into disparate cortical areas to map labeled cells in the BF and cortex, we demonstrated that the BF has a complex topographic organization consisting of segregated or overlapping pools of projection neurons. The overlap in the BF of projecting populations appears to be related to the degree of connectivity between their cortical targets.29 This complex efferent organization pattern and the specific input pattern to target-identified cholinergic neurons,30 together with the regionally selective dendritic orientation of cholinergic neurons,34 led to the hypothesis that the anatomical organization of the BF constitutes a “scaffolding” that may enable the cholinergic system to modulate topographically organized interconnected cortical areas.29 Indeed, the optogenetic activation of cholinergic subgroups in the BF induces modality-selective desynchronization in specific sensory cortical areas in transgenic mice,35 indicating modularity in the organization. Although Buzsaki and colleagues demonstrated decades ago that circumscribed ibotenic acid lesions in the BF result in a dramatic increase of slow delta waves corresponding to the disappearance of cortical AChE fibers,36 it has never been resolved whether cholinergic neurons modulate disparate cortical regions in a globally or a spatially selective fashion, due to the absence of a viable anatomical model to serve as a basis for generating hypotheses about functional relationships.
Here, as an analytical tool to quantify the organization of the BF we created an interactive “virtual BF” database (http://zaborszkylab.org/3DCholinergicSpace/; Figure S1) incorporating the locations of cholinergic cell bodies quantified in over 70 experimental brains with cortical tracer injections, many regions with electrophysiological identification. Some of the brains were used in previous publications.21,29,30,37 All brains are identified with a specific ID and listed in Table S1 with their source. We used this virtual BF to further examine whether the spatial location of cells projecting to 30 ontologically defined cortical targets shows systematic organizational features that deviate from the uniform random distribution suggested by the diffuse model. We quantified the local co-occurrence of functionally related cholinergic neurons by computing the correlation between the local density (in the BF) of neurons classified based on their cortical projection targets. We devised various algorithms collectively referred to as “Spatial Density Correlation” to capture the efferent organizational principles of the BFCS, facilitating the understanding of the underlying mechanisms of its complex functions.
Results
Data production and collections
Cholinergic neurons in the BF were mapped with the NeurolucidaR system in 73 rat brains that received pairwise conventional retrograde (Fast Blue [FB] and Fluoro-Gold [FG]) or virus tracer injections in the cortex (n = 106). After mapping fluorescently labeled cells from 200 μm series, sections were re-stained with Nissl, and images of sections were aligned to a reference brain with 50 μm series. We implemented a relational database schema within the PostgreSQL framework that communicates with the QGIS mapping client to store and visualize 3D vectorial cell data, including delineations and images. This solution provided a complete set of functions for analyzing cell densities, determining spatial relationships, and manipulating delineations with very fast and smooth visualization of high-resolution images as well as vectorial data. We created a reference brain from a full series of 50 μm sections of a single brain in which all cholinergic cells were mapped irrespective of their targets, and then sections were stained for Nissl. These reference sections were imaged with 3 μm/pixel resolution and aligned in 3D. The software (Java, ImageJ) was able to slice this high-resolution 3D stack of images in any plane to match sections from the experimental brains. The spatial registration of experimental sections to the reference brain was carried out using mixed rigid body, affine, and B-Spline-based elastic transformations38 (Figure 1).
Figure 1.
Image and vectorial data integration pipeline
We created a reference brain from a full series of 50 μm sections of a single brain in which all cholinergic cells were mapped irrespective of their targets, and then sections were stained for Nissl. These reference sections were imaged with 3 μm/pixel resolution and aligned in 3D. The software was able to slice this high-resolution 3D stack of images in any plane to match sections from the experimental brains. The spatial registration of experimental sections to the reference brain is carried out using matching with the appropriate template brain sections and defining identical reference points using mixed rigid body, affine, and B-Spline-based elastic transformations.
All injection sites were warped into specific Paxinos atlas plates and collected as a PDF file (Figure S2). Figure S3 validates the precision of warping. These maps are important in the normalization procedures applied to the density analysis (see STAR Methods). Intraoperative electrophysiology was used to identify various motor,39 somatic sensory,39 auditory,21 and visual cortical areas. Table S1 details the percentage participation of each injection in the Paxinos cortical areas, appended with notes about the electrophysiological identification of modality-specific cortical areas. Table S2 explains the cortical ontology categories used in our study.
Spatial density correlations of cholinergic neurons in the basal forebrain
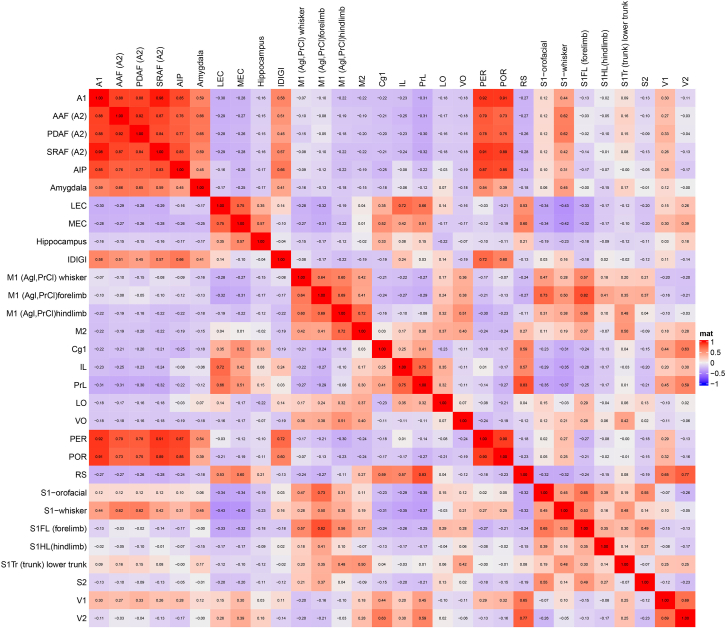
Projection Ch neurons from all experimental brains were co-registered with a reference brain (Figure 1). Next, we defined a 300 μm radius spherical volume around each of the 5,674 projection neurons and counted the number of cells projecting from this volume to any of the 30 cortical ontologically defined regions. This provided us with a 5,674 × 30 matrix of projection counts. In this matrix, each cortical area (column) was associated with a vector of 5,674 rows representing all the Ch neurons projecting to that cortical area, hereafter referred to as the target-projection vector (TPV). Hence, if two cortical areas receive input from the same group of Ch neurons and those neurons display similar cortical projection patterns, their column vectors are expected to be highly correlated. Conversely, if two cortical areas receive input from different groups of Ch neurons, their column vectors are expected to be uncorrelated. Figure 2 displays the methods used for spatial density correlations and Figure 3 represents the correlation matrix of pairwise correlations between each of the 30 cortical areas. The numbers in the matrix represent the coefficients of Pearson correlations between all pairs of TPVs, quantifying the similarity of cortical projection patterns within a local cholinergic ensemble. The symmetrical red (correlated) and blue (anticorrelated) areas represent the most and least likely composition of cortical projection neurons within 300 μm ensembles identified by their cortical projections in the BF, respectively.
Figure 2.
Scheme to generate matrix of spatial correlation
Projection cholinergic neurons from all experimental brains were registered to the reference brain. We then defined a sphere of 300 μm radius around each projection neuron (n = 5,674) and counted the number of neighbors projecting to 30 cortical ontological regions, which provided a target projection vector of 30 integer values. We computed Pearson’s correlations between all pairs of column vectors (30 x 5,674), resulting in a 30 x 30 matrix. We then rearranged the row vectors using hierarchical clustering. Details see in STAR Methods “construction of spatial correlation matrixes.”
Figure 3.
Matrix of the spatial correlation of Ch cells projecting to 30 cortical areas
Symmetrical correlation matrix of local densities between any two cortical targets sampled across the cholinergic space. Each row (or column) represents the composition of co-distributed BF projection neurons for a given projection population. Numbers represent Pearson coefficients. For visualization purposes, we rendered negative, zero, and positive correlations with the shades of blue, white, and red, respectively. Red areas represent pairs of cortical areas sharing a similar composition of cholinergic neurons projecting to them. Cortical regions are listed with their common abbreviations (see list of abbreviations at the end of STAR Methods).
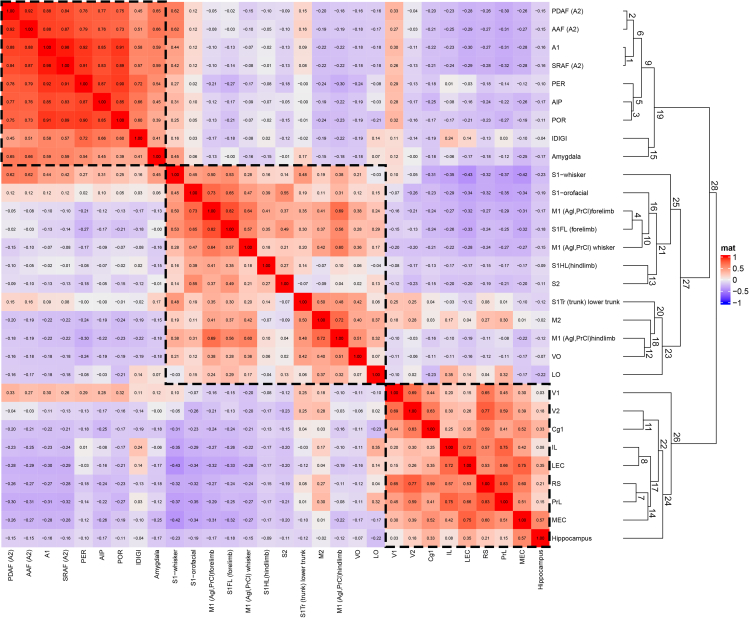
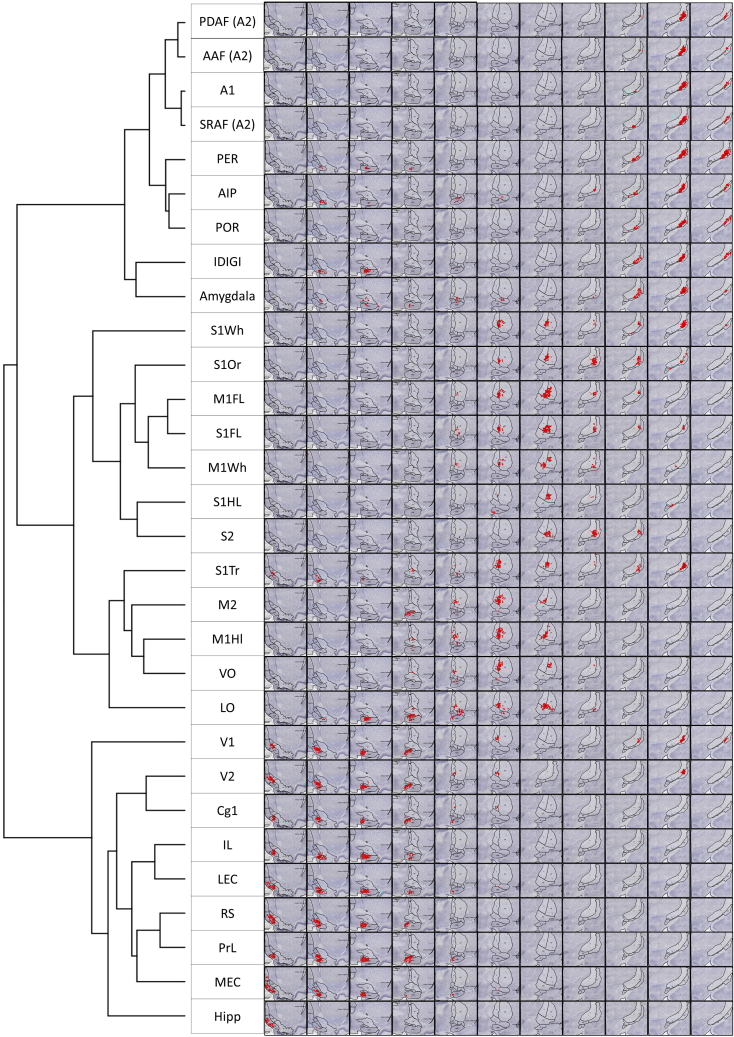
The order of cortical areas in Figure 3 is arbitrary, and the high-correlation areas are fragmented unless we reorganize them by placing similar correlation values next to each other. To achieve this and unravel the underlying architecture, we performed hierarchical clustering on the matrix in Figure 3 and computed a dendrogram using the “complete linkage” procedure. We ordered rows or columns based on their minimum Euclidean distance, which resulted in a scalar value. For example, the algorithm merges the rows “M1-forelimb” (M1FL) and “S1-forelimb” (S1FL), and these values are compared with the next most similar row, which is the “S1-orofacial” cluster, and so on (Figure 4). In other words, this hierarchically reorganized matrix represents the classification of the BF cell populations according to the cellular composition of their neighborhood with respect to their cortical targets. Hierarchical clustering revealed three well-articulated clusters of BF Ch neuron populations classified as: (i) Somatic Sensory-Motor (SSM), (ii) Visual, (iii) and Auditory branches, defined by the sensory modality most predominant within them. This is of particular significance considering that we did not impose any order of structural categories via the experimental design when selecting the injection sites. In Figure 3, we list the cortical areas in alphabetical order, while Figure 4 shows the order after applying hierarchical clustering to the data. This method was agnostic to the structural and topographical relationship between the areas—the three networks emerged exclusively from the correlation between projection patterns expressed by the Ch neurons. Figure 5B shows the three large clusters in a 3D rendering. Please note that the orbitofrontal cortex (OFC: VO, LO) and the medial PFC (mPFC: PrL, IL) are split into two networks; OFC in the SSM, while mPFC, and visual cortex are together with the hippocampus in the visual, while the amygdala is classified with the auditory cortex.
Figure 4.
Matrix of the hierarchical spatial correlation of Ch cells projecting to 30 cortical areas
Hierarchical clustering of correlation matrix using Euclidean distance and complete linkage. This matrix is identical to the correlation matrix of Figure 3; except the rows and columns are reordered according to dendrogram. There are three visually apparent distinct groups of projection populations that are outlined. Numbers in the dendrogram represent linking clusters (clades) according to their hierarchy. Clades (clusters) close to each other in the reordered correlation matrix, such as S1-whisker/amygdala may belong to different major networks because they show increased similarity to clades in other networks. Please note the hyphenated lines on the left represent the cluster boundaries defined by the dendrogram.
Figure 5.
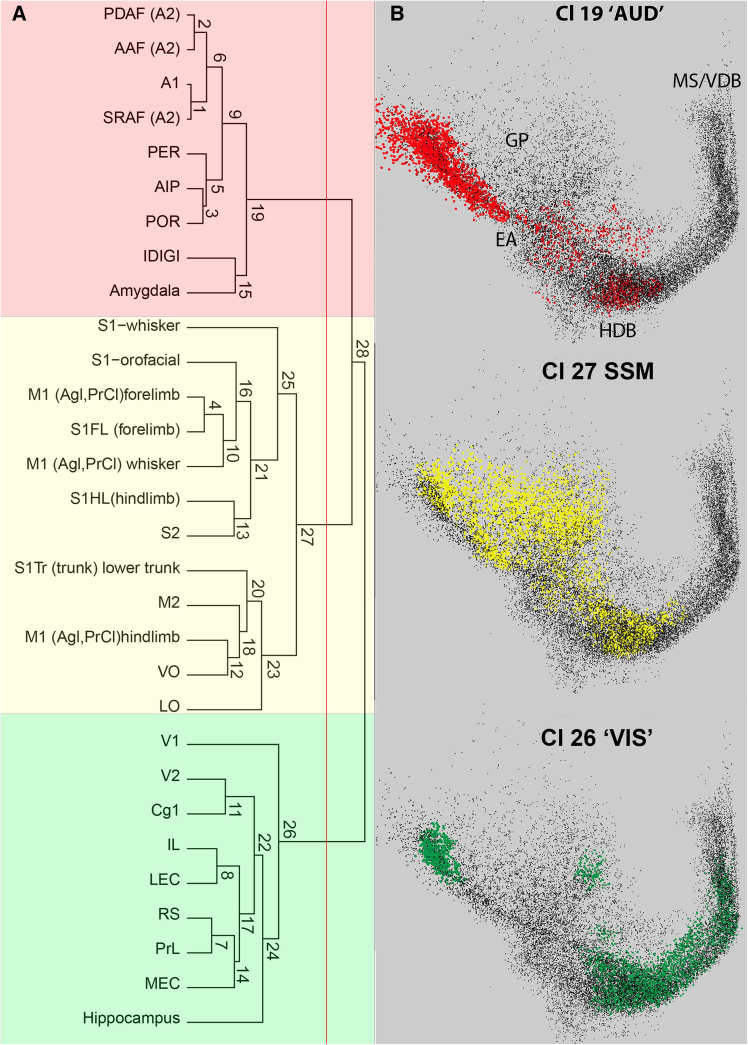
View of the major clusters with the dendrogram
Dendrogram of the hierarchy of Ch cell groups (A) with 3D rendering of the 3 networks (B). Cells of the three distinct projection population groups (Clusters: 26, 27, and 19) visualized in the BF space. AUD = auditory (red); SSM = somatic sensory-motor (yellow); VIS = visual (green). Black dots represent all cholinergic neurons of the template brain. Colored symbols display cells from the individual primary clusters. In the 3D rendering (panel B) medial is right (MSVDB), caudo-lateral is left. For orientation, compare these images with the 3D interactive program (http://zaborszkylab.org/3DCholinergicSpace/). Acronyms label structures with their approximate locations in the rendering; EA: extended amygdala; HDB: horizontal limb of the diagonal band; GP: globus pallidus; MSVDB: medial septum-vertical limb of the diagonal band.
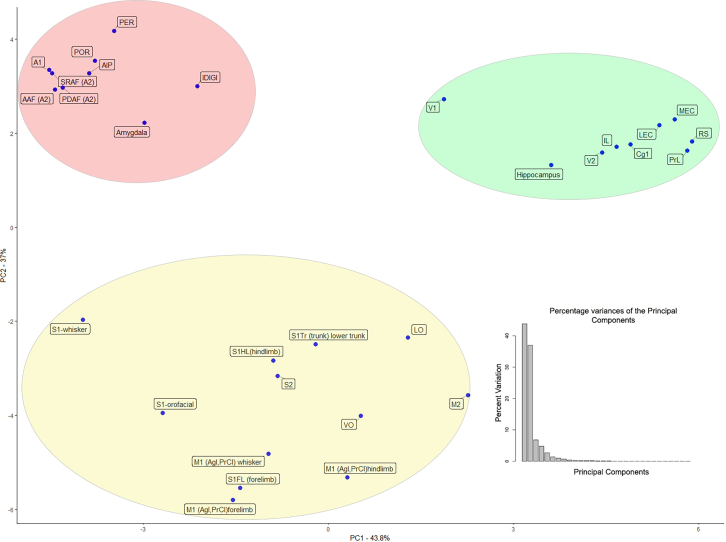
The statistical validation of the resulting dendrogram verified the three large clusters. We calculated the p-values for the dendrogram via multiscale bootstrap resampling.40,41 Figure S4 shows that the large clusters are well within the 90% confidence level. In addition to hierarchical clustering, we applied principal component analysis to the correlation matrix and found that the two largest eigenvalues, representing 37% and 43.8% of the variance, were associated with the same SSM, Visual, and Auditory networks (Figure 6). We conducted an additional clustering on the row vector of correlation values for each cortical area using the Elbow K-means clustering method.42 Running the K-means method at 3 cluster cuts resulted in the same cluster composition (Figure S5). We conclude that the target composition of the local cholinergic ensembles regarding their cortical projections is far from the random uniform distribution. Instead, they associate functionally related cortical targets, within each of the three large functional networks, with high precision.
Figure 6.
Principal component analysis of row vectors in Figure 4
The members of the three major clusters (Clusters: 19, 26, and 27 on Figure 4) of the hierarchical clustering are unambiguously separated in the 2D space as well. These are the three major clusters within which any two projection populations have similar surrounding compositions of other projection populations within a 300 μm radius volume. The first two principal components have remarkably high variance compared to the rest (37%, 43.8%).
The next question was whether the network underlying the axonal projections to functionally related cortical targets is topographically clustered or homogeneously distributed in the BF. In other words, do three functional network projections originate from anatomically confined BF areas, or are they homogeneously distributed? The hierarchical clustering results could afford both. To test this, we selected high-density cell populations among the BF cells with the highest target correlations. The location of the normalized cell populations projecting to individual cortical regions used in the hierarchical clustering was plotted in 2D series of the 3D template brain, arranged at 11 consecutive levels encompassing the whole extent of BF. The rows in Figure 7 represent these images along the antero-posterior axis. To compare the local distribution of normalized projection populations with the hierarchical cluster matrix, the rows are organized in the same order as the dendrogram in Figure 4 or Figure 5A. Each row shows the leaf of the dendrogram representing the hierarchical organization of the BF Ch cell populations. As seen in Figure 7, the distribution of cell populations shows a systematic transition along the antero-posterior axis of the BF while also revealing the three main functional groups labeled after their predominant sensory modality as auditory, SSM, and visual, as evident from Figure 4. In other words, the clusters of highly correlated projections in the matrix of Figure 4 correspond to specific locations in the BF.
Figure 7.
Visualization of the normalized cell population densities in the BF projecting to individual cortical regions
Spatial locations, where the specific normalized cell densities were higher than their own average across the brain (STAR Methods, “visualization methods”) were marked in 2D sections of the 3D template brain arranged at 11 consecutive levels encompassing the whole rostro-caudal extent of the BF. Columns in this figure represent these sections along the antero-posterior axis. To compare the local distribution of normalized projection populations with the hierarchical cluster matrix, the rows are organized in the same order as the dendrogram of Figure 4 or 5A. Each row shows the leaf of the dendrogram representing the hierarchical organization of the BFCS cell populations. Abbreviation of cortical structures: see list of abbreviations in the STAR Methods.
Somatic sensory-motor (SSM) network
According to the dendrogram of Figure 5A, the smallest Euclidean distances in a low-level similarity environment are represented by cluster 4, between the motor forelimb (M1FL)/sensory forelimb (S1FL), and by cluster 13 between the motor hindlimb (M1HL) and S2 cortex. It must be kept in mind that even at this level of similarity, these clusters do not represent independent spaces but show overlap in unique combinations, as can be concluded from viewing Figure 7. At the next similarity level, S1 orofacial joins to clusters 4 and 10, constructing cluster 16 and cluster 16 joins with cluster 13, creating cluster 21. One level higher, clusters 23 and 25 create cluster 27. In cluster 27, all clusters representing the somatic-sensory motor network are merged. The cophenetic distance, a scalar value that is calculated from the Euclidian distance, is the measure of intergroup dissimilarity at which two clusters first combine into a single cluster. In Figure S4, a scale at the left helps to estimate the cophenetic distance, and in Table S3, the numerical values for Euclidian and cophenetic distances for all target combinations can be looked up. For the 29 specific cluster combinations in Figure 5A, these two numerical values are identical (Sheet 2, Table S3). As can be surmised, the SSM network is composed of two subnetworks.
Visual network
V1 and V2 visual areas were defined, following craniotomy and durotomy, by micropipette electrodes inserted into the right cerebral cortex to detect visually evoked potentials in response to the strobe light stimulation of the contralateral eye. Visually responsive areas were marked, and a separate pipette was inserted into the same cortical area at two or more such responsive locations, and the retrograde tracer Fast Blue was deposited iontophoretically at a depth of up to 1.3 mm ventral to pia. In addition to V1 and V2-projecting cholinergic cells, this network also contains cells projecting to the retrosplenial (RS), Cg1 (cingulate), PrL (prelimbic), and IL (infralimbic) cortex. Medial (MEC), lateral entorhinal cortex (LEC), and hippocampal-projecting cholinergic cells cluster to this network using the Euclidian similarity analysis.
Auditory network
Primary (A1) and secondary (A2) auditory fields were identified with multi-unit extracellular recordings as described.21 A1 was defined by a general progression of low to high characteristic frequency (CF) along the posterior to anterior axis. Anterior placements that resulted in a reversal in the CF progression were defined as lying in the anterior auditory field AAF.43 Furthermore, the posterior dorsal auditory field (PDAF) belt area was identified as more responsive to noise than to tone.43 We also identified the suprarhinal auditory field (SRAF44,45), as the auditory-responsive region just above the rhinal sulcus. CF was defined as the frequency that elicited an evoked discharge at the lowest dB sound pressure level (SPL); if more than one stimulus frequency was elicited at the same dB SPL, then the frequency that elicited the greatest number of spikes was identified as the CF. Auditory fields AAF, PDAF, and SRAF are also termed A2 fields.
In addition to auditory cortical-projecting Ch cells, this network, via similarity measurements, contains cholinergic clusters projecting to the perirhinal (PER), postrhinal (POR), insular cortex (AIP, IDIGI), and amygdala. The shortest similarity distance has been found in this network between A1 and SRAF (cluster #1), between PDAF and AAF (cluster #2), between AIP and POR (cluster #3), and between IDIGI (insular cortex, dysgranular, and granular parts) and the Amygdala (cluster #15 in Figure 5A; Figure S4). The anterior belt region (AAF) overlaps with various somatosensory fields, including barrel fields (D-row) and the upper lip region (Figure 4) but belongs to the SSM network based on the Euclidian and cophenetic distances.
Discussion
We found a precise relationship between the cellular composition of local BF Ch ensembles and their cortical targets by analyzing the projection pattern of 5,674 cholinergic neurons in the BF labeled by their cortical targets. Accordingly, local BF ensembles (∼300 μm radius) target functionally related cortical areas. In other words, the cellular composition of local Ch networks seems to reflect the functional relatedness of their cortical targets. This may suggest that these Ch nodes can dynamically coordinate the activity of diverse but functionally connected cortical areas, and thus the BFCS is not a diffuse modulator—as traditionally assumed—but likely has key roles in information transfer between domain-specific cortical areas.
We developed and applied a spatial density correlation (SDC) method to define the target composition of local BF Ch neurons within 300 μm cell-centered volumes. This revealed the diversity of these BF ensembles regarding their cortical projection patterns (Figure 4). While the association between some cortical targets and local BF Ch ensembles was strong (r > 0.7), those highly correlated fields were diffusely scattered over the arbitrarily ordered list of cortical areas in Figure 3. The underlying architecture did not emerge until we subjected the matrix of SDC to hierarchical clustering. The application of hierarchical clustering to cholinergic cell density normalized according to 30 ontologically defined cortical regions resulted in a robust hierarchy organized into three well-defined clusters aligned with (i) auditory, (ii) visual, and (iii) somatosensory/motor functional groups. This hidden organization pattern challenges the alternative model, which assumes that cholinergic projections are part of the diffuse modulatory systems globally affecting cortical regions.22,25
The hierarchical cluster analysis is an unsupervised multivariate statistical method to analyze the internal relationships between data elements. It has been extensively applied, among other clustering methods, to measure connectivity in fMRI resting-state data,46,47 extracellular electrophysiological properties of rat ventral tegmental area neurons48; or the distribution of tau and amyloid deposits in the cerebral cortex.49 Zilles’ group used quantitative multiple-receptor expression to reveal the hierarchical organization of neocortical areas in rats,50 in the human cingulate cortex,51 in Broca region52 and in the inferior parietal lobule.53 An attempt was made in mice, using subjectively weighted strength of connections in anatomic modules to infer cortical organization.54 Using different Cre driver lines to selectively label unique excitatory cell populations and quantitatively analyze axon terminal densities, Harris et al.55 identified six cortical modules in mice: prefrontal, lateral, medial, somatomotor, visual, and auditory. Three of these overlap with the clusters we identified based on the cortical projection patterns of BF Ch neurons. Interestingly, the six-module cortical organization remained after adding thalamocortical input from 29 thalamic nuclei.
We remark that no preconceived hierarchical model was applied to the selection of cortical injection sites during the design of the experiment. The 30 cortical areas were selected purely based on the ontology, without considering their potential associations with the BF Ch system. We applied three additional tests to demonstrate that the observed hierarchical organization is not an artifact generated by the method. Firstly, we computed the significance of the branches of the dendrogram by using a bootstrap method, which confirmed that the 3 primary subnetworks are segregated with above 90% confidence (Figure S4). Secondly, we computed the principal component analysis from the correlation matrix, which revealed the same 3 clusters, including the same cortical areas as identified by the hierarchical clusters, with the three largest eigenvalues (Figure 6). Finally, using a K-means clustering method42 resulted in the same cluster composition as the hierarchical clustering (Figure S5).
Hierarchical clustering: Technical considerations
Single-cell electrophysiology, quantitative anatomical tracing, and single-cell RNAseq furnished ample evidence suggesting that cortico-cortical connections in the mammalian brains are hierarchically organized.55,56,57 Since hierarchy can be investigated at different scales, various morphological or physiological features can be measured. For anatomical features, connection strengths, and distance, markers for myelo-, cyto-, receptor architecture, cell density, and so forth, can be quantified. We calculated cell density from spherical volumes of 300 μm radius centered on the cell body. This 300 μm radius was selected based on the probability of connections with other neurons and the dendritic and axonal arborization pattern of cholinergic neurons.29,34,58 Although analysis with radius values from 100 μm to 500 μm did not change the general shape of the spatial density correlation matrix of Figure 4, using a 300 μm radius articulated the three large networks the best. Since the normalization procedure removed the overlap of injection sites within ontology-defined cortical areas (see STAR Methods), overlapping injection sites did not bias the number of cases in the construction of the correlation matrix. On the other hand, removing whole ontology (input) categories could change the shape of the dendrogram.51 In our analysis, removing the “unidentified” M1 and S1 cases (injections that were targeted only using atlas coordinates without electrophysiology) indeed made a difference in the matrix and the dendrogram: including the “unidentified” categories made the somatic sensory-motor network more compact while removing them resulted in splitting this network into two subnetworks (see Figure 4).
While each cluster can be visualized in 3D and occupies a specific space in the brain (Figure 7), the shape of the dendrogram is a poor indicator of the number of clusters that coexist at each level; therefore, we have numbered them for easy explanation. Since the clusters are arranged in a hierarchy, to associate these clusters with cortical functional group compositions, one needs to draw cut lines parallel with the long axis of the dendrogram; observations that are joined together above the lines are in clusters. In Figure S6, we included 3 scenarios generating three, six, and eighteen clusters and displayed them with the corresponding Silhouette plots59 (see STAR Methods) to explain the composition of clusters. Accordingly, one can envisage combinations along the hierarchy of how the original 3 large clusters can be broken up into smaller and smaller clusters, eventually into many single Ch clusters, segregated along functional cortical targets. These clusters may represent the flow of information.
Anatomical connections and functional interactions within the three networks
Somatic sensory motor network
There is an extensive anatomical and functional interaction between M1FL and S1FL60,61,62, and sensorimotor integration between these body parts could be in part mediated by cholinergic cluster 4 (see Figure 5A). S1 also projects to S260. and cluster 13 may modulate the interaction between S1HL and S2. Furthermore, the S1 orofacial area, M1FL, and S1FL are heavily interconnected63 and clusters 10 and 16 could jointly innervate these body parts. Corroborating that visual, auditory, and entorhinal areas show only very sparse projections to M1 areas, we do not have cholinergic clusters that would jointly innervate these areas. Similarly, there is a lack of spatial density correlation between these areas in Figure 4. Despite the retrosplenial (RS) region projections to M1,64,65,66,67 M1 and RS show negative spatial density correlation (Figure 3) and no joint cholinergic clusters innervating these regions either. The discrepancy between anatomical tracing and the spatial density correlation matrix may be related to the “fibers of passage” problem, a common shortcoming in tracing experiments.
Auditory network
Similarly, decades of research have found both anatomical and functional relationships among the identified auditory clusters (clades 1,2,6; Figure 5A). Most clearly, there are direct projections between A1 and A2 areas as well as shared thalamic inputs.68 There are also reciprocal connections between the auditory thalamus and insular cortex,69,70 and connectivity between both the auditory thalamus and auditory cortex to the amygdala.71,72 Finally, there are auditory cortical projections to postrhinal60 (POR) and the insular cortex is strongly interconnected with the amygdala.73 In addition to the anatomical evidence that supports the relationship among these clusters, many studies have supported the functional connectivity between these regions, including lesions of the insular cortex that interfere with auditory processing74 and lesions of the POR resulting in impairments of auditory fear conditioning.75 Arguably, the most extensively studied among these is the functional relationship between the amygdala and the auditory cortex, especially in relation to auditory learning, memory and auditory cortical plasticity, which is thought to be mediated via the BFCS.76,77 In sum, rather than displaying a simple rostral-caudal relationship, these algorithmically identified clusters potentially reflect a functional network and provide a scaffolding for the cholinergic system to co-modulate these functionally and anatomically related regions.
Visual network
Cortical visual processing is modulated by behavioral context; in V1 visual stimulation, evoked responses are modulated by attention,78,79,80,81 expectation,82,83 reward,84,85 locomotion86,87 and auditory processing.88 In mice, direct cortico-cortical connections were proposed to play a role in the alteration of visual perception during specific behavioral tasks. Yet, the behavioral states during which the modulations occur overlap with brain states known to be regulated by cholinergic cortical neuromodulation such as arousal,89 attention,90 and information processing.91,92,93,94,95 This suggests that cholinergic signaling interacts directly or indirectly (through the modulation of local inhibition) with the cellular mechanisms responsible for the integration of top-down feedback and bottom-up visual inputs. The cholinergic system facilitates activity propagation from V1 to the frontal cortex, and this process is linked to the conscious detection of visual stimuli referred to as global ignition.11
Conspicuously, in the correlation matrix of Figure 3 and the rearranged matrix of Figure 4 V1-A1 spatial density correlation coefficients are higher than average (0.3) and the outlying V1 gravitates toward the auditory cluster (Figure 6; Figure S5) in eigenvalues of the PCA1, corroborating the observation that the distribution of (caudal) V1 projecting cholinergic neurons overlaps with the distribution of auditory projecting cholinergic cells (Figure 7). This overlap may underpin cross-modal sensory integration at the level of BFCS, as evident from experiments demonstrating the alteration of visual feature analysis in V1 by A1 auditory input.88
Our earlier anatomical data29 and the current demonstration of the hierarchical organization of Ch projection strongly support the notion that the BFCS may provide a scaffolding for coordinated activity between remote yet associated cortical areas. It is hypothesized that contextual information provided by the hippocampus can trigger the recall of a past event during episodic memory through the activation of the mPFC neuronal ensembles.96 This interaction may be, in part, mediated by the cholinergic system. Indeed, results show concurrent coordinated ACh release in PFC and hippocampus97 during training on a rewarded working memory task, and this finding corroborates that the cholinergic branches to the hippocampus and the mPFC (PrL and IL) have a cophenetic and Euclidian distance of 1.8 (the max distance is 4.3, Table S3) and are relatively close in the dendrogram (Figure S4).
Across-cluster correlations
It is interesting that PFC and OFC are in different large clusters: OFC (VO, LO in our analysis) belongs to the somatic sensory motor network, and PFC (IL, PrL) to the visual network. Table 3 lists Euclidian and cophenetic distances, two measures of the contextual similarity/dissimilarity based on cell density and projection target. These measures between PFC and OFC are relatively large, yet these regions are physically close. Interestingly, OFC and PFC are also close in the developmental flat map of Swanson98 and in the modified Swanson schema by Puelles.99 However, connectionally, the OFC belongs to a different network from that of the PFC in rodents and primates, including humans.100,101 Furthermore, we ascertained the correlation between the average pairwise distances of cholinergic neurons in the BF and the average pairwise distances of PFC-OFC injection site centroids in the cortex to elucidate the topographic mapping between the cortex and BFCS; however, we did not find evidence for a topographic mapping between the BFCS and cortical injection sites that would explain across-cluster correlations between PFC and OFC (Table S4).
Clustered organization: A functional interpretation
As discussed in a symposium review,102 in vivo electrophysiological data,103 including experiments from our laboratory,104 suggest that the forebrain cholinergic system is capable of behavior-dependent modulation of cortico-cortical functional connectivity, enabling information exchange between interconnected cortical regions. It was noted already in a review more than 30 years ago,105 that certain afferents to BF Ch neurons contact widespread portions of the system while other inputs target relatively restricted portions of the BFCS. Generalized behavioral functions such as sleep/wake cycles may be mediated in part through relatively diffuse inputs, such as noradrenergic afferents from the locus coeruleus.106 Indeed, the structural connectivity between the noradrenergic and cholinergic systems affects the network dynamics required to support adaptive behavior based on human imaging data.107 Restricted afferents, including peptidergic and other forebrain inputs, may be related to more specific cortical processes.108 In a recent study, we identified inputs to cholinergic cell groups projecting to M1/M2, OFC, mPFC,30 entorhinal cortex, and the hippocampus. Accordingly, the BF Ch neurons targeting each of these cortical areas receive a specific combination of afferents. Cholinergic projection neurons to each cortical area show a specific distribution pattern in the defined BF compartments. It is obvious that most of the 30 cortical areas receive their cholinergic input from more than one BF compartment (see Figure 7; Table S5), though it is unclear what input combination reaches neurons in the algorithmically identified cholinergic clusters.
It is possible that the momentary combination of active inputs to cholinergic neurons could select specific Ch nodes according to the hierarchical blueprint (Figures 4 and 5B). This organization may support a mechanism by which the behavior-dependent modulation of specific cognitive processes is aligned with specific input-output wiring30 and selective dendritic orientation34,58 patterns of designated cholinergic cell groups. It is unclear how (i) specific inputs to Ch cells, (ii) local collaterals among Ch cell neurons in the BF, (iii) cortical axonal arborization of Ch cells, in addition to (iv) secondary non-cholinergic connections participate in this mechanism. According to ours and an earlier study109 using well-localized small retrograde tracer injections into homolog areas of S1, S2, and M1, only 1–2% of the projection neurons were double-labeled. While it is obscure how cholinergic clusters participate in the coordination of cognitive processes, our article provides a unified conceptual framework of a hierarchically organized network of nodes to furnish the testing of various functional hypotheses.
Recent studies using ACh indicators and large-scale imaging in mice demonstrate,11 in contrast to the global influence of ACh throughout the cortex,110 that various behavior states are associated with distinct spatiotemporal patterns of ACh release in the cortex. Moreover, cholinergic signaling is independent across different cortical areas,111 consistent with the hierarchical, segregated network model presented in this article. Another study in rats using DREADD technology combined with in vivo resting-state fMRI112 showed that the selective activation of the cholinergic neurons in the BF, resulted in a reduction of resting-state neural activity in the default network, including the cingulate (Cg) and retrosplenial (RS) cortex, but did not change activity in the task-positive networks represented by seed regions in the S1, S2, M1, and M2 cortex (belonging to the SSM network in our article). Again, these results support our data that cholinergic branches in the Cg/RS cortex are separated from the cholinergic clusters in the SSM.
Clustered organization: Potential significance for the progression of pathology in the basal forebrain
Extensive postmortem histology work from the 1980s18 and recent in vivo clinical research on Alzheimer’s (AD)19,113,114 and Parkinson’s115 has demonstrated that degeneration in the BF is closely linked to cognitive impairments in both early AD and PD. The ex vivo localization of putative cholinergic cells in various compartments of the human BF and co-registering them into the MNI single subject reference brain (Colin27) greatly facilitates correlating structural changes with cognitive symptoms.18 These studies suggest a spatial topography of vulnerability in the BF.114 Emerging evidence suggests that the structural degeneration of BF neurons occurs much earlier than was previously thought and may, in fact, precede, predict, and even potentiate cortical degeneration.20 While aggregates of putative cholinergic cells can be easily delineated in postmortem brains using this 3D mask, validated in human imaging studies,116 no systematic, longitudinal postmortem data are available for clarifying whether subgroups of patients with AD with different symptoms show the degeneration of different cell clusters. For example, using a canonical correlation analysis of patients with ADNI, our preliminary analysis discovered that only a subgroup of patients diagnosed with AD has serious memory symptoms, but another group of persons who were also diagnosed with AD showed better than average memory tests but poor language symptoms (Ardekani and Zaborszky, 2022, AAIC Abstract). Considering the hierarchical organization of the Ch system in rodents, it would be important to re-investigate the pathology in neurodegenerative diseases affecting the human BF if the progression of degeneration in the BF follows a trend corresponding to the hierarchical organization described.
Limitations of the study
We argue that the dendrogram in Figures 4 and 5 represents the blueprint of information flow. However, to prove that cholinergic neurons form spatially segregated clusters in the BF to enable spatially independent modulation of (cholinergic) activity by its afferents requires further functional studies. For instance, the binary correlation matrix of Figure 3 may suggest that high spatial correlation between cholinergic neurons projecting to two functionally interrelated cortical targets may occupy specific and exclusive spatial locations in the BF (e.g., PrL and RS with 0.83 Pearson’s correlation coefficient) separated from other projection neurons; this is not the case. The same space contains many other cells with lower densities and correlations. In the listed example, the same space where RS and PrL projection neurons dominate, these neurons are intermingled with V1, V2, and Cg innervating cholinergic neurons. Another limitation of our study is that we used only male rats, which limits the research’s generalizability.
Resource avalability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Laszlo Zaborszky (laszloz@newark.rutgers.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
Data: Cell population vectorial, image and descriptive metadata have been deposited to Zenodo and are publicly available as of the date of the publication. The descriptive data contains the projection cell’s cortical target label, experimental brain identifier, image positional, and image scale data. Images are scans of 50-micron Nissl-stained sections without any gap. DOIs are listed in the key resources table. (https://doi.org/10.5281/zenodo.14502163).
-
•
Code: All original codes for data preprocessing and analyzing have been deposited at Zenodo and are publicly available as of the date of publication. DOIs are listed in the key resources table (https://doi.org/10.5281/zenodo.14502163).
-
•
Additional information: Any additional information required to reanalyze the data reported in this article is available from the lead contact upon request.
Acknowledgments
We thank Drs. Dave Sullivan, Drew Headley and Pierre-Olivier Pollack for reading an earlier version of the article. This work was supported by NIH/NINDS RF1NS023945–28 and R01NS023945 to L.Z.
Author contributions
L.Z. conceived the study, provided funding, and wrote the article with input from Z.N. P.V. wrote the programs for data analysis and analyzed the data. C.C., M.R.G., and H.K. performed some of the experiments. C.C. helped with histology and read an earlier version of the article. P.G. participated in the analysis of initial data and read the article.
Declaration of interests
Z.N. holds options in Zeto, Inc., Santa Clara, CA, and in Visie, Inc., Austin, TX.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Goat anti-Choline-acetyltransferase | EMD Millipore | RRID: AB_2079751 |
| Cy3 donkey anti-goat IgG | Jackson ImmunoResearch | RRID: AB_2307351 |
| Bacterial and virus strains | ||
| AAV-EF1a-FLEX-TVA-mCherry | UNC vector core | |
| AAV-CA-FLEX-RG | UNC vector core | |
| EnvA G-deleted rabies e-GFP | Salk Institute GT3 | 32635 |
| Chemicals, peptides, and recombinant proteins | ||
| Ketamine | Covetrus | 11695-0703-1 |
| Phosphate buffer | Fisher Scientific | S369–500, S375-500 |
| Paraformaldehyde | Alfa Aesar | A11313.OE |
| Fluoro-Gold | Fluorochrome, LLC | Fluorochrome, LLC |
| Fast Blue | Polysciences, Inc | Polysciences, Inc |
| Deposited data | ||
| Visualization of experimental 3D data | http://zaborszkylab.org/3DCholinergicSpace/ | |
| Experimental models: Organisms/strains | ||
| Rat: Sprague-Dawley | Charles River Laboratories | 001 |
| Rat: ChAT::Cre | Gift from Dr. K. Deisseroth, Stanford University | Were backcrossed with wild-type Long-Evans (Harlan) |
| Software and algorithms | ||
| NeurolucidaR | MBF Bioscience | v.7.50.4 |
| PostGIS | Open Geospatial Consortium | https://postgis.net/ |
| PostgreSQL | PostgreSQL Global Development Group | https://www.postgresql.org/ |
| ImageJplugin (Java) | NIH | https://imagej.nih.gov/ij/ |
| QGIS mapping client | Open Source Geospatial Foundation (OSGeo) | https://www.qgis.org/ |
| R statistical framework | The R Foundation | https://www.r-project.org/ |
| Silhouette method | Rousseeuw59 | |
| Pvclust | Suzuki and Shimodaira41 | https://github.com/shimo-lab/pvclust |
| Images, vectorial cell data and related software code | Data: https://doi.org/10.5281/zenodo.14502163 Code:https://doi.org/10.5281/zenodo.14588790 |
|
Experimental models and study participant details
Animals were treated in accordance with the National Research Council’s ‘‘Guide for the Care and Use of Laboratory Animals.’’ Experiments were performed with the approval of the Institutional Animal Care and Use Committee of Rutgers University (PROTO201800148) and by Penn State University College of Medicine. Data from Sprague-Dawley29,37 and ChAT::Cre male rats117 were used as described in the original papers.21,30 Rats were housed in cages of one to three animals on a 12-hr-light/12-hr-dark cycle at Rutgers University. Another group of Sprague-Dawley rats were housed at the Department of Neuroscience and Anatomy at Penn State University College of Medicine in Hershey, Pennsylvania under a collaborative agreement with Prof. Kevin Alloway. Code of the animals, treatment procedures, data related to injection sites, and so forth are summarized in Table S1.
Method details
Surgeries, tracer injections (Fast Blue [FB], Fluoro-Gold [FG] or virus injections)
Each animal was anesthetized with an intramuscular dose of ketamine (20 mg/kg) and xylazine (6 mg/kg) or with isoflurane (1–4%) inhalation in O2. Its head was immobilized in a standard stereotaxic frame, and it received atropine methyl nitrate (0.05 mg/kg, intramuscular), chloramphenicol (50 mg/kg, intramuscular), and dexamethasone (0.5 mg/kg, intramuscular) to aid in ventilation and to help prevent subsequent infection and inflammation.
Electrophysiological recording and stimulation were used to select appropriate sites in the somatosensory, motor, and auditory cortices. For somatosensory cortex injections, specific vibrissae regions were stimulated with fine sticks as a recording electrode was lowered into the cortex to localize sensory activity.39 The somatotopic representation in the motor cortex was determined by using low intensity intracortical microstimulation and was administered to evoke brief twitches of muscles controlling the limbs or whiskers as described.39 The auditory cortex was identified with tungsten micro electrodes lowered to layers IV-V of auditory cortex.21 A1 was defined by a general progression of low to high characteristic frequency (CF) along the posterior to anterior axis. Anterior placements that resulted in a reversal in the CF progression were defined as lying in the anterior auditory field (AAF43). The posterior dorsal auditory field (PDAF43) belt area was identified as more responsive to noise than to tone. The suprarhinal auditory field (SRAF44,45), was identified as the auditory responsive region just above the rhinal sulcus.
Following the electrophysiological identification of selected representations in the somatosensory, motor, and auditory cortices, Fast Blue (FB; Polysciences, Warrington, PA) was deposited by pressure using glass pipettes (tip diameter, 40–80 μm) injections and Fluoro-Gold (FG; Fluorochrome, Denver, CO) by iontophoresis (negative, pulsed DC current, 7 μA; 7-s on–off cycles; 20 min), both 2% solution in purified water. FB and FG injections in the prefrontal,29 insular,29 perirhinal,37 postrhinal,37 entorhinal cortex37 were done only using topographic coordinates of the Paxinos/Watson118 atlas without electrophysiological identification and has been described earlier. Additional FB or FG injections were deposited in the M2, S2 and retrosplenial cortex using atlas coordinates. Visual cortical sites were located via intracortical local field recordings time-locked to stimulus presentation. Visual cortical responsiveness was queried using a 3M NaCl micropipette during presentation of strobe light stimulation of the contralateral visual field. Visually responsive sites received iontophoretic injection of FB.
After a survival period of 6–10 days, the animals were deeply anesthetized with isoflurane supplemented with urethane (1.5 mL of a 0.35 g/mL solution) and transcardially perfused with 0.9% saline, followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB). The brains were removed from the skull, postfixed overnight in the same fixative, and stored in 0.1 M phosphate buffer with 30% sucrose. Similar procedures were followed for anesthesia, postoperative care, and perfusion in both Universities. Brains with somatic sensory and motor injections (n = 35) were shipped from Penn State University College of Medicine to Rutgers University in PB with 30% sucrose between 2001 and 2010.
Virus injections
Rabies monosynaptic virus tracing119 was used in Chat:Cre transgenic rats expressing Cre under the choline acetyltransferase promoter.117 Rats were anesthetized with isoflurane (Isothesia, Henry Schein), and helper viruses120 (AAV-EF1a-FLEXTVA-mCherry and AAV-CA-FLEX-RG, UNC vector core) were first injected in the BF area that is enriched in auditory cortically projecting BF cholinergic cells.21 In rats that were designed to study cholinergic projections to mPFC, orbitofrontal, motor cortex and the amygdala,30 the helper viruses were injected via micropipette across five locations filling the right BF. The helper virus leads to the expression of mCherry fluorescent marker, TVA viral receptor, and rabies virus envelope glycoprotein (RG) in a Cre-dependent manner in cholinergic cells. Following 21 days of recovery, rats were again anesthetized (see above), and the specific cortical areas were injected with modified rabies pseudotyped with the avian virus envelope (EnvA G-deleted rabies e-GFP117 Salk Vector Core). Prior to virus injections, the auditory cortex was electrophysiologically identified as described above. In all other cases, cortical targets were identified only using topographic coordinates of the Paxinos/Watson atlas.118 We limited the size of the rabies injection to prevent any spillover into adjacent cortical regions. In each case, a 0.2 μL injection was made in each identified cortical region. The modified rabies restricts infection to TVA receptor-expressing cells. The rabies virus lacks the RG necessary for transynaptic spread of the virus. This procedure results in specific infection of mCherry/TVA-expressing cholinergic cells (previously infected with helper viruses) and restricts transynaptic spread of the rabies virus to cells monosynaptically connected to these cholinergic neurons.120 Double-labeled e-GFP (modified rabies) and mCherry (helper virus) cells are cholinergic neurons (starter cells) that projected to the target cortical region, whereas e-GFP-positive singly labeled cells indicate monosynaptic (afferent) input to the starter cells as described.21,30
Tissue preparation, immunofluorescence, plotting labeled cells, Nissl staining and delineation of BF areas
50 μm-thick coronal sections were cut using a sliding microtome. Sections were divided into four series, so that a brain may be represented by every fourth section, with a 200 μm distance between adjacent sections. Two of the four series were processed for immunocytochemically identified cholinergic neurons, using fluorescently tagged antibodies against choline-acetyltransferase (ChAT). To confirm specificity of viral targeting, sections from 2 cases of helper virus-injected animal were immunostained for ChAT. For details on tissue processing, see the original papers. A computer-microscopy system equipped with the NeurolucidaR software (v.7.50.4 MBF Bioscience, MicroBrightField, Inc.) was used to trace all sections and significant contours (i.e., Corpus Callosum, Lateral Ventricle, Lateral Olfactory Tract, Optic Chiasm, Anterior Commisure, Internal Capsule, Third Ventricle, Dorsal Third Ventricle, Hippocampus, Fornix, Stria Terminalis, Stria Medullaris, Caudate Nucleus and Zona Incerta), and an epifluorescence microscope was used to visualize and map all retrogradely labeled cells for FB, FG and ChAT present in the BF. After plotting, the coverslips were removed, and the same slides were prepared with a thionin Nissl stain. Nissl-stained sections were illuminated with bright-field and aligned to existing images or to existing Neurolucida maps previously created under fluorescence imaging. Nissl-images of the sections with cholinergic projection neurons were scanned and warped to the template brain (see Figure 1). BF cytoarchitectonic boundaries were drawn on the template brain sections using the QGIS program for comparing the number of neurons in specific BF regions projecting to specific ontologically identified cortical regions (see: “delineations of BF compartments”).
Database, annotation, software
We used PostGIS plugin framework around Postgres database, a relational database management system to store image data and corresponding vectorial data, including cell locations and structure delineations. This solution provides us with a complete set of functions for analyzing geometric components, determining spatial relationships, and manipulating delineations. For 2D data visualization we used the QGIS Mapping Client. This framework provides us with very fast and smooth visualization of high-resolution images as well as vectorial data.
Image and vectorial data registration
We created an image registration tool (Java) for anatomic image and vectorial data registration. We extended an ImageJ plugin to be suitable for this purpose.120 This tool can use delineations as well as image data to guide the registration and can communicate directly with the Postgres database. The tool is using the combination of affine and BSpline-based elastic transformation for registration. The registration process starts with section matching. Each section of the experimental brain is visually matched with the corresponding section in the reference brain. Since there were differences in terms of cutting angle between experiments, we created a special tool to aid section matching. This tool (Java/ImageJ) can generate high resolution virtual sections of the reference brain based on user inputs by cutting it in different angles real time and provide the high-resolution image that matches with the experimental brain section image. The used cutting angle has been considered during the final computation of cell positions in the reference brain. After section matching, we visually inspect identical reference points on both the experimental and reference section. Based on those reference points we apply the registration transformation. We registered 106 cortical injections in 73 brains into a single reference brain using the registration tool described above. Fluorescent-labeled cholinergic and non-cholinergic neurons were mapped in the BF using the NeurolucidaR (NL, MBF Bioscience) data-acquisition system. After mapping, coverslips were removed and sections were Nissl-stained, realigned with the corresponding maps, and scanned using NL system. To validate the registration process of experimental sections we compared the registered cytoarchitectonic zones to their correspondent zone on the reference section they registered into. On randomly selected sections we used the Dice coefficient121 to measure the difference,
| (Equation 1) |
where X, Y are arbitrary geometries and A is the area function (Figure S3).
Injection sites in paxinos-coronal-space
First, we adjusted the images of the Paxinos-atlas with the corresponding outline and registered the injections sites from the Nissl-images of the brain sections using elastic transformation. We measured the participation of injection site I in cortical region R by dividing the volume of their intersection by the volume of I (Equation 2). Additionally, we expressed how much injection site‘A’is covered by injection site ‘B’ by measuring the volume of intersection of injection site A with injection site B and divide it by the volume of injection site A (Equation 3).
| (Equation 2) |
| (Equation 3) |
Construction of spatial correlation matrices
Projection cholinergic neurons from all experimental brains were registered to the reference brain. Originally, we collected 7,401 cholinergic projection neurons, however, 1,727 cholinergic neurons were sampled from cases, where the motor or the S1 cortex was identified topographically without electrophysiology. To avoid nested cases in the hierarchical analysis, we removed these ‘unidentified’ cholinergic neurons. We sampled the BF cholinergic space at multiple points to determine the composition of cortical projection neurons according to their cortical targets. The sampling points were the projection neuron positions in the BF. We defined a sphere of 300 μm radius around each projection neuron (n = 5,674) and counted the number of neighbors projecting to any cortical injection sites. The injection sites were grouped according to 30 ontological categories (Table S2). Thus, for each sampling point, we made 30 measurements at its possible target projections, which provided a target-projection-vector (TPV) of 30 integer values. Then, we combined these vectors into a matrix where each row represented a TPV with the number of cortical targets shared within the proximity of the sampling location. When counted the projection neurons around each cell, we also had to account for overlapping injection sites. We applied a normalization to compensate for the multiple labeling of the same projection area. At each measurement point of the cholinergic cell space, we checked whether any neighboring cell projected to cortical targets with overlapping injection sites and computed a normalization constant (Equation 4) to be applied to the TPV. This normalization constant (Cj) was defined for a given injection site Ij covering cortical region R as:
| (Equation 4) |
where IR is the set of injections covering cortical region R, and V is the volume of injections.
At any given sampling location p, we calculated the normalized number of projection neurons of projection population j
| (Equation 5) |
where N p,j is the original number of projection neurons from the cholinergic population j, and Cj is the normalization constant (Equation 4).
After normalization, the dataset was ready to be analyzed for the composition similarity of Ch ensembles regarding their cortical projection patterns. To do that, we computed Pearson’s correlations (Equation 6) between all pairs of column vectors (30 x 5,674) except the column vector itself, resulting in a 30 x 30 matrix of Figure 3 representing the most likely composition of neurons within 300 μm ensembles of neurons identified by their cortical projections.
| (Equation 6) |
For visualization purposes, we rendered negative, zero and positive correlations with the shades of blue, white and red, respectively. Red areas represent pairs of cortical areas sharing similar composition of cholinergic neurons projecting to them and other cortical areas from local ensemble of Ch neurons in the BF. Next, we applied hierarchical clustering122 to the correlation matrix using Euclidian distance and MAX (complete) linkage using the open-source R statistical framework. The program first generates the distance matrix between the rows. Each row is handled as a single cluster. The algorithm is iterative and at each iteration it tries to find the closest pair of clusters - according to MAX linkage - and merge them into one. At each iteration point the program reorganizes the matrix rows so that the in between cluster will be minimal. As a result, the final matrix was organized to reflect the compositional similarity of Ch neurons of the BF in terms of the similarity of their projection patterns to cortical targets.
Quantification and statistical analysis
Hierarchical clustering generates dendrograms no matter how segregated and hierarchically organized the dataset is. To assert the level of uncertainty associated with the clusters we used pvclust in R package41 that implemented multiscale bootstrap resampling. This method associates a p value (0 < p < 1) with each branch based on estimating how unlikely is to obtain a given cluster configuration by-chance after random resampling of the dataset. In Figure S5 is the P-values extended dendrogram from Figure 4. The red numbers at branching points represent Approximate Unbiased p values.40 We used cophenetic correlation123 to measure how faithfully the dendrogram preserves the pairwise Euclidean distances between row vectors of correlation coefficients for each cortical area. Cophenetic distance between two leaves on a dendrogram is the intergroup dissimilarity at which the two are first combined into a single cluster. We used ‘complete linkage’ in our hierarchical clustering method, which is the distance between the outermost data points between two clusters. The cophenetic correlation coefficient (rc = 0.88) is the result of the Pearson correlation between the cophenetic distance and the Euclidean distance for each row vector pair.
Furthermore, to partition the dendrogram we used the Silhouette method,59 which finds the optimal cut of the dendrogram (cluster number) that represent the most coherent cluster separation in our data. The silhouette value shows how similar an object is to its own cluster compared to the other clusters. The silhouette score59 ranges from −1 to +1. A high value indicates that the object well matches the cluster where it belongs and poorly matches the other clusters. For the Silhouette, we compute a score by measuring the object’s average distance (Euclidean) from objects of the other clusters and iterating that for each cluster, including its own. Then, we compare the average of its cluster to the neighboring clusters’ averages. We find the neighboring cluster with the smallest difference and compute the Silhouette score as the ratio of this difference to the absolute average distance of this neighbor. We obtained the highest silhouette score (s = 0.55) when we cut the dendrogram into three parts, hence confirming the 3 main clusters also apparent by visual observation (Figure S6).
Visualization methods
The aim is to find high-density spaces for single target populations. Using TPV (see above), we assess the normalized projection neuron densities (cell numbers around each projection cell within its 300 μm radius) at all sampling points (n = 5,674) in the cholinergic space. We computed the average neuron density for each target across all sampling points. Next, we visualize all the sampling points where the density of a specific target is above its average (Figures 5B and 7).
Nomenclature of cortical and basal forebrain areas (ontology hierarchies). Delineations of compartments in the basal forebrain
Table S2 lists in column A cortical regions based on cytoarchitectonic delineations. Columns B-G lists categories where a specific region is encompassed in larger and larger volumes. We used in our work mostly the terms and abbreviations defined in column B. While we tried to adhere as much as possible to terms used in the fifth edition of the Paxinos/Watson atlas,118 we applied a few updated terms based on our studies using electrophysiology in the auditory, somatic sensory and motor cortical areas. For example, body parts in M1 and S1 cortex were specified using intraoperative electrophysiology. Auditory cortical areas were designated as A1, AAF, PDAF, SRAF as defined electrophysiologically as explained under “FB and FG tracer injections in cortical areas.” Table S1 in column G explains the percentage involvement of injections in cortical areas according to the Paxinos/Watson atlas118 that was used for registering injection sites. For example, injection case 13082FG (yellow highlighted in Table S1) was designated as located in the AAF cortex, this injection encompasses parts of the secondary dorsal auditory area, barrel field, S1 upper lip and S2 regions as delineated in the Paxinos/Watson atlas (compare Figure S1; Table S1). Perirhinal (PER, areas 35 and 36) and postrhinal (POR) areas were defined as Burwell and Amaral.124 MEC and LEC for the medial and lateral entorhinal areas are used as defined by Witter et al.125
Delineations of BF compartments
On the Nissl-series of the template brain, using the tracing feature of the QGIS program on each 50 μm sections, BF structures, including MSVDB, HDB, VP, GP, SIB, EA, EAM, AL, ‘ic’ and “a” (see list of abbreviations below) were delineated, while projection cholinergic neurons were hidden, thus not to be biased by the presence of cholinergic neurons that in fact do not observe artificial borders. In general, we followed letter acronyms used in the Paxinos-Atlas. Structure ‘a’ is the caudal continuation of HDB. Additional to the original Nissl-stained sections of the template brain, while viewing them in the microscope, brain sections stained for Calbindin, Calretinin, Parvalbumin, Glutamic Acid Decarboxylase, Enkephalin, Substance P, Cholecystokinin, Vaso Intestinal Peptide, Luxol Fast Blue Cresyl Violet and Timm-staining were consulted. The border between GP and ‘ic’ is based upon the presence of compact fibers; as the arrangement of fibers became less compact and the large neuron of the GP appears, we designated the structure as GP.
List of Abbreviations
a = caudal continuation of HDB
A1 = primary auditory cortex
A2 = secondary auditory cortex
AAF = anterior auditory field
Ach = acetylcholine
Agl = lateral agranular (frontal) cortex
AIP = agranular posterior insular cortex
AL = nucleus of the ansa lenticularis
BF = basal forebrain
BFC = basal forebrain cholinergic
BFCS = basal forebrain cholinergic system
Cg1 = cingulate cortex
Ch = cholinergic
ChAT = choline acetyltransferase
AIP = anterior insular cortex, posterior part
EA = extended amygdala
FB = Fast Blue
FG = Fluoro-Gold
GP = globus pallidus
HDB = horizontal limb of the diagonal band
ic = internal capsule
IDIGI = insular cortex, dysgranular and granular parts
IL = infralimbic cortex
LEC = lateral entorhinal cortex
LO = lateral orbital cortex
M1 = primary motor cortex (Agl, PrCl, earlier used abbreviations)
M1FL = primary motor cortex, forelimb
M1-hindlimb = primary motor cortex, hindlimb (M1-HL)
M1-whisker = primary motor cortex, whisker (M1Wh)
M2 = secondary motor cortex
MEC = medial entorhinal cortex
mPFC = medial prefrontal cortex
MSVDB = medial septum-vertical limb of the diagonal band
OFC = orbitofrontal cortex
PDAF = posterior dorsal auditory field
PER = perirhinal cortex
PFC = prefrontal cortex
POR = postrhinal cortex
PrCl = lateral precentral area
PrL = prelimbic cortex
RS = retrosplenial cortex
S1 = primary somatosensory cortex
S1-orofacial = primary somatosensory cortex, orofacial area (S1Or)
S1FL = primary somatosensory cortex, forelimb
S1HL = primary somatosensort cortex, hindlimb
S1Tr = primary somatosensensory cortex, trunk
S1-whisker = primary somatosensort cortex, whisker (S1Wh)
S2 = secondary somatosensory cortex
SDC = spatial density correlation
SPL = sound pressure level
SRAF = suprarhinal auditory field
SSM = somatic sensory-motor network
SIB = substantia innominata, basal part
TPV = target-projection vector
V1 = primary visual cortex, monocular area
V2 = secondary visual cortex, mediolateral area
VDB = vertical limb of the diagonal band
VO = ventral orbital cortex
VP = ventral pallidum
Published: February 11, 2025
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2025.112001.
Supplemental information
Categories in Column B (in red) are used in this article. Column C–G contain increasingly larger volumes that encompass volume in previous order.
References
- 1.Minces V., Pinto L., Dan Y., Chiba A.A. Cholinergic shaping of neural correlations. Proc. Natl. Acad. Sci. USA. 2017;114:5725–5730. doi: 10.1073/pnas.1621493114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prado V.F., Janickova H., Al-Onaizi M.A., Prado M.A.M. Cholinergic circuits in cognitive flexibility. Neuroscience. 2017;345:130–141. doi: 10.1016/j.neuroscience.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Conner J.M., Kulczycki M., Tuszynski M.H. Unique contributions of distinct cholinergic projections to motor cortical plasticity and learning. Cerebr. Cortex. 2010;20:2739–2748. doi: 10.1093/cercor/bhq022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballinger E.C., Ananth M., Talmage D.A., Role L.W. Basal Forebrain Cholinergic Circuits and Signaling in Cognition and Cognitive Decline. Neuron. 2016;91:1199–1218. doi: 10.1016/j.neuron.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ananth M.R., Rajebhosale P., Kim R., Talmage D.A., Role L.W. Basal forebrain cholinergic signalling: development, connectivity and roles in cognition. Nat. Rev. Neurosci. 2023;24:233–251. doi: 10.1038/s41583-023-00677-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bottorff J., Padgett S., Turrigiano G.G. Basal forebrain cholinergic activity is necessary for upward firing rate homeostasis in the rodent visual cortex. Proc. Natl. Acad. Sci. USA. 2024;121 doi: 10.1073/pnas.2317987121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mishra J., Fellous J.M., Sejnowski T.J. Selective attention through phase relationship of excitatory and inhibitory input synchrony in a model cortical neuron. Neural Netw. 2006;19:1329–1346. doi: 10.1016/j.neunet.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tiesinga P., Fellous J.M., José J., Sejnowski T.J. Optimal information transfer in synchronized neocortical neurons. Neurocomputing. 2001;38–40:397–402. [Google Scholar]
- 9.Schmitz T.W., Duncan J. Normalization and the Cholinergic Microcircuit: A Unified Basis for Attention. Trends Cognit. Sci. 2018;22:422–437. doi: 10.1016/j.tics.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Xu M., Chung S., Zhang S., Zhong P., Ma C., Chang W.C., Weissbourd B., Sakai N., Luo L., Nishino S., Dan Y. Basal forebrain circuit for sleep-wake control. Nat. Neurosci. 2015;18:1641–1647. doi: 10.1038/nn.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li B., Ma C., Huang Y.A., Ding X., Silverman D., Chen C., Darmohray D., Lu L., Liu S., Montaldo G., et al. Circuit mechanism for suppression of frontal cortical ignition during NREM sleep. Cell. 2023;186:5739–5750. doi: 10.1016/j.cell.2023.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Hangya B., Ranade S.P., Lorenc M., Kepecs A. Central Cholinergic Neurons Are Rapidly Recruited by Reinforcement Feedback. Cell. 2015;162:1155–1168. doi: 10.1016/j.cell.2015.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrison T.C., Pinto L., Brock J.R., Dan Y. Calcium Imaging of Basal Forebrain Activity during Innate and Learned Behaviors. Front. Neural Circ. 2016;10:36. doi: 10.3389/fncir.2016.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo W., Robert B., Polley D.B. The Cholinergic Basal Forebrain Links Auditory Stimuli with Delayed Reinforcement to Support Learning. Neuron. 2019;103:1164–1177. doi: 10.1016/j.neuron.2019.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crouse R.B., Kim K., Batchelor H.M., Girardi E.M., Kamaletdinova R., Chan J., Rajebhosale P., Pittenger S.T., Role L.W., Talmage D.A., et al. Acetylcholine is released in the basolateral amygdala in response to predictors of reward and enhances the learning of cue-reward contingency. Elife. 2020;9 doi: 10.7554/eLife.57335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robert B., Kimchi E.Y., Watanabe Y., Chakoma T., Jing M., Li Y., Polley D.B. A functional topography within the cholinergic basal forebrain for encoding sensory cues and behavioral reinforcement outcomes. Elife. 2021;10 doi: 10.7554/eLife.69514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimchi E.Y., Burgos-Robles A., Matthews G.A., Chakoma T., Patarino M., Weddington J.C., Siciliano C., Yang W., Foutch S., Simons R., et al. Reward contingency gates selective cholinergic suppression of amygdala neurons. Elife. 2024;12 doi: 10.7554/eLife.89093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zaborszky L., Hoemke L., Mohlberg H., Schleicher A., Amunts K., Zilles K. Stereotaxic probabilistic maps of the magnocellular cell groups in human basal forebrain. Neuroimage. 2008;42:1127–1141. doi: 10.1016/j.neuroimage.2008.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cantero J.L., Zaborszky L., Atienza M. Volume Loss of the Nucleus Basalis of Meynert is Associated with Atrophy of Innervated Regions in Mild Cognitive Impairment. Cerebr. Cortex. 2017;27:3881–3889. doi: 10.1093/cercor/bhw195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmitz T.W., Zaborszky L. In: Handbook of Clinical Neurology, The Human Hypothalamus: Anterior Region. Swaab F., editor. Elsevier; 2021. Spatial topography of the basal forebrain cholinergic projections: Organization and vulnerability to degeneration; pp. 159–173. [DOI] [PubMed] [Google Scholar]
- 21.Chavez C., Zaborszky L. Basal Forebrain Cholinergic-Auditory Cortical Network: Primary Versus Nonprimary Auditory Cortical Areas. Cerebr. Cortex. 2017;27:2335–2347. doi: 10.1093/cercor/bhw091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saper C.B. In: Handbook of Physiology, The Nervous System, Section 1 Vol 5. Plum F., Geiger S., editors. American Physiological Society; 1987. Diffuse cortical projection systems: anatomical organization and role in cortical function; pp. 169–210. [Google Scholar]
- 23.Pfaff D. Path toward Consciousness. Cambridge University Press; 2019. How brain arousal mechanisms work. [Google Scholar]
- 24.Butcher L.L. In: Neurotransmitter Interactions and Cognitive Functions. Levin E., et al., editors. Birkhauser; 1992. The cholinergic basal forebrain and its telencephalic targets: interrelations and implications for cognitive function; pp. 15–26. [Google Scholar]
- 25.Sarter M., Bruno J.P. Trans-synaptic stimulation of cortical acetylcholine and enhancement of attentional functions: a rational approach for the development of cognition enhancers. Behav. Brain Res. 1997;83:7–14. doi: 10.1016/s0166-4328(97)86039-1. [DOI] [PubMed] [Google Scholar]
- 26.Hasselmo M.E. The role of acetylcholine in learning and memory. Curr. Opin. Neurobiol. 2006;16:710–715. doi: 10.1016/j.conb.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarter M. Cholinergic control of attention to cues guiding established performance versus learning: theoretical comment on Maddux, Kerfoot, Chatterjee, and Holland (2007) Behav. Neurosci. 2007;121:233–235. doi: 10.1037/0735-7044.121.1.233. [DOI] [PubMed] [Google Scholar]
- 28.Parikh V., Kozak R., Martinez V., Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron. 2007;56:141–154. doi: 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zaborszky L., Csordas A., Mosca K., Kim J., Gielow M.R., Vadasz C., Nadasdy Z. Neurons in the basal forebrain project to the cortex in a complex topographic organization that reflects corticocortical connectivity patterns: an experimental study based on retrograde tracing and 3D reconstruction. Cerebr. Cortex. 2015;25:118–137. doi: 10.1093/cercor/bht210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gielow M.R., Zaborszky L. The Input-Output Relationship of the Cholinergic Basal Forebrain. Cell Rep. 2017;18:1817–1830. doi: 10.1016/j.celrep.2017.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bloem B., Schoppink L., Rotaru D.C., Faiz A., Hendriks P., Mansvelder H.D., van de Berg W.D.J., Wouterlood F.G. Topographic mapping between basal forebrain cholinergic neurons and the medial prefrontal cortex in mice. J. Neurosci. 2014;34:16234–16246. doi: 10.1523/JNEUROSCI.3011-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allaway K.C., Munoz W., Tremblay R., Sherer M., Herron J., Rudy B., Machold R., Fishell G. Cellular birthdate predicts laminar and regional cholinergic projection topography in the forebrain. Elife. 2020;9 doi: 10.7554/eLife.63249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarter M., Lustig C. Forebrain Cholinergic Signaling: Wired and Phasic, Not Tonic, and Causing Behavior. J. Neurosci. 2020;40:712–719. doi: 10.1523/JNEUROSCI.1305-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaborszky L., Duque A., Gielow M., Gombkoto P., Nadasdy Z., Somogyi J. In: The Rat Nervous System. 4th edition. Paxinos G., editor. Elsevier; 2015. Organization of the basal forebrain cholinergic projection system: specific or diffuse? pp. 491–507. [Google Scholar]
- 35.Kim J.H., Jung A.H., Jeong D., Choi I., Kim K., Shin S., Kim S.J., Lee S.H. Selectivity of Neuromodulatory Projections from the Basal Forebrain and Locus Ceruleus to Primary Sensory Cortices. J. Neurosci. 2016;36:5314–5327. doi: 10.1523/JNEUROSCI.4333-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buzsaki G., Bickford R.G., Ponomareff G., Thal L.J., Mandel R., Gage F.H. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J. Neurosci. 1988;8:4007–4026. doi: 10.1523/JNEUROSCI.08-11-04007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kondo H., Zaborszky L. Topographic organization of the basal forebrain projections to the perirhinal, postrhinal, and entorhinal cortex in rats. J. Comp. Neurol. 2016;524:2503–2515. doi: 10.1002/cne.23967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sorzano C.O.S., Thévenaz P., Unser M. Elastic registration of biological images using vector-spline regularization. IEEE Trans. Biomed. Eng. 2005;52:652–663. doi: 10.1109/TBME.2005.844030. [DOI] [PubMed] [Google Scholar]
- 39.Smith J.B., Alloway K.D. Rat whisker motor cortex is subdivided into sensory-input and motor-output areas. Front. Neural Circ. 2013;7:4. doi: 10.3389/fncir.2013.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shimodaira H. Approximately unbiased tests of regions using multistep-multiscale bootstrap resampling. Ann. Stat. 2004;32:2616–2641. [Google Scholar]
- 41.Suzuki R., Shimodaira H. Pvclust: an R package for assessing the uncertainty in hierarchical clustering. Bioinformatics. 2006;22:1540–1542. doi: 10.1093/bioinformatics/btl117. [DOI] [PubMed] [Google Scholar]
- 42.Kiraly B., Hangya B. Navigating the statistical minefield of model selection and clustering in neuroscience. eNeuro. 2022;94 doi: 10.1523/ENEURO.0066-22.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rutkowski R.G., Miasnikov A.A., Weinberger N.M. Characterisation of multiple physiological fields within the anatomical core of rat auditory cortex. Hear. Res. 2003;181:116–130. doi: 10.1016/s0378-5955(03)00182-5. [DOI] [PubMed] [Google Scholar]
- 44.Polley D.B., Read H.L., Storace D.A., Merzenich M.M. Multiparametric auditory receptive field organization across five cortical fields in the albino rat. J. Neurophysiol. 2007;97:3621–3638. doi: 10.1152/jn.01298.2006. [DOI] [PubMed] [Google Scholar]
- 45.Profant O., Burianová J., Syka J. The response properties of neurons in different fields of the auditory cortex in the rat. Hear. Res. 2013;296:51–59. doi: 10.1016/j.heares.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 46.Cordes D., Haughton V., Carew J.D., Arfanakis K., Maravilla K. Hierarchical clustering to measure connectivity in fMRI resting-state data. Magn. Reson. Imaging. 2002;20:305–317. doi: 10.1016/s0730-725x(02)00503-9. [DOI] [PubMed] [Google Scholar]
- 47.Liu X., Zhu X.H., Qiu P., Chen W. A correlation-matrix-based hierarchical clustering method for functional connectivity analysis. J. Neurosci. Methods. 2012;211:94–102. doi: 10.1016/j.jneumeth.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Miceli M., Husson Z., Ruel P., Layé S., Cota D., Fioramonti X., Bosch-Bouju C., Gronier B. In silico Hierarchical Clustering of Neuronal Populations in the Rat Ventral Tegmental Area Based on Extracellular Electrophysiological Properties. Front. Neural Circ. 2020;14:51. doi: 10.3389/fncir.2020.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sepulcre J., Grothe M.J., Sabuncu M., Chhatwal J., Schultz A.P., Hanseeuw B., El Fakhri G., Sperling R., Johnson K.A. Hierarchical Organization of Tau and Amyloid Deposits in the Cerebral Cortex. JAMA Neurol. 2017;74:813–820. doi: 10.1001/jamaneurol.2017.0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palomero-Gallagher N., Zilles K. In: The Rat Nervous System. 4th Edition. Paxinos G., editor. Elsevier Science & Technology; 2015. Isocortex; pp. 601–625. [Google Scholar]
- 51.Palomero-Gallagher N., Vogt B.A., Schleicher A., Mayberg H.S., Zilles K. Receptor architecture of human cingulate cortex: evaluation of the four-region neurobiological model. Hum. Brain Mapp. 2009;30:2336–2355. doi: 10.1002/hbm.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amunts K., Lenzen M., Friederici A.D., Schleicher A., Morosan P., Palomero-Gallagher N., Zilles K. Broca's region: novel organizational principles and multiple receptor mapping. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caspers S., Schleicher A., Bacha-Trams M., Palomero-Gallagher N., Amunts K., Zilles K. Organization of the human inferior parietal lobule based on receptor architectonics. Cerebr. Cortex. 2013;23:615–628. doi: 10.1093/cercor/bhs048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zingg B., Hintiryan H., Gou L., Song M.Y., Bay M., Bienkowski M.S., Foster N.N., Yamashita S., Bowman I., Toga A.W., Dong H.W. Neural networks of the mouse neocortex. Cell. 2014;156:1096–1111. doi: 10.1016/j.cell.2014.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harris J.A., Mihalas S., Hirokawa K.E., Whitesell J.D., Choi H., Bernard A., Bohn P., Caldejon S., Casal L., Cho A., et al. Hierarchical organization of cortical and thalamic connectivity. Nature. 2019;575:195–202. doi: 10.1038/s41586-019-1716-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Markov N.T., Kennedy H. The importance of being hierarchical. Curr. Opin. Neurobiol. 2013;23:187–194. doi: 10.1016/j.conb.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 57.Wang X.J., Pereira U., Rosa M.G., Kennedy H. Brain connectomes come of age. Curr. Opin. Neurobiol. 2020;65:152–161. doi: 10.1016/j.conb.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zaborszky L., Csordas A., Buhl D.L., Duque A., Somogyi J., Nadasdy Z. In: Computational Neuroanatomy: Principles and Methods. Ascoli A., editor. Humana Press; 2002. Computational anatomical analysis of the basal forebrain corticopetal system; pp. 171–197. [Google Scholar]
- 59.Rousseeuw P.J. Silhouettes: A graphical aid to the interpretation and validation of cluster analysis. Comput. Appl. Math. 1987;20:53–65. [Google Scholar]
- 60.Hoffer Z.S., Arantes H.B., Roth R.L., Alloway K.D. Functional circuits mediating sensorimotor integration: quantitative comparisons of projections from rodent barrel cortex to primary motor cortex, neostriatum, superior colliculus, and the pons. J. Comp. Neurol. 2005;488:82–100. doi: 10.1002/cne.20579. [DOI] [PubMed] [Google Scholar]
- 61.Aronoff R., Matyas F., Mateo C., Ciron C., Schneider B., Petersen C.C.H. Long-range connectivity of mouse primary somatosensory barrel cortex. Eur. J. Neurosci. 2010;31:2221–2233. doi: 10.1111/j.1460-9568.2010.07264.x. [DOI] [PubMed] [Google Scholar]
- 62.Zakiewicz I.M., Bjaalie J.G., Leergaard T.B. Brain-wide map of efferent projections from rat barrel cortex. Front. Neuroinf. 2014;8:5. doi: 10.3389/fninf.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamawaki N., Raineri Tapies M.G., Stults A., Smith G.A., Shepherd G.M. Circuit organization of the excitatory sensorimotor loop through hand/forelimb S1 and M1. Elife. 2021;10 doi: 10.7554/eLife.66836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Colechio E.M., Alloway K.D. Differential topography of the bilateral cortical projections to the whisker and forepaw regions in rat motor cortex. Brain Struct. Funct. 2009;213:423–439. doi: 10.1007/s00429-009-0215-7. [DOI] [PubMed] [Google Scholar]
- 65.Vogt B.A., Miller M.W. Cortical connections between rat cingulate cortex and visual, motor, and postsubicular cortices. J. Comp. Neurol. 1983;216:192–210. doi: 10.1002/cne.902160207. [DOI] [PubMed] [Google Scholar]
- 66.Miyashita E., Keller A., Asanuma H. Input-output organization of the rat vibrissal motor cortex. Exp. Brain Res. 1994;99:223–232. doi: 10.1007/BF00239589. [DOI] [PubMed] [Google Scholar]
- 67.Shibata H., Kondo S., Naito J. Organization of retrosplenial cortical projections to the anterior cingulate, motor, and prefrontal cortices in the rat. Neurosci. Res. 2004;49:1–11. doi: 10.1016/j.neures.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 68.Winer J.A., Schreiner C.E. Springer; New York: 2010. The Auditory Cortex. [Google Scholar]
- 69.Winer J.A., Diamond I.T., Raczkowski D. Subdivisions of the auditory cortex of the cat: the retrograde transport of horseradish peroxidase to the medial geniculate body and posterior thalamic nuclei. J. Comp. Neurol. 1977;176:387–417. doi: 10.1002/cne.901760307. [DOI] [PubMed] [Google Scholar]
- 70.Winer J.A., Diehl J.J., Larue D.T. Projections of auditory cortex to the medial geniculate body of the cat. J. Comp. Neurol. 2001;430:27–55. [PubMed] [Google Scholar]
- 71.LeDoux J.E., Farb C.R., Romanski L.M. Overlapping projections to the amygdala and striatum from auditory processing areas of the thalamus and cortex. Neurosci. Lett. 1991;134:139–144. doi: 10.1016/0304-3940(91)90526-y. [DOI] [PubMed] [Google Scholar]
- 72.Agster K.L., Burwell R.D. Cortical efferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. Hippocampus. 2009;19:1159–1186. doi: 10.1002/hipo.20578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gehrlach D.A., Weiand C., Gaitanos T.N., Cho E., Klein A.S., Hennrich A.A., Conzelmann K.K., Gogolla N. A whole-brain connectivity map of mouse insular cortex. Elife. 2020;9 doi: 10.7554/eLife.55585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Karimi M., Farahani S., Nasirinezhad F., Jalaei S., Mokrian H., Shahbazi A. Does insular cortex lesion cause tinnitus in rats? Iran. J. Basic Med. Sci. 2022;25:1177–1182. doi: 10.22038/IJBMS.2022.63698.14083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.DeAngeli N.E., Fournier D.I., Gulledge A.T., Burwell R.D., Todd T.P., Bucci D.J. Postrhinal cortex contributions to the expression of auditory fear conditioning. Neurobiol. Learn. Mem. 2022;191 doi: 10.1016/j.nlm.2022.107609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chavez C.M., McGaugh J.L., Weinberger N.M. The basolateral amygdala modulates specific sensory memory representations in the cerebral cortex. Neurobiol. Learn. Mem. 2009;91:382–392. doi: 10.1016/j.nlm.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chavez C.M., McGaugh J.L., Weinberger N.M. Activation of the basolateral amygdala induces long-term enhancement of specific memory representations in the cerebral cortex. Neurobiol. Learn. Mem. 2013;101:8–18. doi: 10.1016/j.nlm.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roelfsema P.R., Lamme V.A., Spekreijse H. Object-based attention in the primary visual cortex of the macaque monkey. Nature. 1998;395:376–381. doi: 10.1038/26475. [DOI] [PubMed] [Google Scholar]
- 79.Ito M., Gilbert C.D. Attention modulates contextual influences in the primary visual cortex of alert monkeys. Neuron. 1999;22:593–604. doi: 10.1016/s0896-6273(00)80713-8. [DOI] [PubMed] [Google Scholar]
- 80.McAdams C.J., Reid R.C. Attention modulates the responses of simple cells in monkey primary visual cortex. J. Neurosci. 2005;25:11023–11033. doi: 10.1523/JNEUROSCI.2904-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chalk M., Herrero J.L., Gieselmann M.A., Delicato L.S., Gotthardt S., Thiele A. Attention reduces stimulus-driven gamma frequency oscillations and spike field coherence in V1. Neuron. 2010;66:114–125. doi: 10.1016/j.neuron.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jaramillo S., Zador A.M. The auditory cortex mediates the perceptual effects of acoustic temporal expectation. Nat. Neurosci. 2011;14:246–251. doi: 10.1038/nn.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Keller G.B., Bonhoeffer T., Hübener M. Sensorimotor mismatch signals in primary visual cortex of the behaving mouse. Neuron. 2012;74:809–815. doi: 10.1016/j.neuron.2012.03.040. [DOI] [PubMed] [Google Scholar]
- 84.Shuler M.G., Bear M.F. Reward timing in the primary visual cortex. Science. 2006;311:1606–1609. doi: 10.1126/science.1123513. [DOI] [PubMed] [Google Scholar]
- 85.Stanisor L., van der Togt C., Pennartz C.M., Roelfsema P.R. A unified selection signal for attention and reward in primary visual cortex. Proc. Natl. Acad. Sci. USA. 2013;110:9136–9141. doi: 10.1073/pnas.1300117110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Niell C.M., Stryker M.P. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron. 2010;65:472–479. doi: 10.1016/j.neuron.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Polack P.O., Friedman J., Golshani P. Cellular mechanisms of brain state-dependent gain modulation in visual cortex. Nat. Neurosci. 2013;16:1331–1339. doi: 10.1038/nn.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ibrahim L.A., Mesik L., Ji X.Y., Fang Q., Li H.F., Li Y.T., Zingg B., Zhang L.I., Tao H.W. Cross-Modality Sharpening of Visual Cortical Processing through Layer-1-Mediated Inhibition and Disinhibition. Neuron. 2016;89:1031–1045. doi: 10.1016/j.neuron.2016.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Phillis J.W. Acetylcholine release from the cerebral cortex: its role in cortical arousal. Brain Res. 1968;7:378–389. doi: 10.1016/0006-8993(68)90004-8. [DOI] [PubMed] [Google Scholar]
- 90.Herrero J.L., Roberts M.J., Delicato L.S., Gieselmann M.A., Dayan P., Thiele A. Acetylcholine contributes through muscarinic receptors to attentional modulation in V1. Nature. 2008;454:1110–1114. doi: 10.1038/nature07141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Disney A.A., Aoki C., Hawken M.J. Gain modulation by nicotine in macaque v1. Neuron. 2007;56:701–713. doi: 10.1016/j.neuron.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Goard M., Dan Y. Basal forebrain activation enhances cortical coding of natural scenes. Nat. Neurosci. 2009;12:1444–1449. doi: 10.1038/nn.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kosovicheva A.A., Sheremata S.L., Rokem A., Landau A.N., Silver M.A. Cholinergic enhancement reduces orientation-specific surround suppression but not visual crowding. Front. Behav. Neurosci. 2012;6:61. doi: 10.3389/fnbeh.2012.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bhattacharyya A., Veit J., Kretz R., Bondar I., Rainer G. Basal forebrain activation controls contrast sensitivity in primary visual cortex. BMC Neurosci. 2013;14:55. doi: 10.1186/1471-2202-14-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Soma S., Shimegi S., Suematsu N., Tamura H., Sato H. Modulation-specific and laminar-dependent effects of acetylcholine on visual responses in the rat primary visual cortex. PLoS One. 2013;8 doi: 10.1371/journal.pone.0068430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Morici J.F., Weisstaub N.V., Zold C.L. Hippocampal-medial prefrontal cortex network dynamics predict performance during retrieval in a context-guided object memory task. Proc. Natl. Acad. Sci. USA. 2022;119 doi: 10.1073/pnas.2203024119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Teles-Grilo Ruivo L.M., Baker K.L., Conway M.W., Kinsley P.J., Gilmour G., Phillips K.G., Isaac J.T.R., Lowry J.P., Mellor J.R. Coordinated Acetylcholine Release in Prefrontal Cortex and Hippocampus Is Associated with Arousal and Reward on Distinct Timescales. Cell Rep. 2017;18:905–917. doi: 10.1016/j.celrep.2016.12.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Swanson L.W., Koehler C., Bjorklund A. In: Handbook of Chemical Neuroanatomy. Vol. 5. Integrated system of the CNS. Part I: Hypothalamus, hippocampus, amygdala, retina. Bjorklund A., Hokfelt T., Swanson L., editors. Elsevier; Amsterdam: 1987. The limbic region: I: The septohippocampal system; pp. 125–277. [Google Scholar]
- 99.Puelles L., Alonso A., García-Calero E., Martínez-de-la-Torre M. Concentric ring topology of mammalian cortical sectors and relevance for patterning studies. J. Comp. Neurol. 2019;527:1731–1752. doi: 10.1002/cne.24650. [DOI] [PubMed] [Google Scholar]
- 100.Ongur D., Price J.L. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebr. Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 101.Samara Z., Evers E.A.T., Goulas A., Uylings H.B.M., Rajkowska G., Ramaekers J.G., Stiers P. Human orbital and anterior medial prefrontal cortex: Inttrinsic connectivity parcellation and functional organization. Brain Struct. Funct. 2017;222:2941–2960. doi: 10.1007/s00429-017-1378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zaborszky L., Gombkoto P., Varsanyi P., Gielow M.R., Poe G., Role L.W., Ananth M., Rajebhosale P., Talmage D.A., Hasselmo M.E., et al. Specific Basal Forebrain-Cortical Cholinergic Circuits Coordinate Cognitive Operations. J. Neurosci. 2018;38:9446–9458. doi: 10.1523/JNEUROSCI.1676-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tingley D., Alexander A.S., Quinn L.K., Chiba A.A., Nitz D. Multiplexed oscillations and phase rate coding in the basal forebrain. Sci. Adv. 2018;4 doi: 10.1126/sciadv.aar3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gombkoto P., Gielow M., Varsanyi P., Chavez C., Zaborszky L. Contribution of the basal forebrain to corticocortical network interactions. Brain Struct. Funct. 2021;226:1803–1821. doi: 10.1007/s00429-021-02290-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zaborszky L., Cullinan W.E., Braun A. In: Basal Forebrain: Anatomy to Function. Napier T.C., et al., editors. Plenum Press; 1991. Afferents to basal forebrain cholinergic projection neurons: An update; pp. 43–100. [DOI] [PubMed] [Google Scholar]
- 106.Zaborszky L., Cullinan W.E., Luine V.N. Catecholaminergic-cholinergic interaction in the basal forebrain. Prog. Brain Res. 1993;98:31–49. doi: 10.1016/s0079-6123(08)62379-1. [DOI] [PubMed] [Google Scholar]
- 107.Taylor N.L., D'Souza A., Munn B.R., Lv J., Zaborszky L., Müller E.J., Wainstein G., Calamante F., Shine J.M. Structural connections between the noradrenergic and cholinergic system shape the dynamics of functional brain networks. Neuroimage. 2022;260 doi: 10.1016/j.neuroimage.2022.119455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zaborszky L., Gombkoto P. In: Handbook of Brain Microcircuits. Shepherd G., Grillner S., editors. Oxford Univ PR; 2018. The cholinergic multicompartmental basal forebrain microcircuit; pp. 163–183. [Google Scholar]
- 109.Baskerville K.A., Chang H.T., Herron P. Topography of cholinergic afferents from the nucleus basalis of Meynert to representational areas of sensorimotor cortices in the rat. J. Comp. Neurol. 1993;335:552–562. doi: 10.1002/cne.903350407. [DOI] [PubMed] [Google Scholar]
- 110.Hasselmo M.E., Sarter M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology. 2011;36:52–73. doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lohani S., Moberly A.H., Benisty H., Landa B., Jing M., Li Y., Higley M.J., Cardin J.A. Spatiotemporally heterogeneous coordination of cholinergic and neocortical activity. Nat. Neurosci. 2022;25:1706–1713. doi: 10.1038/s41593-022-01202-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Peeters L.M., van den Berg M., Hinz R., Majumdar G., Pintelon I., Keliris G.A. Cholinergic Modulation of the Default Mode Like Network in Rats. iScience. 2020;23 doi: 10.1016/j.isci.2020.101455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Grothe M., Zaborszky L., Atienza M., Gil-Neciga E., Rodriguez-Romero R., Teipel S.J., Amunts K., Suarez-Gonzalez A., Cantero J.L. Reduction of basal forebrain cholinergic system parallels cognitive impairment in patients at high risk of developing Alzheimer's disease. Cerebr. Cortex. 2010;20:1685–1695. doi: 10.1093/cercor/bhp232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cantero J.L., Atienza M., Lage C., Zaborszky L., Vilaplana E., Lopez-Garcia S., Pozueta A., Rodriguez-Rodriguez E., Blesa R., Alcolea D., et al. Atrophy of Basal Forebrain Initiates with Tau Pathology in Individuals at Risk for Alzheimer's Disease. Cerebr. Cortex. 2020;30:2083–2098. doi: 10.1093/cercor/bhz224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pereira J.B., Hall S., Jalakas M., Grothe M.J., Strandberg O., Stomrud E., Westman E., van Westen D., Hansson O. Longitudinal degeneration of the basal forebrain predicts subsequent dementia in Parkinson's disease. Neurobiol. Dis. 2020;139 doi: 10.1016/j.nbd.2020.104831. [DOI] [PubMed] [Google Scholar]
- 116.Butler T., Zaborszky L., Pirraglia E., Li J., Wang X.H., Li Y., Tsui W., Talos D., Devinsky O., Kuchna I., et al. Comparison of human septal nuclei MRI measurements using automated segmentation and a new manual protocol based on histology. Neuroimage. 2014;97:245–251. doi: 10.1016/j.neuroimage.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Witten I.B., Steinberg E.E., Lee S.Y., Davidson T.J., Zalocusky K.A., Brodsky M., Yizhar O., Cho S.L., Gong S., Ramakrishnan C., et al. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron. 2011;72:721–733. doi: 10.1016/j.neuron.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Paxinos G., Watson C. Fifth edition. Elsevier Academic Press; 2005. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- 119.Wickersham I.R., Finke S., Conzelmann K.K., Callaway E.M. Retrograde neuronal tracing with a deletion-mutant rabies virus. Nat. Methods. 2007;4:47–49. doi: 10.1038/nmeth999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Callaway E.M., Luo L. Monosynaptic Circuit Tracing with Glycoprotein-Deleted Rabies Viruses. J. Neurosci. 2015;35:8979–8985. doi: 10.1523/JNEUROSCI.0409-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hunnicutt B.J., Long B.R., Kusefoglu D., Gertz K.J., Zhong H., Mao T. A comprehensive thalamocortical projection map at the mesoscopic level. Nat. Neurosci. 2014;17:1276–1285. doi: 10.1038/nn.3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rohlf F.J., Fisher D.R. Tests for hierarchical structure in random data sets. Syst. Biol. 1968;17:407–412. [Google Scholar]
- 123.Suzuki R., Terada Y., Shimodaira H. Pvclust: hierarchical clustering with p-values via multiscale bootstrap resampling. 2019. https://CRAN.R-project.org/package=pvclust
- 124.Burwell R.D., Amaral D.G. Perirhinal and postrhinal cortices of the rat: interconnectivity and connections with the entorhinal cortex. J. Comp. Neurol. 1998;391:293–321. doi: 10.1002/(sici)1096-9861(19980216)391:3<293::aid-cne2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 125.Witter M.P., Doan T.P., Jacobsen B., Nilssen E.S., Ohara S. Architecture of the Entorhinal Cortex A Review of Entorhinal Anatomy in Rodents with Some Comparative Notes. Front. Syst. Neurosci. 2017;11:46. doi: 10.3389/fnsys.2017.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Categories in Column B (in red) are used in this article. Column C–G contain increasingly larger volumes that encompass volume in previous order.
Data Availability Statement
-
•
Data: Cell population vectorial, image and descriptive metadata have been deposited to Zenodo and are publicly available as of the date of the publication. The descriptive data contains the projection cell’s cortical target label, experimental brain identifier, image positional, and image scale data. Images are scans of 50-micron Nissl-stained sections without any gap. DOIs are listed in the key resources table. (https://doi.org/10.5281/zenodo.14502163).
-
•
Code: All original codes for data preprocessing and analyzing have been deposited at Zenodo and are publicly available as of the date of publication. DOIs are listed in the key resources table (https://doi.org/10.5281/zenodo.14502163).
-
•
Additional information: Any additional information required to reanalyze the data reported in this article is available from the lead contact upon request.