Abstract
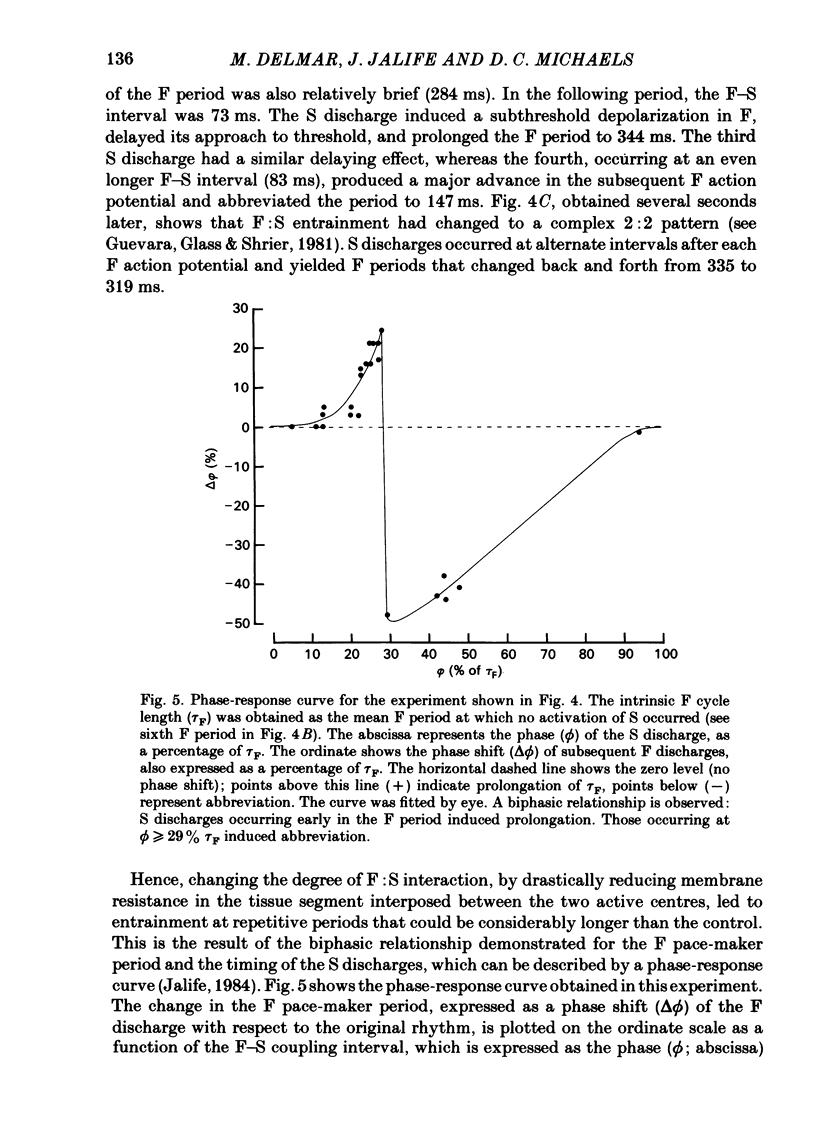
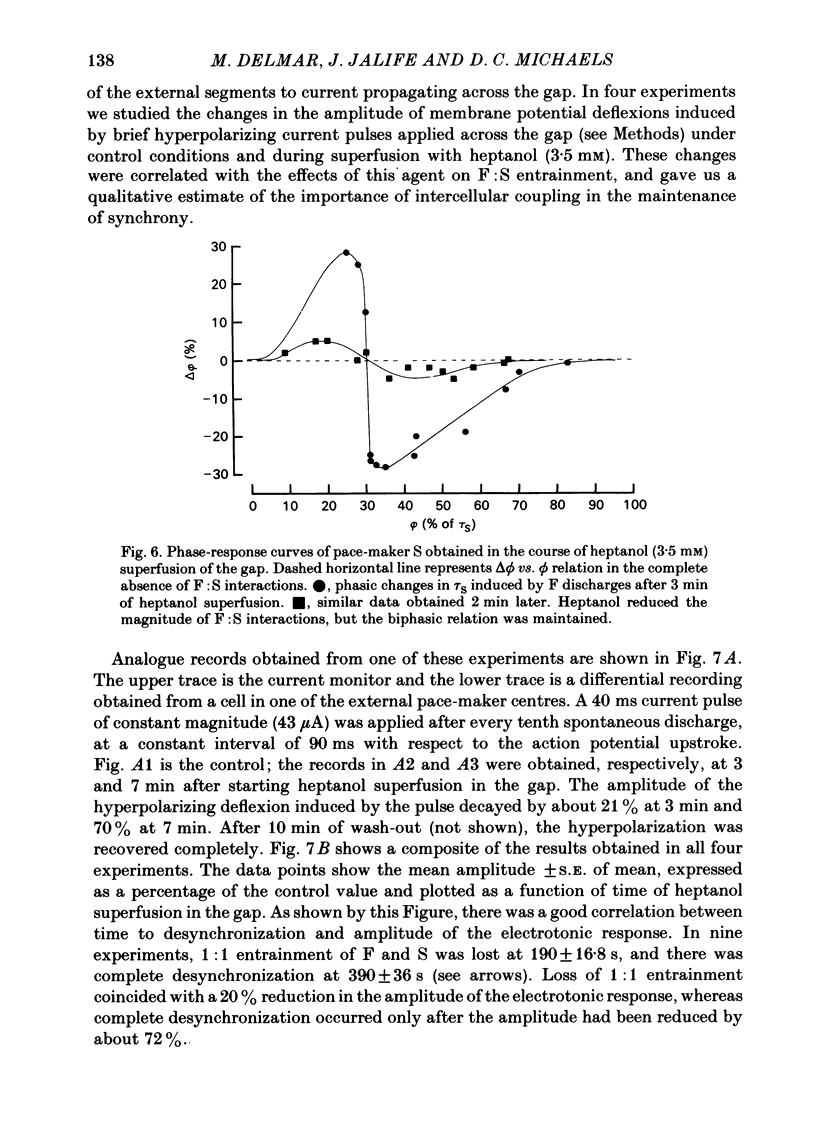
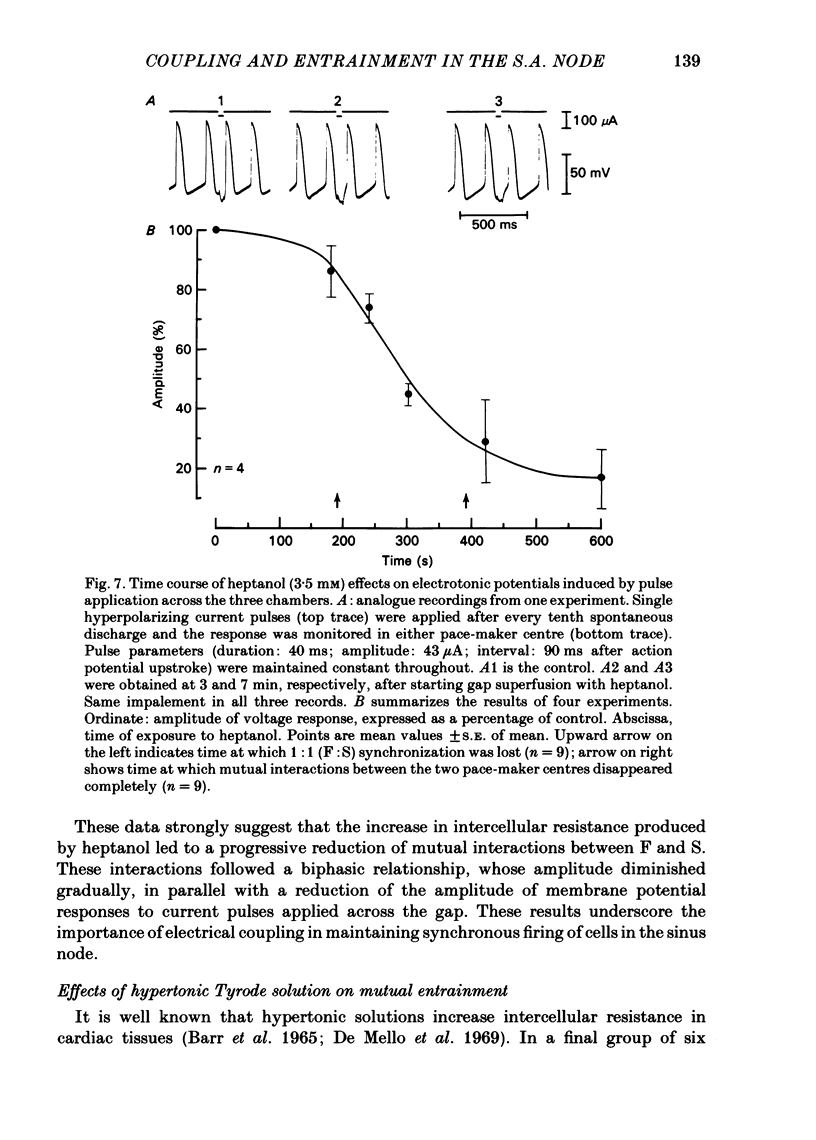
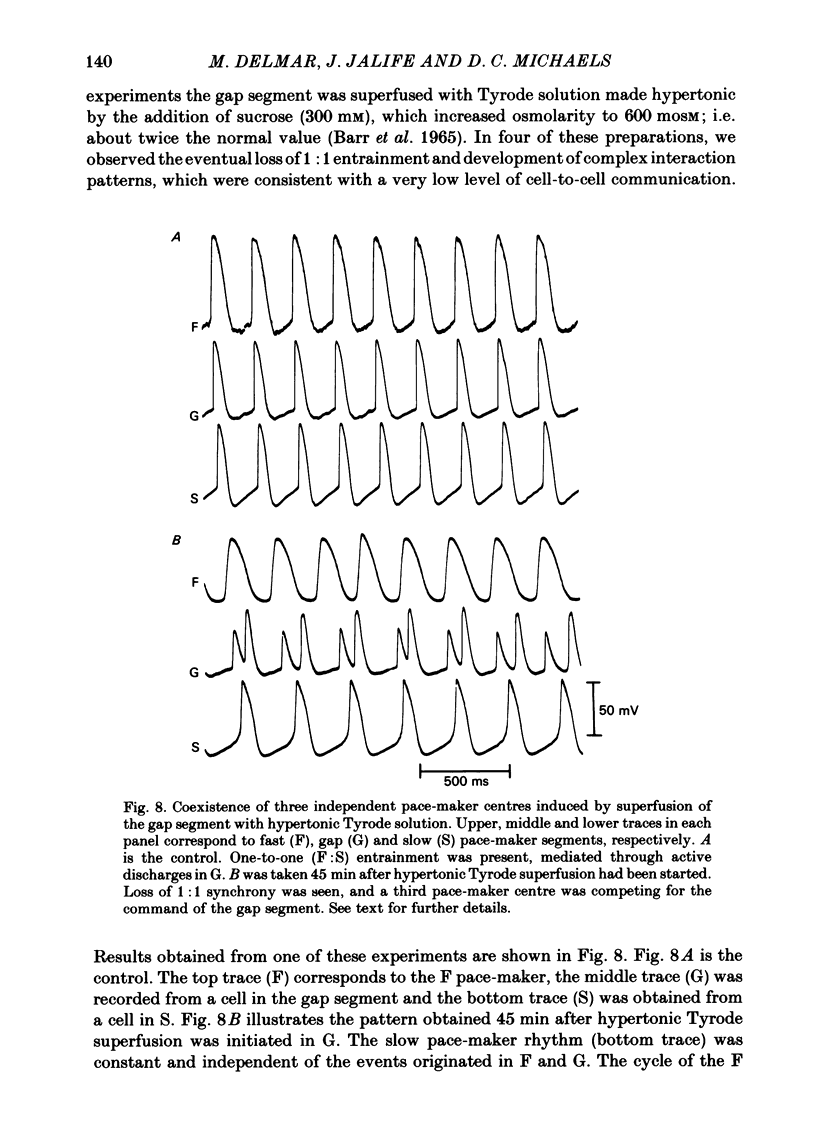
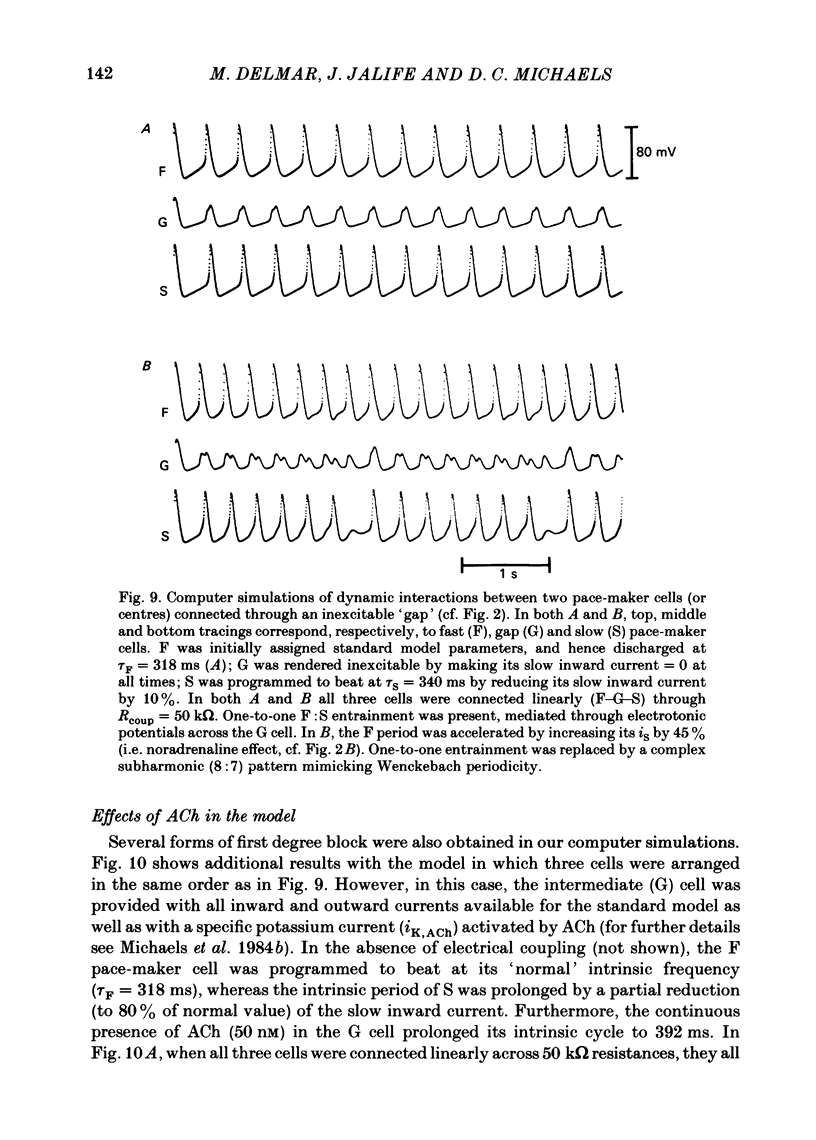
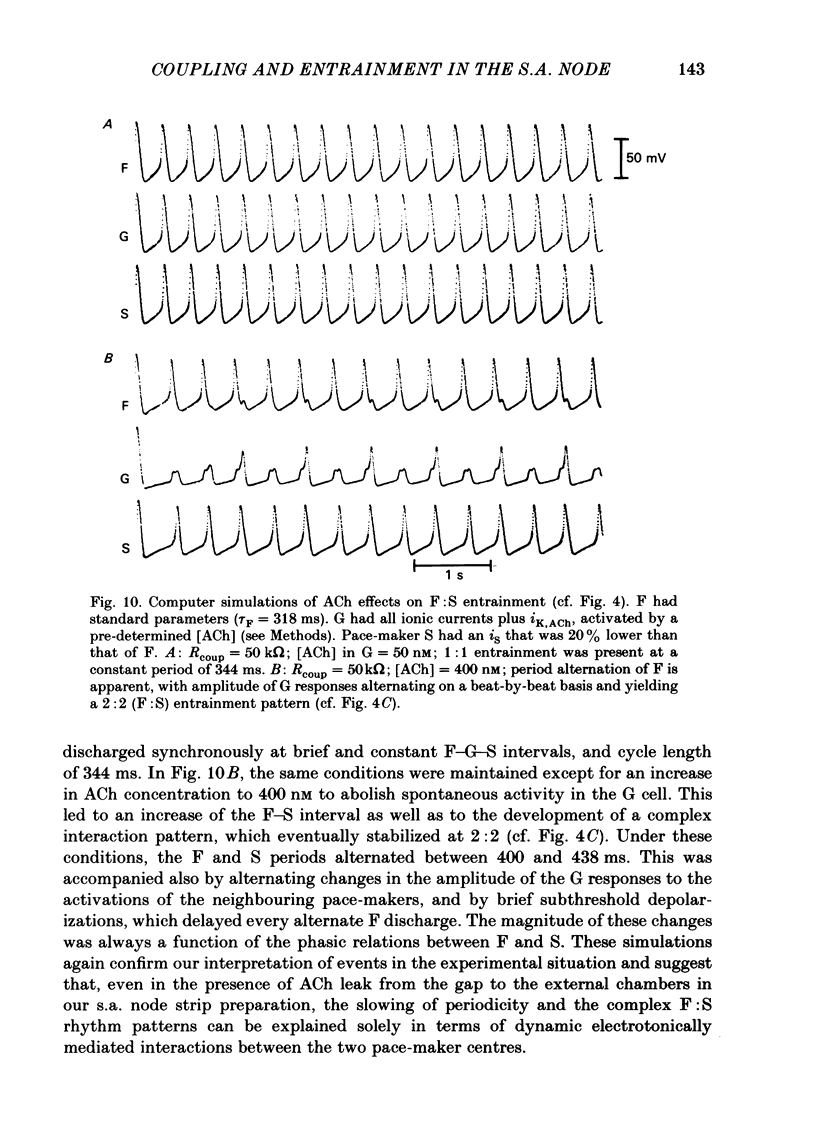
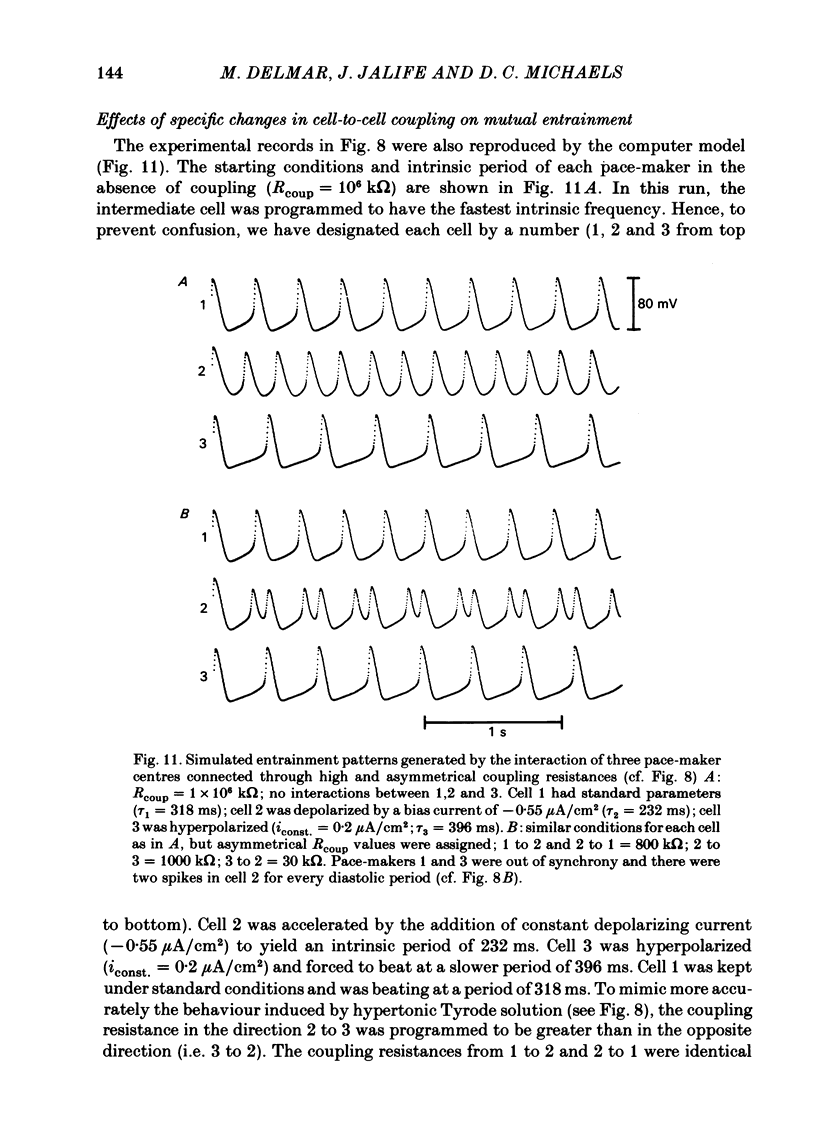
The mechanisms of synchronization between sino-atrial pace-maker cells were studied in biological preparations from rabbit hearts, and in computer simulations of the Hodgkin & Huxley type. For biological experiments, thin strips of sino-atrial node were placed in a three-compartment bath. The electrical properties of the tissue in the middle segment (the 'gap') were manipulated pharmacologically to alter electrical coupling and/or excitability of cells in that segment, and to study the patterns of interaction between two pace-maker centres in the external segments. Superfusion of the gap segment with either verapamil (2 microM) or acetylcholine (10 microM) produced a loss of 1:1 synchrony (entrainment) of spontaneous discharges generated by the external pace-makers but subharmonic (i.e. 3:2; 5:4; 9:8; etc.) entrainment was always maintained. When the gap segment was superfused with heptanol (3.5 mM), which is known to increase intercellular resistance, the pace-maker centres in the external chambers beat independently of one another. Progressive loss of synchrony paralleled reductions in amplitude of electrotonic responses to current pulses applied across the gap. Gap superfusion with hypertonic Tyrode solution (600 mosM) produced a major reduction in the degree of synchronization between the external pace-makers, even though the cells in the central compartment maintained their excitability. Under these conditions, as many as three independent pace-maker centres, one in each chamber, coexisted in a given preparation. Using computerized simulations based on equations of time- and voltage-dependent membrane currents, three 'cells', each capable of maintaining spontaneous activity, were connected in a linear array through ohmic resistances. When selective parameters (e.g. membrane conductances, coupling resistance) were modified appropriately, the mathematical simulations reproduced very closely the interaction patterns observed in the experimental preparations. Our results show that synchronization in the sinus node results from mutual interactions and entrainment between all the cells in this region. These interactions are of the kind expected for a population of coupled, self-sustained oscillators, and are mediated through electrotonic propagation of current across low-resistance junctions.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARR L., DEWEY M. M., BERGER W. PROPAGATION OF ACTION POTENTIALS AND THE STRUCTURE OF THE NEXUS IN CARDIAC MUSCLE. J Gen Physiol. 1965 May;48:797–823. doi: 10.1085/jgp.48.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleeker W. K., Mackaay A. J., Masson-Pévet M., Bouman L. N., Becker A. E. Functional and morphological organization of the rabbit sinus node. Circ Res. 1980 Jan;46(1):11–22. doi: 10.1161/01.res.46.1.11. [DOI] [PubMed] [Google Scholar]
- Clapham D. E., Shrier A., DeHaan R. L. Junctional resistance and action potential delay between embryonic heart cell aggregates. J Gen Physiol. 1980 Jun;75(6):633–654. doi: 10.1085/jgp.75.6.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHaan R. L., Hirakow R. Synchronizatin of pulsation rates in isolated cardiac myocytes. Exp Cell Res. 1972 Jan;70(1):214–220. doi: 10.1016/0014-4827(72)90199-1. [DOI] [PubMed] [Google Scholar]
- Délèze J., Hervé J. C. Effect of several uncouplers of cell-to-cell communication on gap junction morphology in mammalian heart. J Membr Biol. 1983;74(3):203–215. doi: 10.1007/BF02332124. [DOI] [PubMed] [Google Scholar]
- Goshima K. Synchronized beating of and electrotonic transmission between myocardial cells mediated by heterotypic strain cells in monolayer culture. Exp Cell Res. 1969 Dec;58(2):420–426. doi: 10.1016/0014-4827(69)90523-0. [DOI] [PubMed] [Google Scholar]
- Guevara M. R., Glass L., Shrier A. Phase locking, period-doubling bifurcations, and irregular dynamics in periodically stimulated cardiac cells. Science. 1981 Dec 18;214(4527):1350–1353. doi: 10.1126/science.7313693. [DOI] [PubMed] [Google Scholar]
- Jalife J., Hamilton A. J., Lamanna V. R., Moe G. K. Effects of current flow on pacemaker activity of the isolated kitten sinoatrial node. Am J Physiol. 1980 Mar;238(3):H307–H316. doi: 10.1152/ajpheart.1980.238.3.H307. [DOI] [PubMed] [Google Scholar]
- Jalife J., Hamilton A. J., Moe G. K. Desensitization of the cholinergic receptor at the sinoatrial cell of the kitten. Am J Physiol. 1980 Apr;238(4):H439–H448. doi: 10.1152/ajpheart.1980.238.4.H439. [DOI] [PubMed] [Google Scholar]
- Jalife J., Moe G. K. Effect of electrotonic potentials on pacemaker activity of canine Purkinje fibers in relation to parasystole. Circ Res. 1976 Dec;39(6):801–808. doi: 10.1161/01.res.39.6.801. [DOI] [PubMed] [Google Scholar]
- Jalife J., Moe G. K. Phasic effects of vagal stimulation on pacemaker activity of the isolated sinus node of the young cat. Circ Res. 1979 Nov;45(5):595–608. doi: 10.1161/01.res.45.5.595. [DOI] [PubMed] [Google Scholar]
- Jalife J. Mutual entrainment and electrical coupling as mechanisms for synchronous firing of rabbit sino-atrial pace-maker cells. J Physiol. 1984 Nov;356:221–243. doi: 10.1113/jphysiol.1984.sp015461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalife J., Slenter V. A., Salata J. J., Michaels D. C. Dynamic vagal control of pacemaker activity in the mammalian sinoatrial node. Circ Res. 1983 Jun;52(6):642–656. doi: 10.1161/01.res.52.6.642. [DOI] [PubMed] [Google Scholar]
- Johnston M. F., Simon S. A., Ramón F. Interaction of anaesthetics with electrical synapses. Nature. 1980 Jul 31;286(5772):498–500. doi: 10.1038/286498a0. [DOI] [PubMed] [Google Scholar]
- Joyner R. W., Picone J., Veenstra R., Rawling D. Propagation through electrically coupled cells. Effects of regional changes in membrane properties. Circ Res. 1983 Oct;53(4):526–534. doi: 10.1161/01.res.53.4.526. [DOI] [PubMed] [Google Scholar]
- Kameyama M. Electrical coupling between ventricular paired cells isolated from guinea-pig heart. J Physiol. 1983 Mar;336:345–357. doi: 10.1113/jphysiol.1983.sp014585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith A, Flack M. The Form and Nature of the Muscular Connections between the Primary Divisions of the Vertebrate Heart. J Anat Physiol. 1907 Apr;41(Pt 3):172–189. [PMC free article] [PubMed] [Google Scholar]
- Michaels D. C., Matyas E. P., Jalife J. A mathematical model of the effects of acetylcholine pulses on sinoatrial pacemaker activity. Circ Res. 1984 Jul;55(1):89–101. doi: 10.1161/01.res.55.1.89. [DOI] [PubMed] [Google Scholar]
- Nakayama T., Kurachi Y., Noma A., Irisawa H. Action potential and membrane currents of single pacemaker cells of the rabbit heart. Pflugers Arch. 1984 Nov;402(3):248–257. doi: 10.1007/BF00585507. [DOI] [PubMed] [Google Scholar]
- Osterrieder W., Noma A., Trautwein W. On the kinetics of the potassium channel activated by acetylcholine in the S-A node of the rabbit heart. Pflugers Arch. 1980 Jul;386(2):101–109. doi: 10.1007/BF00584196. [DOI] [PubMed] [Google Scholar]
- Pavlidis T., Pinsker H. M. Oscillator theory and neurophysiology: introduction. Fed Proc. 1977 Jun;36(7):2033–2035. [PubMed] [Google Scholar]
- Sano T., Sawanobori T., Adaniya H. Mechanism of rhythm determination among pacemaker cells of the mammalian sinus node. Am J Physiol. 1978 Oct;235(4):H379–H384. doi: 10.1152/ajpheart.1978.235.4.H379. [DOI] [PubMed] [Google Scholar]
- Tuganowski W., Kopeć P., Tarnowski W. Mechanism of intercellular synchronization in the rabbit sinus node. Experientia. 1981 May 15;37(5):485–487. doi: 10.1007/BF01986151. [DOI] [PubMed] [Google Scholar]
- Wit A. L., Cranefield P. F. Effect of verapamil on the sinoatrial and atrioventricular nodes of the rabbit and the mechanism by which it arrests reentrant atrioventricular nodal tachycardia. Circ Res. 1974 Sep;35(3):413–425. doi: 10.1161/01.res.35.3.413. [DOI] [PubMed] [Google Scholar]
- Yanagihara K., Noma A., Irisawa H. Reconstruction of sino-atrial node pacemaker potential based on the voltage clamp experiments. Jpn J Physiol. 1980;30(6):841–857. doi: 10.2170/jjphysiol.30.841. [DOI] [PubMed] [Google Scholar]
- Ypey D. L., Clapham D. E., DeHaan R. L. Development of electrical coupling and action potential synchrony between paired aggregates of embryonic heart cells. J Membr Biol. 1979 Dec 12;51(1):75–96. doi: 10.1007/BF01869344. [DOI] [PubMed] [Google Scholar]
- de Mello W. C., Motta G. E., Chapeau M. A study on the healing-over of myocardial cells of toads. Circ Res. 1969 Mar;24(3):475–487. doi: 10.1161/01.res.24.3.475. [DOI] [PubMed] [Google Scholar]


