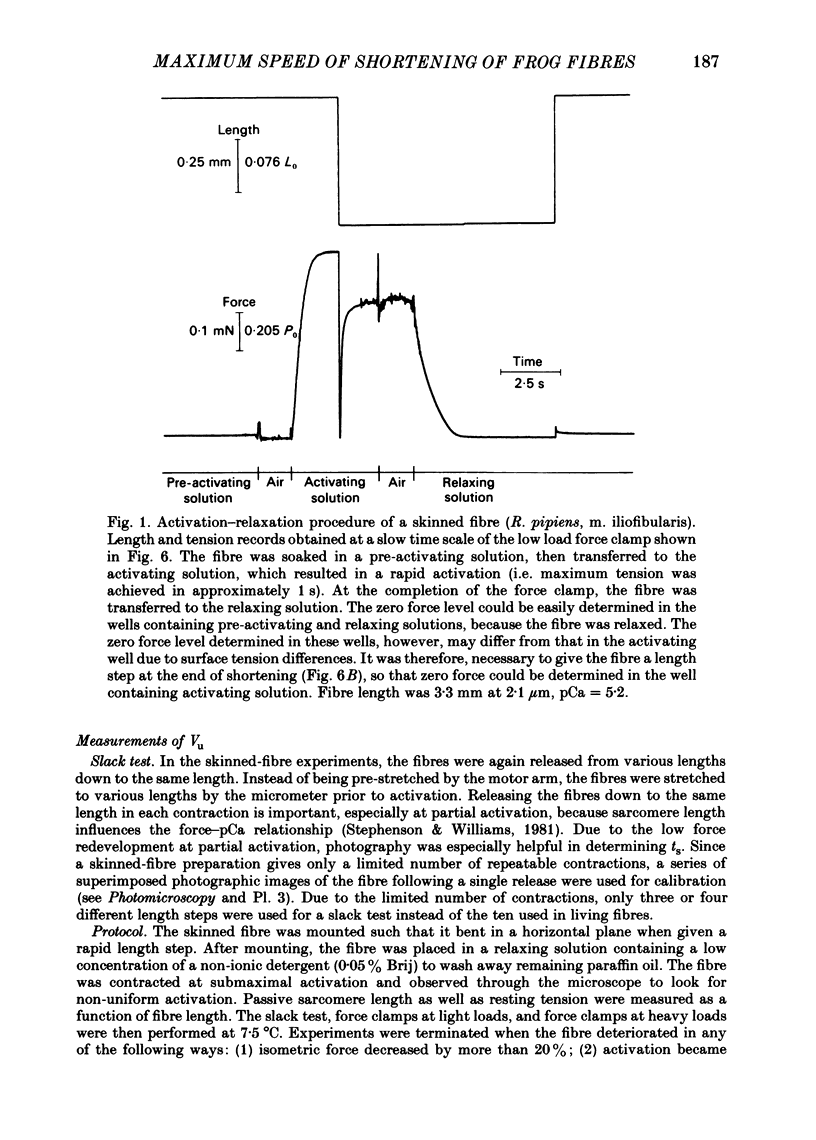
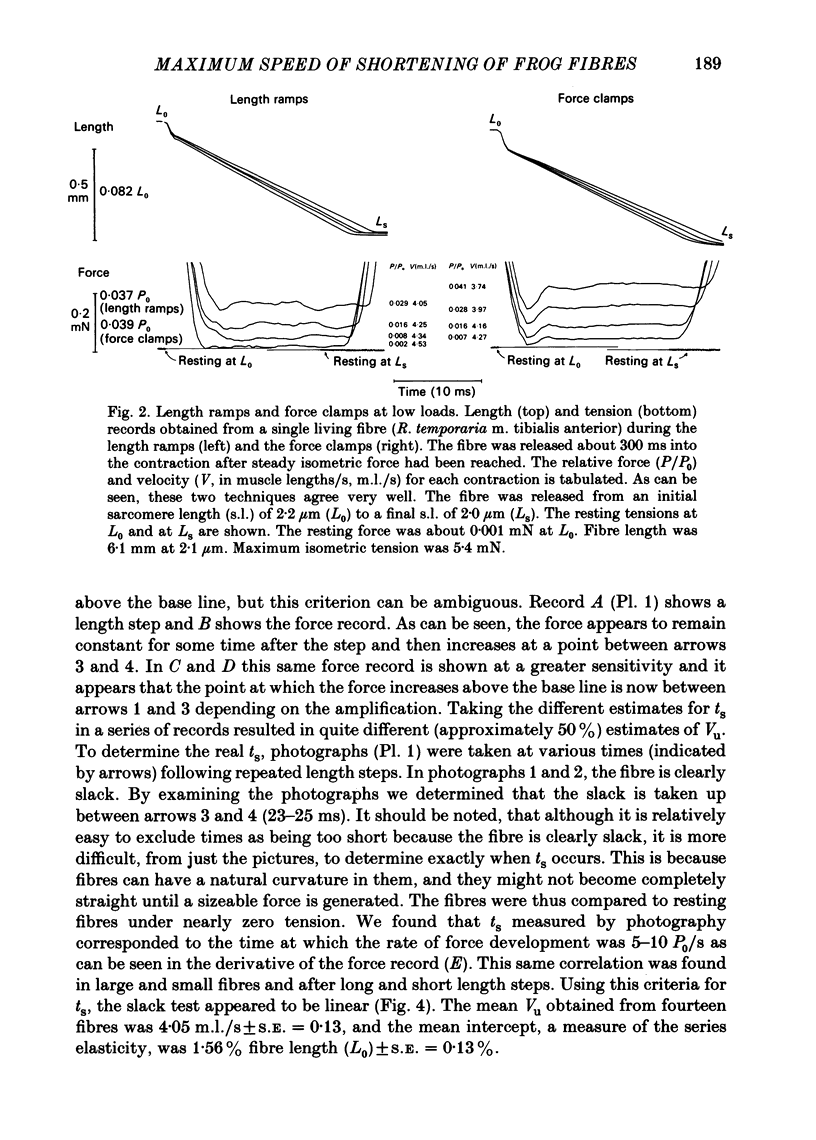
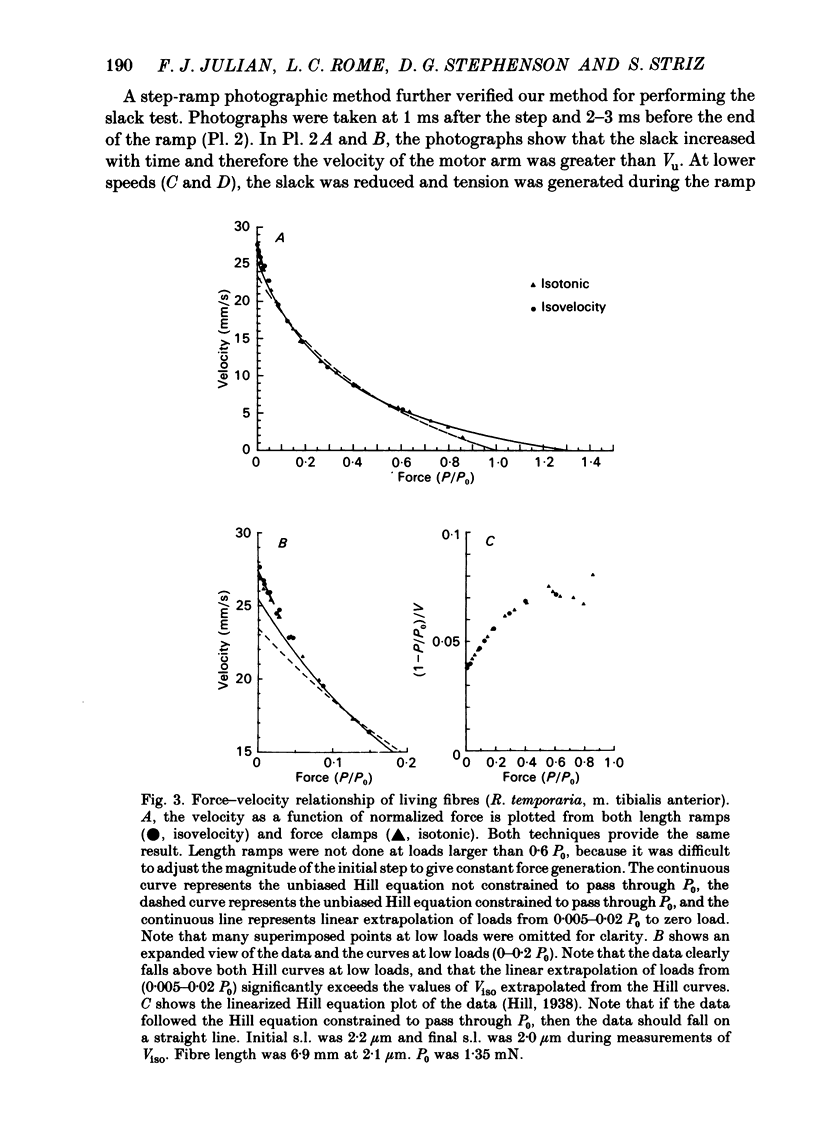
Abstract
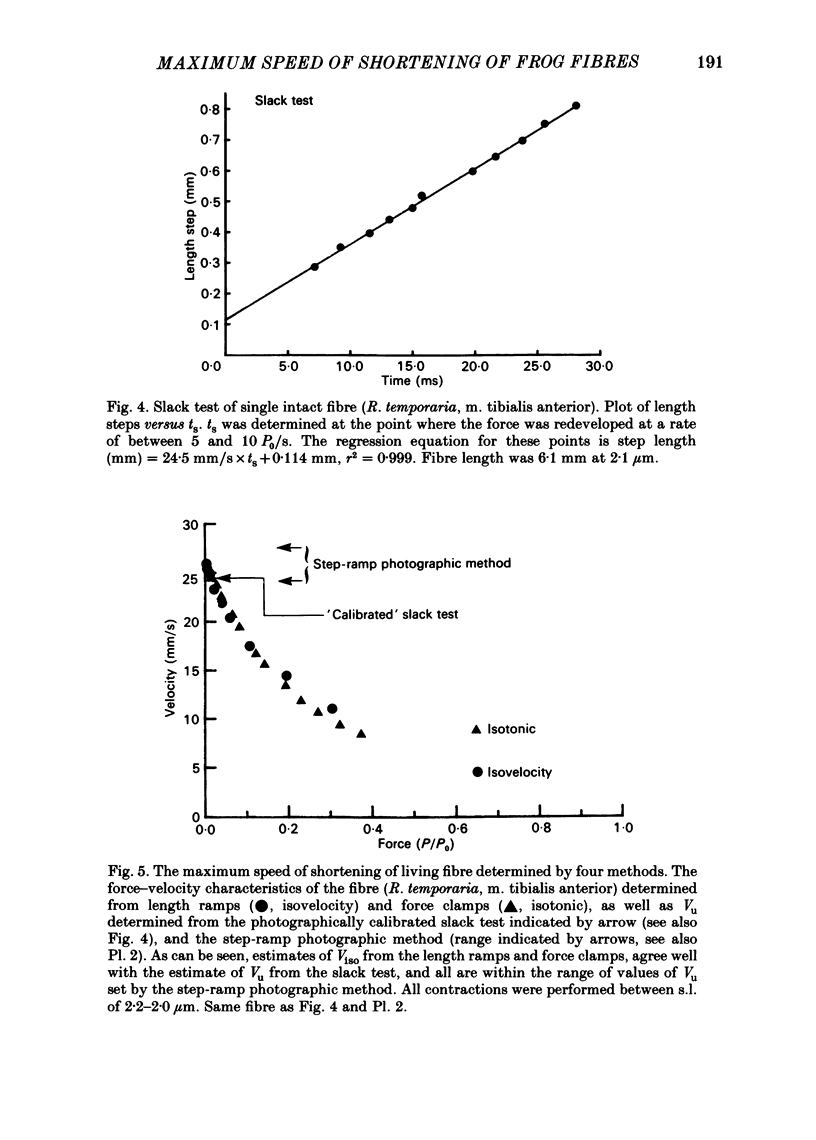
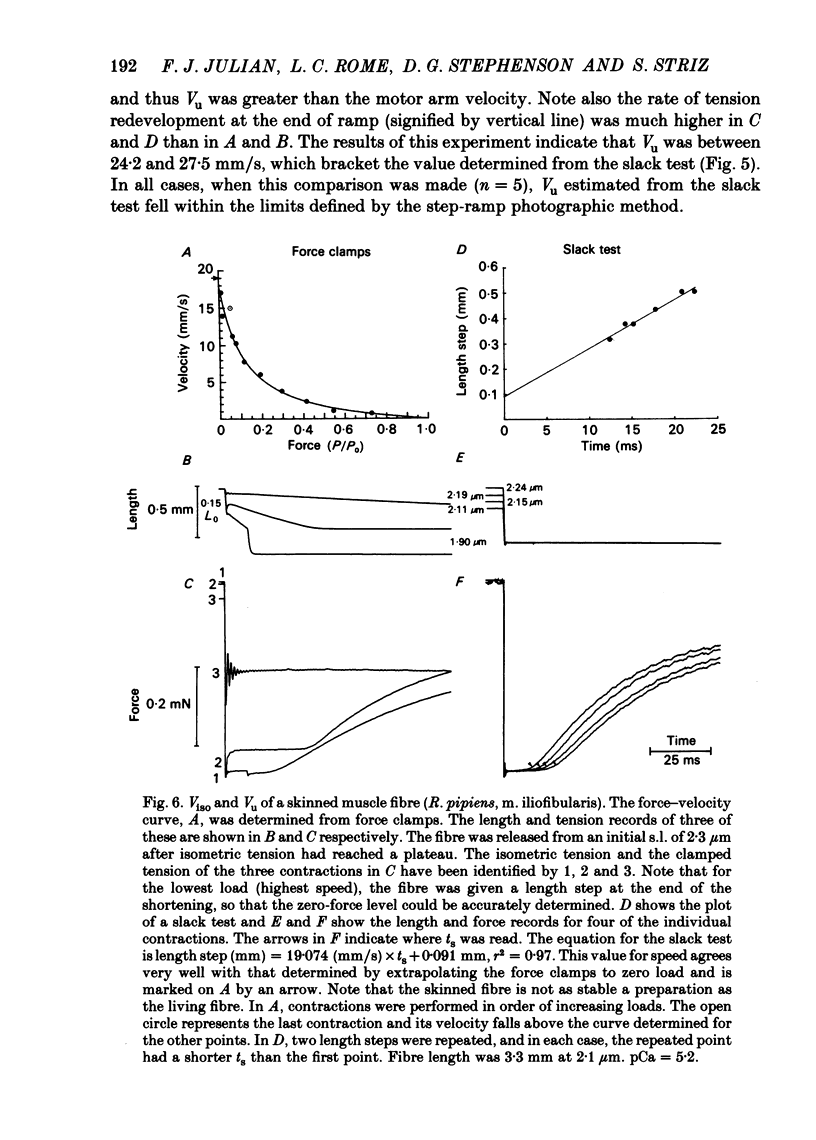
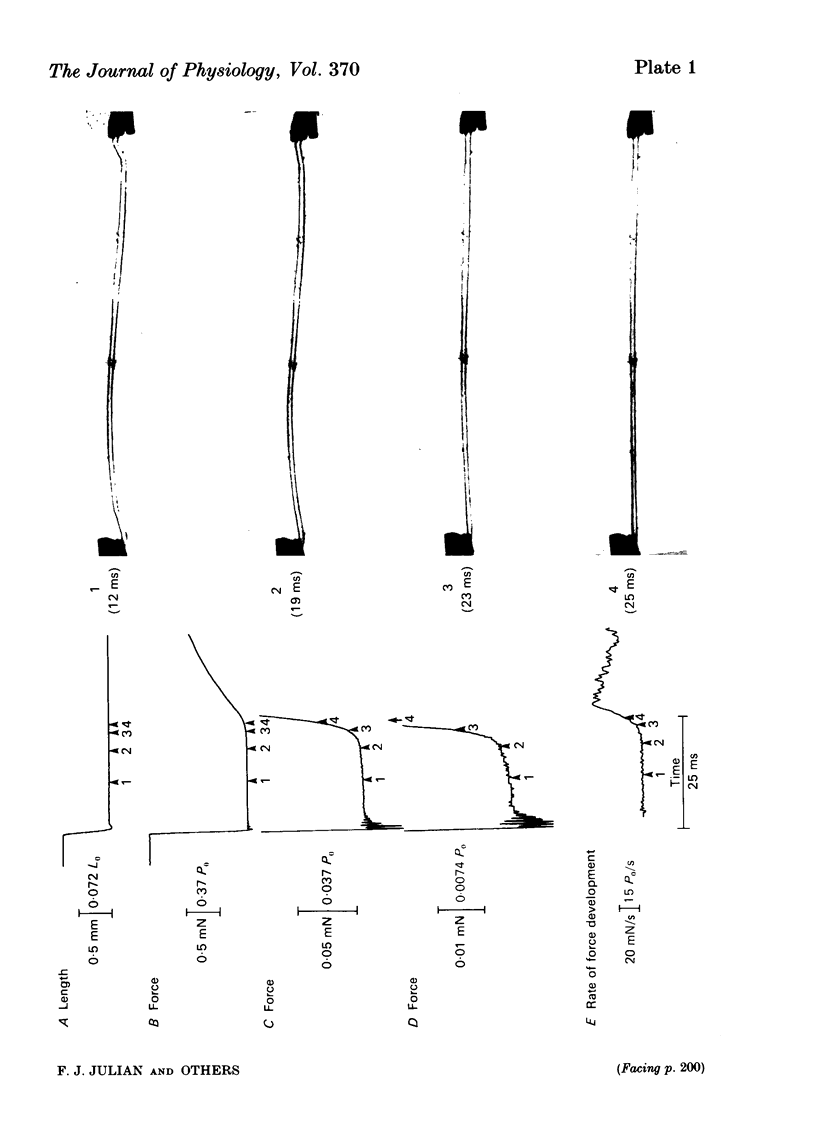
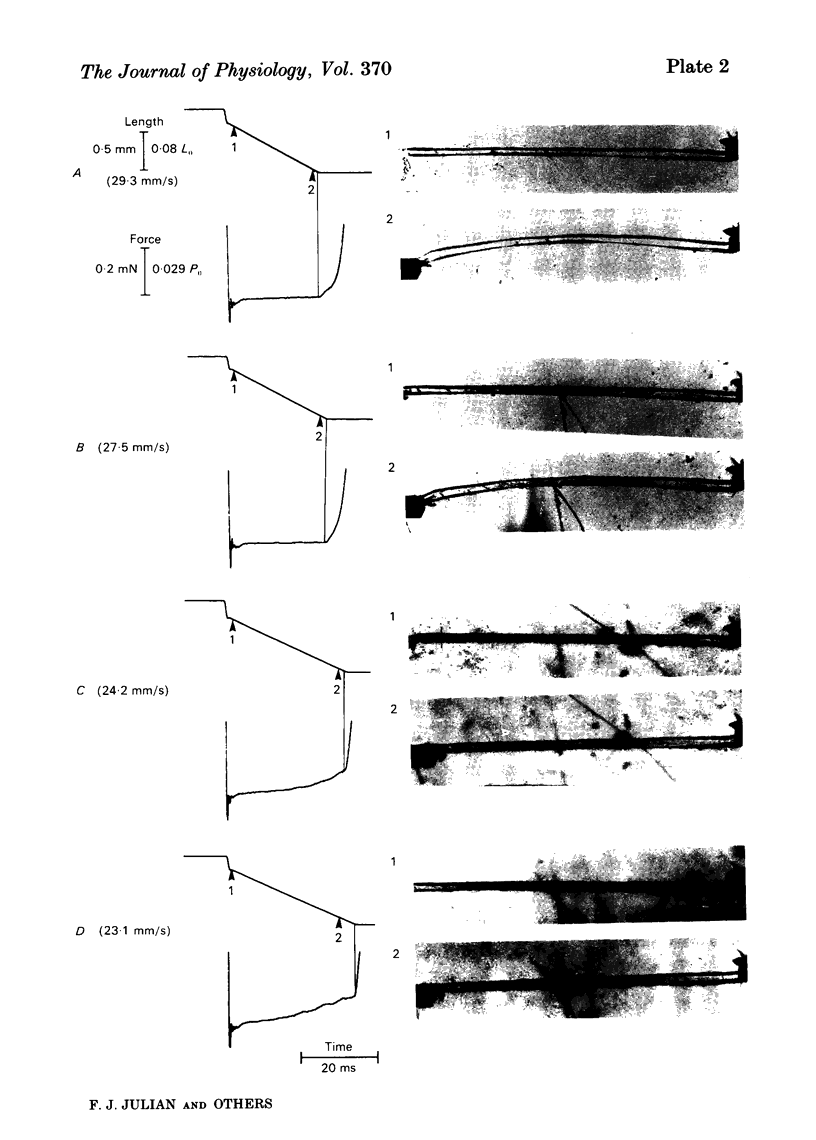
This study was performed to determine whether Viso (the maximum speed of shortening extrapolated from force-velocity curves) equalled Vu (the unloaded speed of shortening determined by the slack test) in both living fibres from Rana temporaria and mechanically skinned fibres from Rana pipiens. In living fibres (R. temporaria) we obtained improved estimates of Viso by performing force clamps (isotonic) and length ramps (isovelocity) down to very low loads (0.005 isometric tension, P0). Force-velocity characteristics determined by force clamps and length ramps were the same. The hyperbolic Hill curves deviated from the force-velocity data at both high and low loads and underestimated Viso by varying degrees. A better estimate of Viso was obtained by linear extrapolation of data at loads from 0.005-0.02 P0 and the mean Viso at 7.5 degrees C was 4.08 muscle lengths/s +/- 0.11 (mean +/- S.E., n = 14). Improved estimates of Vu in living fibres were obtained by photographically calibrating the slack test. The mean Vu was 4.05 muscle lengths/s +/- 0.13 (mean +/- S.E., n = 14) and the intercept was 0.0156 fibre lengths (L0) +/- 0.0013 (mean +/- S.E., n = 14). The step-ramp photographic method, in which the motor speed is matched to Vu, was developed as an independent way to measure Vu in living fibres. Vu measured in this way agreed well with Vu measured by the slack test. In all living fibres, the improved estimates of Vu agreed well with the improved estimates of Viso. Vu/Viso = 0.99 +/- 0.01 (mean +/- S.E., n = 14). In mechanically skinned R. pipiens fibres, force clamps were performed down to loads of 0.01 mN. The force-velocity curve of the skinned fibres differed in shape from that of the living fibres. Although there was significant deviation from the Hill equation at low loads, the data at high loads were well fitted by the Hill curve. Viso determined by extrapolating the Hill equation to zero load was 5.87 muscle lengths/s +/- 0.38 (mean +/- S.E., n = 9) at 7.5 degrees C. In five fibres, the linear extrapolation of low loads (0.01-0.05 P0) showed that the Hill equation underestimated the true Viso by 6%. The slack test with mechanically skinned fibres was calibrated by taking a series of photographic exposures of the fibre at various times following each length step. Vu = 6.12 muscle lengths/s +/- 0.44 (mean +/- S.E., n = 10) and the intercept was 0.0585 L0 +/- 0.0069 (mean +/- S.E., n = 10).(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF













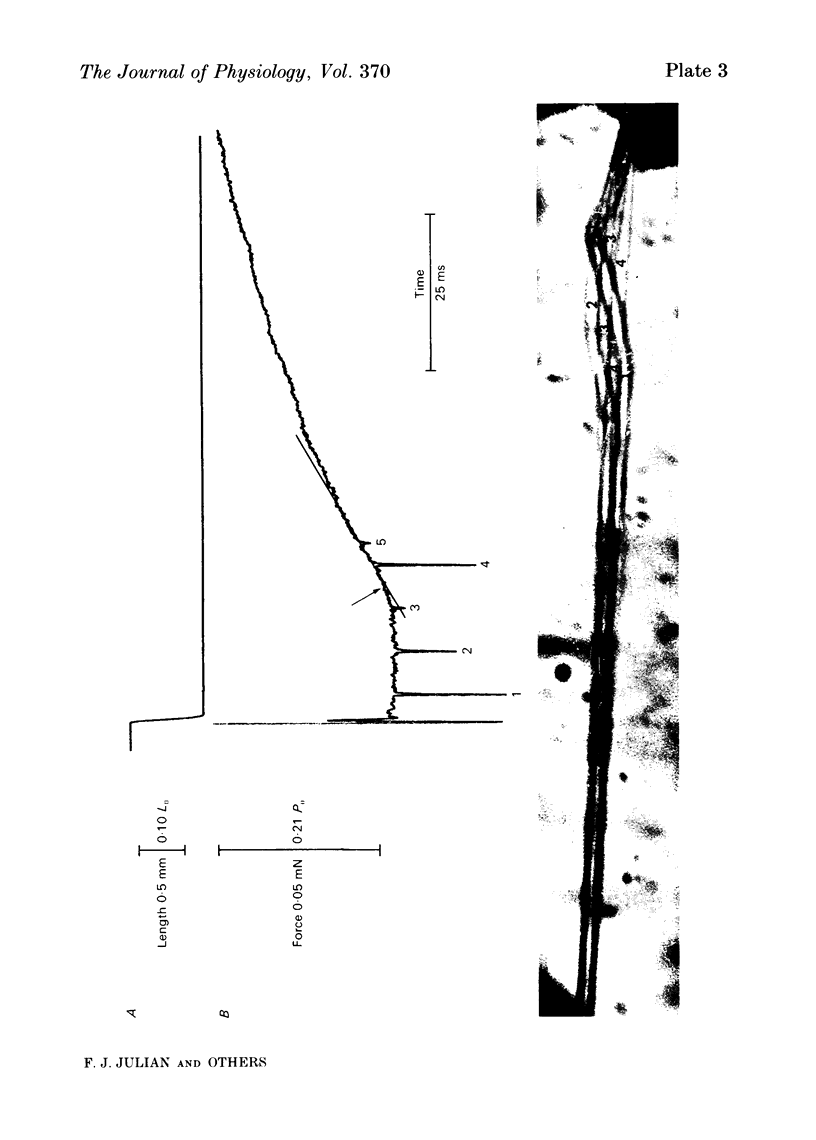
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley C. C., Moisescu D. G. Effect of changing the composition of the bathing solutions upon the isometric tension-pCa relationship in bundles of crustacean myofibrils. J Physiol. 1977 Sep;270(3):627–652. doi: 10.1113/jphysiol.1977.sp011972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claflin D. R., Faulkner J. A. Shortening velocity extrapolated to zero load and unloaded shortening velocity of whole rat skeletal muscle. J Physiol. 1985 Feb;359:357–363. doi: 10.1113/jphysiol.1985.sp015589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Mulieri L. A., Scubon-Mulieri B. Non-hyperbolic force-velocity relationship in single muscle fibres. Acta Physiol Scand. 1976 Oct;98(2):143–156. doi: 10.1111/j.1748-1716.1976.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Edman K. A. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979 Jun;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczi M. A., Goldman Y. E., Simmons R. M. The dependence of force and shortening velocity on substrate concentration in skinned muscle fibres from Rana temporaria. J Physiol. 1984 May;350:519–543. doi: 10.1113/jphysiol.1984.sp015216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J., Morgan D. L. Intersarcomere dynamics during fixed-end tetanic contractions of frog muscle fibres. J Physiol. 1979 Aug;293:365–378. doi: 10.1113/jphysiol.1979.sp012894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J., Morgan D. L. Tension, stiffness, unloaded shortening speed and potentiation of frog muscle fibres at sarcomere lengths below optimum. J Physiol. 1981;319:205–217. doi: 10.1113/jphysiol.1981.sp013902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J., Moss R. L. Effects of calcium and ionic strength on shortening velocity and tension development in frog skinned muscle fibres. J Physiol. 1981 Feb;311:179–199. doi: 10.1113/jphysiol.1981.sp013580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J., Lindblom P., Johansson B. Contractile properties of two varieties of twitch muscle fibres in Xenopus laevis. Acta Physiol Scand. 1982 Apr;114(4):523–535. doi: 10.1111/j.1748-1716.1982.tb07020.x. [DOI] [PubMed] [Google Scholar]
- Moisescu D. G., Thieleczek R. Calcium and strontium concentration changes within skinned muscle preparations following a change in the external bathing solution. J Physiol. 1978 Feb;275:241–262. doi: 10.1113/jphysiol.1978.sp012188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson D. G., Williams D. A. Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. J Physiol. 1981 Aug;317:281–302. doi: 10.1113/jphysiol.1981.sp013825. [DOI] [PMC free article] [PubMed] [Google Scholar]