Abstract
Invasive fungal infections are emerging diseases that kill over 1.5 million people per year worldwide. With the increase of immunocompromised populations, the incidence of invasive fungal infections is expected to continue to rise. Vaccines for viral and bacterial infectious diseases have had a transformative impact on human health worldwide. However, no fungal vaccines are currently in clinical use. Recently, interest in fungal vaccines has grown significantly. One Candida vaccine has completed phase 2 clinical trials, and research on vaccines against coccidioidomycosis continues to advance. Additionally, multiple groups have discovered various Cryptococcus mutant strains that promote protective responses to subsequent challenge in mouse models. There has also been progress in antibody-mediated fungal vaccines. In this review, we highlight recent fungal vaccine research progress, outline the wealth of data generated, and summarize current research for both fungal biology and immunology studies relevant to fungal vaccine development. We also review technological advancements in vaccine development and highlight the future prospects of a human vaccine against invasive fungal infections.
Keywords: fungal infections, fungal vaccines, adaptive immunity, innate immunity, trained immunity, T cells
THE HISTORICAL IMPORTANCE OF VACCINES
Vaccines are considered one of the most significant achievements in public health in human history. The importance of vaccines cannot be overstated. As Stanley Plotkin wrote in his book Vaccines, “The impact of vaccination on the health of the world’s peoples is hard to exaggerate. With the exception of safe water, no other modality has had such a major effect on mortality reduction and population growth” (95). The development of vaccines against viral and bacterial infections has been highly successful. Although the idea of vaccination may have been developed much earlier, the term vaccine was first used by Edward Jenner in 1796 when he treated a young boy by inoculating him with the pus from a cowpox blister that contained vaccinia virus. The first smallpox vaccine was subsequently developed in 1798, and its mass use led to the eradication of smallpox almost two centuries later in 1979. A century after Edward Jenner’s experiment, Louis Pasteur spear-headed the development of a live attenuated cholera vaccine and an inactivated anthrax vaccine for use in humans. Around the same time, a vaccine against Yersinia pestis, the cause of plague, was invented. Between 1890 and 1950, bacterial vaccine development expanded greatly, including the BCG (bacillus Calmette-Guérin) vaccine for preventing Mycobacterium tuberculosis infection. The availability of viral tissue culture methods in the 1950s led to the development of polio vaccines, which helped eradicate polio from many regions around the world (95). Vaccine development has now enabled the containment of a number of once highly prevalent infectious diseases in the United States, including polio, flu, hepatitis A, rubella (three-day measles), Hib (Haemophilus influenzae type B), measles, whooping cough, pneumococcal pneumonia, rotavirus infection, mumps, chickenpox, diphtheria, etc. An outbreak of a SARS-CoV-2 in late 2019 has caused a global pandemic of COVID-19. The urgency to control this ongoing pandemic has led to successful development of vaccines in record time, which is redefining vaccine development procedures.
However, despite these enormous successes, fungal vaccines have remained significantly underdeveloped. No fungal vaccines are available for clinical use in humans, and due to increases in chronic diseases and immunodeficiencies, invasive fungal infections have become more prevalent and more deadly. This has led to increased public awareness about the severity of these diseases and the importance of fungal vaccines. However, advances in our collective knowledge of fungal biology, fungal pathogenesis, and fungal immunology are required for breakthroughs in fungal vaccine development. Exciting new research and developments may finally help break the barrier for a successful fungal vaccine. In this review, we summarize the current understanding of host immunity against fungal infections and its role in vaccine development.
THE NEED FOR FUNGAL VACCINES
The fungal kingdom contains over 1.5 million known species, and likely many more (42). Among these, several hundred fungal species have been reported to cause infections in humans and animals. Some fungal species cause invasive mycoses that are often life-threatening. Although some invasive fungal infections occur in healthy people, most infections occur opportunistically in immunocompromised hosts. Numerous factors have contributed to a dramatic increase in immunocompromised populations, including the HIV/AIDS epidemic, increased life span due to the advancement of modern medicine, and improved living conditions. This demographic change has led to an increased risk for invasive fungal infections in immunocompromised populations, often with deadly consequences. Therefore, invasive fungal infections have become a significant public health concern, and current antifungal drugs are inadequate to effectively treat fungal infections. Because fungi are eukaryotic organisms, they share many cellular mechanisms with their mammalian hosts. Thus, developing suitable drug targets has proved difficult, with only three major drug classes currently available: polyenes (e.g., amphotericin B), azoles (e.g., fluconazole), and echinocandins (e.g., caspofungin). 5-Fluorocytosine, which targets DNA and RNA synthesis, has been used in combinational therapies together with amphotericin B to treat severe candidiasis and cryptococcal meningitis. While all these drugs have been successfully utilized for treatment of some fungal infections, several factors such as drug toxicity, high cost, and restrictions on administration methods often prevent timely access for many patients in critical need of them. In addition, the development of resistance to some antifungals has become a factor, and new species such as Candida auris have emerged that are highly resistant to our current arsenal of antifungals. Therefore, in addition to development of new antifungal drugs, fungal vaccines are highly desired and will likely significantly improve the well-being of immunocompromised populations who are at high risk, especially in regions with underdeveloped health care systems. The reasons for the lack of fungal vaccine development are complicated and include the complexity of fungal cell surface structures, an inadequate understanding of fungal immunology, and the fact that the majority of susceptible hosts are immunocompromised. However, recent advances in fungal biology and immunology have provided a much-improved understanding of invasive fungal infections.
IMMUNE MECHANISMS OF VACCINE-INDUCED PROTECTION AGAINST FUNGAL INFECTIONS
The fact that the majority of invasive fungal infections occur in individuals with specific immune deficiencies, such as HIV/AIDS patients, suggests host immunity plays a critical role in controlling fungal infections. Furthermore, due to much of the fungal-infection-susceptible population being immunocompromised, developing a vaccine for this population could be quite challenging. A better understanding of host immunity against fungal infections and how a vaccine candidate harnesses the host immune system could provide critical information. Recent advancements in fungal immunology and encouraging results in vaccine-induced protection in immunocompromised animal hosts have provided much-needed support for fungal vaccine development.
Adaptive Immunity
Protection against a variety of fungal infections strongly depends on effective host T cell responses (34, 36, 78). There is extensive evidence for the critical role of CD4+ T cells in protection against multiple clinically important fungal pathogens, including Candida, Pneumocystis, Blastomyces, Histoplasma, Coccidioides, and Cryptococcus (112, 135, 145). Animal models of infection show that removal of CD4+ T cells results in enhanced susceptibility to fungal infection and is linked to an impaired control of fungal cells at the infection site (31, 44, 50, 51, 58, 109, 110, 131). Clinically, patients with reduced CD4+ T cell counts are at increased risk of developing deadly fungal infections (68, 76, 105, 111). In fact, at the height of the HIV epidemic, fungal infections, including Pneumocystis and Cryptococcus infections, were AIDS-defining illnesses (19, 91, 94). To date, opportunistic fungal infections continue to be a significant cause of AIDS-related mortality (4, 71, 122). Given the importance of CD4+ T cells in orchestrating protective immunity, it is not surprising that vaccine-induced protection in various models of fungal infection has been shown to depend on appropriate activation of T cell responses (29, 81, 130, 152). Activation and differentiation of IFN-γ-producing Th1 cells is also critical in vaccine-mediated protection against multiple fungal pathogens (3). Th1 cell–derived cytokines activate phagocyte responses that help contain and eliminate fungal cells (6, 57, 80, 153). Th17 cells have also emerged as critical mediators of defense against diverse fungal infection (1, 21, 43, 113, 139). Th17-derived cytokines, IL-17 and IL-22, promote the recruitment of neutrophils, critical innate immune cells that contribute to the eradication of multiple fungal pathogens (21, 53). In addition, Th17 responses promote the secretion of antimicrobial peptides that can restrain fungal cells (65). Thus, it is not surprising that vaccine-induced fungal protection has also been linked to activation of Th17 responses, with potent protection often being elicited by the coordinated activation of both Th1 and Th17 responses (82, 147).
Given the high susceptibility of CD4+ T cell–deficient patients to fungal infections, effective vaccines against fungal pathogens would ideally confer protection in the setting of CD4+ T cell deficiency. Various animal models have provided promising evidence for the possibility of eliciting CD4+ T cell–independent protection against multiple fungal infections (25, 67, 142, 151). In a model of blastomycosis, CD8+ T cells were found to compensate for the absence of CD4+ T cells (83). Similarly, several candidate vaccine strains confer protection against cryptococcosis in CD4+ T cell–depleted mice (87, 98, 138, 142). Mechanistically, it has been proposed that in the absence of CD4+ T cells, CD8+ T cells can expand and serve as a critical cytokine source, especially of IFN-γ and occasionally also IL-17 (66, 67, 80). Thus, emerging evidence suggests the possibility of developing fungal vaccine formulations that can promote broad activation of host immunity in a manner that can still protect in settings of selected immune dysfunction. It remains unclear whether similar vaccine-induced antifungal immunity mechanisms can operate in patients living with HIV. Important factors to consider include the restoration of stable CD4+ T cell levels in HIV-positive patients receiving highly active antiretroviral therapy (HAART) that may allow for effective vaccination (22). Additionally, the timing of vaccination may allow for effective immunization in patients treated during early stages of HIV infection (38). Clinical recommendations stress the importance of vaccinations in HIV-infected individuals, and studies show activation of protective responses following immunization to influenza, hepatitis B virus and pneumococcus (8,33). Thus, fungal vaccines could be selected for their ability to stimulate CD4-independent protection in HIV patients with reduced CD4+ T cell counts. Another possible approach is the use of vaccines that mediate protection via generation of antibodies or development of monoclonal antibodies to boost fungal growth control (reviewed in 11, 132).
An exciting aspect of preclinical studies in fungal vaccines has been the identification of conserved epitopes that provide protection across diverse fungal pathogens (128). Given the complex nature of fungal pathogens and the various challenges faced in the effective implementation of antifungal vaccination programs, the development of a universal vaccine against fungi would overcome many of these challenges (100). Proof-of-concept studies have demonstrated the presence of conserved, protective T cell epitopes across Ascomycetes (143). Calnexin-specific T cells were uncovered to mediate protection against diverse dimorphic fungi (143). Calnexin-specific T cells were also activated in response to Aspergillus and dermatophytes (143). Thus, it may be possible to design vaccine formulations that confer broad protection against multiple clinically relevant fungal pathogens. The identification of the fungal antigen Engl2 as a molecule with combined features of adjuvant and T cell epitopes is another intriguing proof-of concept finding that could guide studies of various fungal pathogens and help identify molecules with similar features (137).
Various studies support inclusion of fungus-derived pathogen-associated molecular patterns (PAMPs) as potent adjuvants that can help tailor vaccine-induced immune responses (Figure 1). Members of the C-type lectin family of receptors have emerged as crucial activators of antifungal innate immune responses (40, 41). Important roles for Dectin-1, Dectin-2, and Mincle have been identified, not only as activators of innate effector functions but also as regulators of T cell differentiation toward protective Th1 and Th17 lineages (114). Fungal vaccine formulations that include combinations of fungal PAMPs have shown great promise in mouse models as mediators of tailored antifungal immunity. Among these, glucan particles, which are enriched with β-glucans and chitin, can be loaded with various antigens and used as a delivery platform for antifungal vaccines (discussed in more detail in sections below). The use of glucan particles facilitates delivery of relevant fungus-derived antigens together with adjuvants optimized for programming antifungal defenses. In this setting, optimal vaccine-induced protection was mediated by Th1 and Th17 cells and involved engagement of host innate immune receptors Dectin-1 and Dectin-2 and the downstream signaling partner CARD-9.
Figure 1.
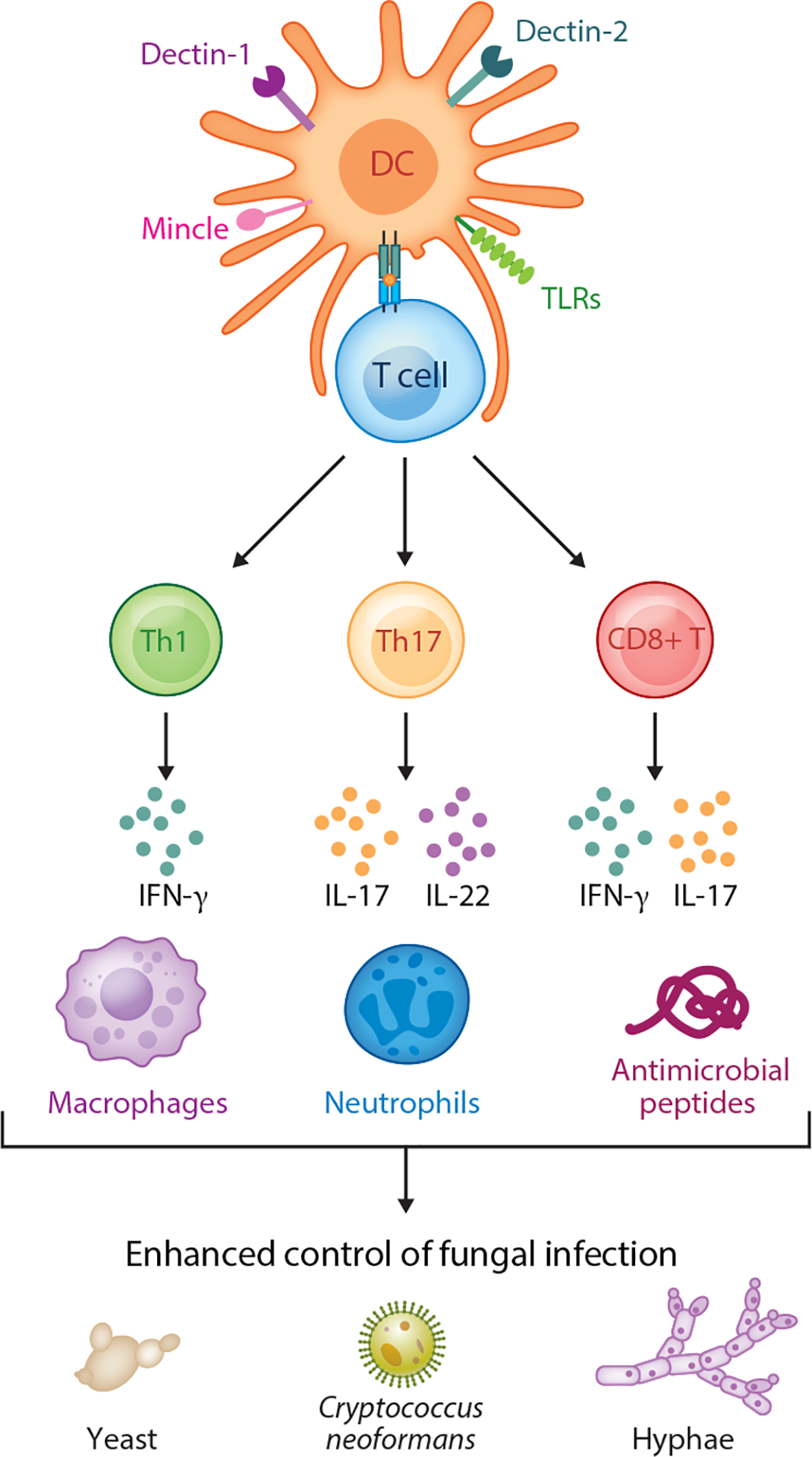
Overview of adaptive immunity. Innate cells recognize molecular patterns present in fungal pathogens via innate receptors. The C-type lectins Dectin-1, Dectin-2, and Mincle have been identified as critical activators of host immune responses in the context of fungal infection. Toll-like receptors (TLRs) are also engaged during fungal infection and together with C-type lectins help activate dendritic cells (DCs). In turn, DCs are critical for the activation of T cell responses via presentation of fungus-derived antigens in the context of MHC together with proper costimulation and secretion of cytokines that shape T cell differentiation. The differentiation of CD4+ T cells toward Th1 and Th17 cells is critical for antifungal defense and the activation of effective vaccine-mediated immunity. Th1 and Th17 cells produce critical cytokines, such as IFN-γ, IL-17, and IL-22, that act on innate cell effectors to further amplify the effective control of fungal infection. Effector cytokines also help induce the expression of antimicrobial peptides that can have direct toxic effects on fungal cells. Effector cytokines also act on innate cell targets. These include macrophages and neutrophils, which are significantly involved in direct eradiation of various fungal pathogens and morphotypes that range from yeast to hyphal forms. The activation of robust antifungal immunity also involves CD8+ T cells, which can also serve as an important source of protective cytokines. CD8+ T cells are particularly important in the context of CD4+ T cell deficiency, where an expansion of CD8+ T cells can compensate for loss of CD4+ T cells and help mediate vaccine-induced protection.
Innate Cells and Trained Immunity
The potent adaptive immune responses that need to be elicited by antifungal vaccines are critically influenced by proper activation of innate immune cells and, in particular, dendritic cells that help shape T cell differentiation. C-type lectins are critical innate receptors that shape Th1 and Th17 cell differentiation via effects on innate immune cells and promote cytokine secretion that supports antifungal immunity (40). Toll-like receptors, especially TLR2, TLR4, and TLR9, are also involved in the activation of antifungal responses and vaccine-induced protection (5). Thus, vaccine formulations that include adjuvants to engage these pathways have shown promise in various studies. In classic vaccination strategies, the primary role of innate cells has been understood to be coordination of adaptive immune cells, which then act as the ultimate effectors of protection. Exciting developments in recent years have expanded the role for innate cells in vaccine-induced protection, as well as their ability to retain memory features originally associated with only T and B lymphocytes. The most accepted term for these features is trained immunity (30, 86). Seminal studies have shown that innate cells can undergo innate training and be programmed with long-term changes that promote enhanced responses upon secondary challenges (30). In these studies, fungal PAMPs were critical mediators of trained immunity (Figure 2). Murine studies show prior immunization with heterologous vaccines such as BCG (bacillus Calmette-Guérin) vaccine can confer protection against candidiasis in a T cell–independent manner (17). Importantly, heterologous protection involved the engagement of C-type lectins. Moreover, ex vivo studies showed β-glucan recognition (via Dectin-1 on monocytes) induced trained immunity in monocytes through HIF-α signals that subsequently induced epigenetic changes (17). These findings suggest that mechanistically, fungus-derived PAMPs can help program epigenetic changes in innate immune cells and thus endow innate effectors with enhanced responses to fungal infections. Importantly, trained innate cell immunity has the critical feature of promoting protection not only against the priming antigen but also against heterologous infections. Thus, future studies in trained immunity promise to provide the basis for tailored designs of potent antifungal vaccines capable of exploiting both innate and adaptive immune cells and providing broad protection against fungal pathogens. The potential detrimental impact of trained immunity via activation of inflammatory pathology remains to be fully explored, but there are indications that this is an important factor to be considered in future studies (85).
Figure 2.
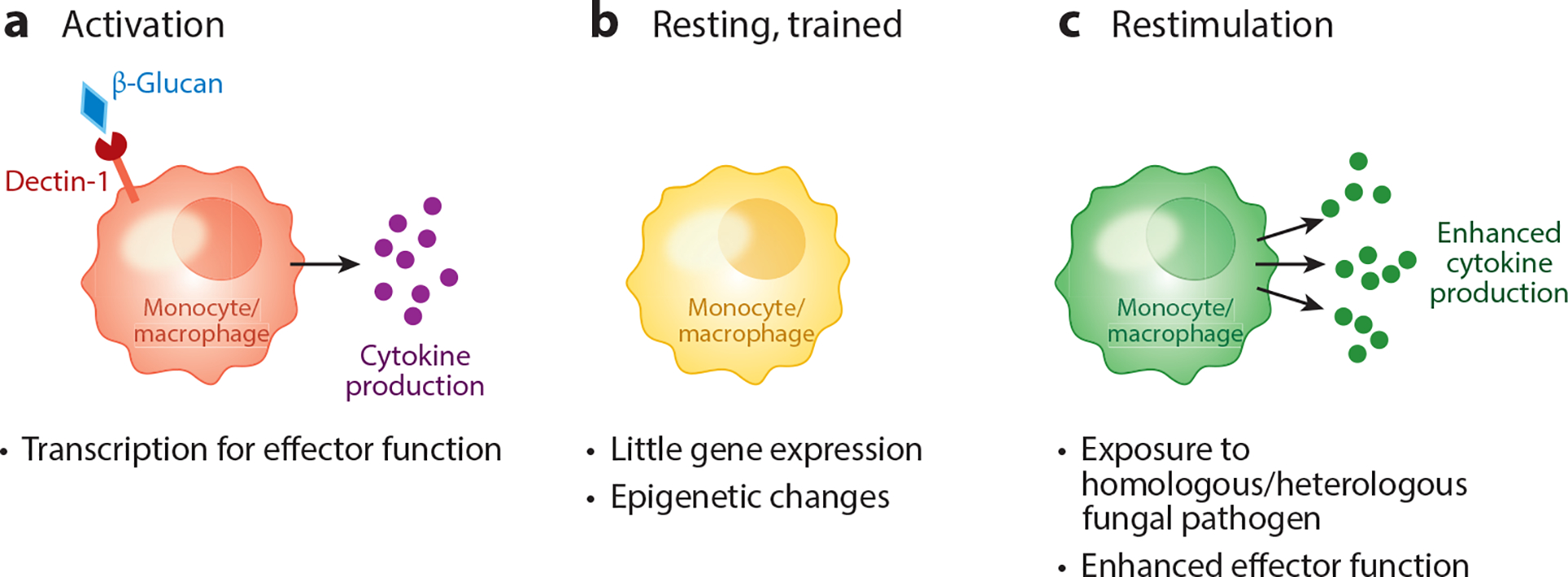
Overview of trained immunity. In the context of fungal infection, vaccination with formulations that include fungus-derived adjuvants such as β-glucan can mediate the programing of monocytes and macrophages for enhanced secondary responses, known as trained immunity. (a) Recognition of β-glucan via Dectin-1, for example, has been found to activate innate cells for increased transcription, cytokine secretion, and effector functions during initial encounter with fungi. (b) This primary response also triggers epigenetic changes in the now experienced cells and leads to alterations in their chromatin structure such that they are poised for rapid and robust production of cytokines during a secondary insult. (c) Importantly, enhanced secondary responses in trained innate cells not only help mediate homologous protection but also can be potent inducers of heterologous defense.
CHALLENGES TO DEVELOPING FUNGAL VACCINES
Despite rapid advances in fungal immunology and the identification of many immunogenic mutant strains and fungal factors that are antigenic in animal models, a very limited number of vaccine candidates have been further developed and tested in human clinical trials. Several complex factors likely contribute to the slow progress toward important fungal vaccine development and commercialization. As immunodeficiency is a major risk factor for fungal infections, an ideal fungal vaccine would be effective in immunocompromised hosts. This efficacy is required for targeting large immunocompromised populations and having a significant impact on public health. Although many cases of fungal infection have been reported in healthy people and some fungal pathogens are primary pathogens, the fungus-infected population may not be large enough to attract industry interest, especially if vaccines only work in individuals with normal immunity. Furthermore, identifying at-risk populations could prove challenging. Recently, a number of vaccine candidates have been effective in immunocompromised hosts. Multiple Cryptococcus vaccine candidates have successfully immunized mice with continued protection against Cryptococcus, including in CD4+ T cell–depleted mice. Additionally, mice vaccinated with heat-killed fbp1Δ mutant (HK-fbp1) cells and treated with the immunosuppression drug cyclophosphamide remained protected against Aspergillus fumigatus (138). Both Cryptococcus H99γ and sgl1Δ vaccine strains are effective in CD4+ T cell– or CD8+ T cell–depleted mice, but not in dually depleted mice (87, 142). These promising developments suggest it is possible to develop vaccines against fungal infections, even in immunocompromised hosts.
Another factor limiting fungal vaccine development is the number of different fungal pathogens that can cause infections. Although collectively fungal infections constitute a major public health burden on a global scale, individual fungal pathogens typically impact specific populations in geographically restricted areas. Additionally, people susceptible to fungal infection, e.g., those with HIV/AIDS, are often at risk for infections by multiple fungal pathogens. Ideally, a pan-fungal vaccine capable of protecting at-risk populations from infections by multiple fungal pathogens would significantly increase the impact on public health and attract the interest of the pharmaceutical industry. A few studies have reported vaccine candidates capable of protecting immunized animals against multiple invasive fungal pathogens. For example, HK-fbp1-vaccinated mice are protected against Cryptococcus neoformans, Cryptococcus gattii, and A. fumigatus (138), while mice immunized with the rAls3p-N vaccine (NDV-3A) are also protected against the bacterial pathogen Staphylococcus aureus.
Fungal cells have complex cell wall structures, which also increases the difficulty of identifying antigenic factors for vaccine development. Antigenic factors will not necessarily be a single component in many whole cell–based vaccine candidates. It may also be challenging to identify antigenic factors and optimize the vaccine, both of which would increase vaccine development costs and affect potential efficacy.
The limitations described above may have an impact on the size of the market for fungal vaccines, as well as the interest of the pharmaceutical industry and private funders. Funding sources beyond industry partners, such as government agencies and foundations, may provide support to bridge funding gaps for fungal vaccine development.
STATUS OF FUNGAL VACCINE RESEARCH AND DEVELOPMENT
In recent years, rapid advances in fungal immunology and fungal gene functional analyses have identified several mutant fungal strains capable of inducing Th1 and Th17 protective immunity. These strains also impart protection against virulent fungal strains in animal models. These findings have sparked new research interest in fungal vaccine development. Although many vaccine candidates have been proposed or reported for a variety of fungal pathogens, as summarized in several excellent review articles (3, 7, 23, 59, 79, 88, 118) (Table 1), more systematic research has also been done on several well-studied fungal infections. These thorough studies have led to a better understanding of fungal biology and immunology. Here, we provide a broad summary but also focus on a handful of major fungal pathogens.
Table 1.
Fungal vaccine candidates
| Vaccine | Background | Mechanism | Target diseases | Status | Reference(s) |
|---|---|---|---|---|---|
| Whole-cell vaccines (live attenuated or killed cells) | |||||
| PCA-2 | Live Candida albicans strain lacking yeast-hypha conversion | Increased polymorphonuclear cells and macrophage activities | Candidiasis Staphylococcus aureus infection | Murine model | 2 |
| CNC13 | Live C. albicans strain deleted of kinase Hog1 | Immunoglobulins and IgG2a | Candidiasis | Murine model | 35 |
| Ter-NRG1 | Live C. albicans overexpressing transcription factor Nrg1 | T cell response | Candidiasis | Murine model | 108 |
| RML2U | Live C. albicans strain deleted of Ecm33 | Antibody response | Candidiasis | Murine model | 74 |
| cph1/efg1 | Live C. albicans strain deleted of transcription factors Cph1 and Efg1 | Not defined | Candidiasis | Murine model | 149 |
| H99γ | Live Cryptococcus neoformans strain expressing human IFN-γ | T cell response | Cryptococcosis | Murine model | 141 |
| Cda123 | Live or heat-killed C. neoformans strain lacking chitin deacetylase activity | T cell response | Cryptococcosis | Murine model | 134 |
| Znf2OE | C. neoformans strain overexpressing transcription factor Znf2 | T cell response | Cryptococcosis | Murine model | 150 |
| HK-fbp1 | Heat-killed C. neoformans strain deleted of Fbp1 | T cell response | Cryptococcosis Aspergillosis | Murine model | 75, 138 |
| Sgl1 | Live C. neoformans strain deleted of Sgl1 | T cell response | Cryptococcosis | Murine model | 20 |
| Killed spherules | Formalin-killed Coccidioides immitis strain | Not defined | Coccidioidomycosis | Human phase 3 trial | 90 |
| Cps1 | Live C. immitis strain deleted of Cps1 | T cell response | Coccidioidomycosis | Murine model | 84 |
| Bad1 | Live Blastomyces dermatitis strain deleted of Bad1 | Multiple arms of immune responses | Blastomycosis | Murine model | 144 |
| Recombinant or subunit vaccines | |||||
| NDV-3A | Agglutinin-like adhesion protein Als3, with Alhydrogel adjuvant | T cell– and antibody-mediated responses | Candidiasis S. aureus infection | Human phase 2a trial | 32, 119 |
| PEV7 | Secreted aspartyl protease Sap, with cholera toxin adjuvant | Antibody response | Candidiasis | Human phase 1 trial | 24 |
| CWSP | β-Mercaptoethanol-extracted Candida cell wall proteins, with liposomal adjuvant | Antibody and Th17 responses | Candidiasis | Murine model | 126 |
| Mannan-glycopeptides | C. albicans glycopeptides conjugated with β-mannan | Antibody response | Candidiasis | Murine model | 148 |
| β-Glucan-CRM197 | C. albicans β-glucan-CRM197 conjugates, with complete Freund’s adjuvant | Antibody response | Candidiasis | Murine model | 37 |
| GXM-TT | C. neoformans GXM conjugated with tetanus toxoid | Antibody response | Cryptococcosis | Murine model | 37 |
| GXM-BSA | C. neoformans GXM conjugated with BSA | Antibody response | Cryptococcosis | Murine model | 18 |
| Glucan particles | Glucan particles packaged with C. neoformans alkaline extracts | Antibody and T cell responses | Cryptococcosis | Murine model | 115, 116 |
| Aspf2 | Recombinant allergen; only works in immunocompetent hosts | T cell response | Aspergillosis | Murine model | 13 |
| Crf1 | Cell wall glucanase Crf1, with CpG adjuvant | T cell response | Aspergillosis Candidiasis | Murine model | 123 |
| AF.KEX1 | Protease KexB; protects immunocompromised mice | Antibody response | Aspergillosis | Murine model | 97 |
| Antigen 2 | Proline-rich antigen on cell surface, with CpG adjuvant | T cell response | Coccidioidomycosis | Murine model | 56 |
| rHsp60 | Histoplasmosis capsulatum recombinant glycoprotein Hsp60 | T cell response | Histoplasmosis | Murine model | 39 |
| H antigen | Recombinant β-glucosidase protein in H. capsulatum | T cell response | Histoplasmosis | Murine model | 27 |
| Antibody-based vaccines | |||||
| Mycograb | C. albicans Hsp90 recombinant antibody | Therapeutic antibody | Candidiasis | Human trials | 89 |
| GXM antibody 18B7 | C. neoformans GXM monoclonal antibody | Therapeutic antibody | Cryptococcosis | Human phase 1 trial | 62 |
| P13 | A peptide mimic of C. neoformans GXM | Antibody response | Cryptococcosis | Murine model | 37 |
| Anti-Crf1 | Neutralizing anybody against Crf1 protein | Therapeutic antibody | Aspergillosis | Murine model | 15 |
| β-Glucan antibody | β-Glucan monoclonal antibody | Therapeutic antibody | Cryptococcosis | Murine model | 96 |
| Glucosylceramide antibody | Monoclonal antibody | Therapeutic antibody | Cryptococcosis | Murine model | 104 |
Abbreviations: BSA, bovine serum albumin; GXM, glucuronoxylomannan; HSP60, heat shock protein 60; rHSP60, recombinant HSP60; TT, tetanus toxoid.
Candida Vaccine Candidates
Candidiasis, typically that caused by Candida albicans, is the most common yeast infection in humans and the fourth-most common bloodstream infection in hospitalized patients in the United States (140). C. albicans is a commensal human gut microbiota species. In immunocompromised or other impaired host conditions, Candida spp. can undergo morphological changes to produce infectious pseudohyphae and hyphae. Successful infection by C. albicans relies on multiple virulence strategies, including high plasticity in morphological switching and robust biofilm formation (124). C. albicans then disseminates through the bloodstream to cause systemic infection. While C. albicans is the most common Candida species causing candidiasis, several other Candida species can cause invasive candidiasis, including C. glabrata, C. parapsilosis, C. tropicalis, and C. krusei. Due to the increased use of antifungal drugs, some Candida species are increasingly drug resistant. Furthermore, certain drug-resistant species, such as C. glabrata, have become more abundant in certain areas, such as Europe (136). The recent outbreak of C. auris in hospital settings has raised significant concern, as this species is resistant to multiple antifungal drug classes. Due to the limited number of antifungal drugs, increased drug resistance, and scarce antifungal drug availability in large parts of the world, non-drug-based treatment strategies are urgently needed. Ideally, an effective vaccine against Candida infection would also overcome the increasing challenges of antifungal drug resistance.
Over the years, multiple fungal vaccine candidates have shown efficacy in animal models (Table 1); however, only a few have been tested in human clinical trials. Several C. albicans mutant strains have shown efficacy in animal models and have been proposed for human vaccine development. As the yeast-to-hyphae transition is a major virulence factor in C. albicans, proteins required for such a dimorphic switch have been characterized for their role in host-pathogen interaction and infection. Several mutants defective in the yeast-to-hyphae transition have been shown to induce protective immunity in mice against wild-type C. albicans challenge. Among these hyphal defective strains are the PCA-2 strain that is resistant to echinocandins and agerminative (2), the CNC13 strain that lacks the MAP kinase Hog1 protein (35), the NRG1 strain that overexpresses the filamentation repressor Nrg1 (108), the RML2U strain that lacks the cell wall protein Ecm33(74), and the cph1/efg1 double mutant strain that locks fungal cells in yeast phase (149). These mutants have all been tested vaccines to protect against challenge by their respective wild-type strains, and many showed positive outcomes in animal models. In addition, the PCA-2 vaccine also shows cross protection against S. aureus, suggesting a conserved antigenic factor in these organisms or nonspecific host immune activation mechanisms that remain poorly understood. Despite positive protection in murine models, none of these candidates has been tested in human clinical trials. Lack of development for human trials may be due to the effect of most of these fungal infections on immunocompromised hosts. Use of live attenuated vaccines in immunocompromised hosts has clear safety concerns, including the chance of the host developing disease. C. albicans is a commensal human gut organism, and a minor concern also exists that a Candida vaccine may affect the normal host gut microbiota.
Beyond using live attenuated vaccines based on virulence-defective mutants, exciting developments have been reported for recombinant-protein-based approaches. These approaches have mainly focused on the Al (agglutinin-like sequence) and Sap (secreted aspartyl protease) protein families. Als are fungal surface glycoproteins that play an important role in yeast adhesion and are required for Candida infection (46). This eight-member protein family shares a four-domain structure consisting of (a) a high-complexity N-terminal domain that mediates interaction with host cells or other substrates, (b) a threonine-rich domain, (c) a central domain, and (d) a C-terminal domain that anchors the protein to the fungal cell wall through a glycosylphosphatidylinositol anchor (46). Following the first report of an α-agglutinin-like protein in 1998 (47), studies on Als gene family members have provided a deep understanding of their functions, especially their role in Candida adhesion to host surfaces and medical implants. Among the eight Al proteins in C. albicans, Als1 and Als3 have been extensively studied in genetic and biochemical analyses to understand their roles in adhesion, biofilm formation, and fungal virulence (16, 54, 92, 121). Since both of these Als proteins are expressed on the fungal surface and are important for virulence, recombinant Als1 and Als3 protein fragment vaccine approaches have been proposed. The N termini of Als1 (rAls1p-N) and Als3 (rAls3p-N) have both been successfully utilized for vaccine development with or without adjuvants (54, 70, 121). rAls1p-N was expressed and purified in Saccharomyces cerevisiae and was used in combination with complete Freund’s adjuvant, being administrated subcutaneously. A booster was given at 21 days after immunization, before challenge with wild-type C. albicans. This vaccine approach was deemed effective, reducing fungal burden and displaying an improved murine survival rate of 50–57%. The vaccine also protected neutropenic mice from oropharyngeal candidiasis as well as vaginal candidiasis (54, 121). Compared to rAls1p-N, the rAls3p-N recombinant vaccine shows an even stronger antibody response and improved animal survival in murine models (120). The rAls3p-N vaccine, based on the N terminus of Als3, also protects mice against the bacterial pathogen S. aureus (119). This cross protection is likely due to the similarity of antigenic factor, as Als3 is structurally similar to a clumping factor in S. aureus. Encouragingly, an rAls3p-N vaccine formulated with an Alhydrogel adjuvant has been tested in women in a phase 1b/2a clinical trial for recurrent vulvovaginal candidiasis (RVVC), with promising results (32).
The ten-member Sap gene family represents a new virulence factor that is required for Candida virulence through regulation of the yeast-to-hyphae transition, as well as for host adhesion and deep-tissue penetration of the fungus (49, 61). Among this family, Sap2 is most abundant in C. albicans. A vaccine based on recombinant Sap2 (rSap2) is capable of clearing vaginal candidiasis in animal models. PEV7, a modified version of the rSap2 vaccine that consists of a truncated recombinant Sap2 incorporated into influenza virosomes, induces anti-Sap2 IgG and IgA. Furthermore, PEV7 induces long-lasting protection when administered as an intravaginal immunization (24). PEV7 was also safe in a repeated-dose toxicological study in rats. PEV7 is being tested in a phase 1 clinical trial for RVVC; the vaccine has yielded positive outcomes for all immunized patients and produced mucosal immune responses with high titers.
Mycograb, codeveloped by Novartis, is a human recombinant antibody against fungal Hsp90 that consists of Hsp90 peptide (NKILKVIRKNIVKK) cross-linked with human heavy-chain antibody. The heat shock protein Hsp90 is a highly conserved chaperone protein on fungal cell walls that is induced by stress stimuli. Hsp90 is abundant and immunogenic and is required for cell viability in C. albicans (77). In combination with Amphotericin B, Mycograb significantly improves survival in a murine model of systemic candidiasis (89). However, Mycograb did not gain regulatory approval and was later found that its ability to potentiate amphotericin B effects were likely not Hsp90-specific (99).
Cell wall extracts and some cell wall components, such as mannans and β-glucans, are conjugated with an antigenic factor or adjuvants that have been tested for potential vaccine development against Candida spp. Mice immunized with β-mercaptoethanol-extracted C. albicans cell wall protein mixtures in combination with the Ribi Adjuvant System (RAS) R-700 showed improved survival when challenged with C. albicans (126). Fungal glycoconjugates, such as mannans and β-glucans, are major PAMPs and can be recognized by pathogen recognition receptors. As such, they have been investigated as vaccine candidates. Cell surface mannans are among such PAMPs and are recognized by multiple host receptors. Consistent with the observation that mannosylated antigens demonstrate more effective presentation than nonmannosylated antigens, cell surface peptides linked with mannans have been utilized as vaccine candidates in murine models of C. albicans immunization (148). C. albicans β-glucan is another cell surface antigenic component that elicits Dectin-1 receptor–mediated innate immunity (125). Fungal β-glucan in combination with MF59 adjuvant or diphtheria toxoid (Lam-CRM197) has shown protection against murine vaginal candidiasis (93, 127). However, although highly immunogenic, the complex nature of mannans and β-glucan exposure on fungal cell surfaces may also result in varied host protection. Better mechanistic understanding of polysaccharide-mediated immune responses will likely be required before translation of these findings into safe and effective human antifungal vaccines (129).
Cryptococcus Vaccine Candidates
Cryptococcosis is a life-threatening fungal infection mostly caused by inhaling environmental fungal spores and yeasts produced by C. neoformans and C. gattii. Inhaled fungal cells lodge deep into lung alveoli and cause pulmonary infection. Cells often then disseminate to the central nervous system and cause cryptococcal meningoencephalitis, which is uniformly fatal without proper treatment (52). Cryptococcus infection is the leading cause of fungal meningitis and accounts for roughly 15% of HIV/AIDS-related deaths. Although C. neoformans is considered an opportunistic pathogen that often infects immunocompromised populations, C. gattii can infect immunocompetent individuals and is thus considered a primary pathogen. Similar to treatments for candidiasis and other invasive mycoses, cryptococcosis treatment is limited, and specific vaccines are needed. Although no vaccine is available, there are exciting ongoing research activities to develop prophylactic vaccines against cryptococcus infection.
The C. neoformans cell surface capsule has a unique structure and is likely a key virulence factor. The C. neoformans polysaccharide capsule comprises primarily glucuronoxylomannan (GXM) and galactoxylomannan, with a minimal amount of mannoproteins. This polysaccharide capsule enables cryptococcal cells to evade the host immune system and cause systemic infection. EarlyC. neoformans vaccine research used polysaccharide antigens. However, the polysaccharide capsule is generally considered a T cell–independent type 2 antigen, has anti-inflammation and antiphagocytosis functions, and is thought to suppress Th1 protective immunity—making capsule GXM a poor immunogenic antigen. Indeed, GXM-only vaccines do not induce the strong immune responses associated with long-term immune memory. To overcome this, polysaccharide-based vaccines were later constructed of capsule protein conjugated to other antigenic carriers, e.g., tetanus toxoid (TT) (28, 37) or bovine serum albumin (BSA) (18, 37). These GXM conjugated vaccines showed an improved animal survival rate after fungal challenge in murine models (10). Recently, a GXM monoclonal antibody, 18B7, has been developed as a potential neutralizing antibody. A phase 1 clinical trial of 18B7 in HIV patients with cryptococcal antigenemia has been completed. 18B7 displays a modest reduction in cryptococcal antigen titers (9, 62). However, this titer reduction is not long-lasting, suggesting further modifications may be necessary for a sustained response. Other monoclonal antibodies generated against Cryptococcus cell wall components such as β-glucan (96), glucosylceramide (104), and melanin (106) have also been utilized to passively immunize mice, with only modestly positive outcomes. However, all these antibodies did reduce lung fungal burden and prolong survival of treated mice.
In addition to capsules, other fungal antigenic factors have been identified, potentially providing opportunities for single-component-based vaccine development. Analysis of fungal antigen fractions revealed mannoprotein as an important antigenic component for stimulating cell-mediated immunity. These findings led to the identification of MP98, a chitin deacetylase motif required for converting chitin to chitosan, and MP88, whose function remains unknown (48, 63). Studies of these mannoproteins have demonstrated the importance of posttranslational modification in recombinant-protein-based vaccine design, as N- and O-linked mannosylation is essential for antigen recognition and optimal T cell responses (64, 117). However, not all mannoproteins display the same proinflammatory responses, and a more detailed understanding of individual mannoprotein fractions is required. Furthermore, the combination of certain adjuvants with mannoprotein antigens may be required to induce desired host immune responses (72). One fungus-based adjuvant is β-glucan particles (GPs), which have been developed as an antigen delivery platform. Cryptococcus alkaline extracts packaged in GPs have been tested as a vaccine strategy against cryptococcosis. Mice immunized with GPs containing fungal extracts and single-antigen proteins produced antigen-specific CD4+ T cell recall responses (115, 116). Vaccinated mice also exhibited prolonged protection against C. neoformans or C. gattii challenge (115, 116). One exciting aspect of this system is that GPs act both as a delivery system and as an adjuvant. This property allows for a vaccine strategy where GPs are used to package different antigenic fractions and are mixed with different antigens into a single vaccine—increasing vaccine development feasibility and flexibility.
Recently, multiple groups have reported exciting findings toward the development of whole-cell-based vaccine candidates derived from genetically modified Cryptococcus strains. Given the importance of Th1 cytokine IFN-γ in anticryptococcal activity, C. neoformans wild-type strain H99 expressing murine IFN-γ (H99γ) was engineered and tested in a murine model of systemic cryptococcosis (141). Mice infected with live H99γ resolved their infection and showed full protection against subsequent challenge of H99, with complete clearance (141). Impressively, follow-up studies showed that protection remains even in mice depleted of CD4+ or CD8+ T cells by antibodies (142). Trained innate immunity was also proposed to be important in this H99γ-mediated vaccine protection (45). Several other genetically modified Cryptococcus strains have also shown strong protection in murine models against H99 challenge. These strains include a Cryptococcus strain locked in pseudohypha form due to overexpression of the mating-specific transcription factor Znf2 (Znf2OE) (150), a strain where chitin deacetylase genes (cda1Δ2Δ3Δ) are deleted (133, 134), a strain lacking sterol glucosidase (sgl1Δ) (20, 87, 98), and an fbp1Δ null mutant strain that lacks SCF E3 ligase subunit Fbp1 function (69, 75, 138). Vaccination with H99γ and sgl1Δ live cells, but not their heat-inactivated counterparts, conferred protection in mice. Furthermore, vaccination with heat-inactivated cda1Δ2Δ3Δ, Znf2OE, and fbp1Δ cells also conferred strong protection. Impressively, some of these vaccine candidates (H99γ, cda1Δ2Δ3Δ, fbp1Δ, sgl1Δ) conferred protection even in T cell–depleted mice, suggesting that they may be effective in immunocompromised patients with low CD4+ T cell counts. Mice immunized with heat-killed fbp1Δ cells showed a broad spectrum of protection, with strong protection not only against C. neoformans and C. gattii challenge, but also against A. fumigatus, another major fungal pathogen. It will be interesting to determine whether this fbp1Δ vaccine contains a conserved antigenic factor or other immune mechanism that could trigger trained innate immunity. Although GXM secretion is important for sgl1Δ vaccine protection, the detailed mechanisms of protection in many of these strains, including fbp1Δ, remain elusive. In the future, it would be informative to compare these diverse vaccine strains to determine whether they share specific common core antigenic factors or immune mechanisms that allow them to confer protection. It will also be important to identify the specific factors that are sufficient to confer protection in each vaccine candidate. This would provide a better understanding of the unique protection mechanisms for each strain. Such studies may require joint efforts and greater collaboration among groups interested in Cryptococcus vaccine research and development.
Aspergillus Vaccine Candidates
A. fumigatus is a major filamentous fungal pathogen that causes significant invasive infection with a high mortality rate. As A. fumigatus infects mostly people with severe immunodeficiencies, including neutropenic patients, vaccine research and development has been limited. With an improved understanding of fungal immunology, we now understand some vaccines can be protective even in immunocompromised individuals. A prophylactic Aspergillus vaccine would still be very valuable for specific patient populations, such as individuals undergoing organ transplantation or cancer chemotherapy. Early studies showed that A. fumigatus crude culture filtrate antigens and the recombinant allergen Aspf2 can induce development of local and peripheral protective Th1 memory responses. This approach resulted in protective antifungal responses in mice with invasive pulmonary aspergillosis. However, this induction was only detected in immunocompetent mice and not IFN-γ- or IL-12-deficient immunocompromised mice (13). The complex nature of the cell extract mixture used in this approach may make it difficult to determine the specific mechanism of antigenicity and to produce vaccines on a large scale consistent outcomes.
A vaccine candidate based on an immunogenic epitope of the A. fumigatus cell wall glucanase Crf1 has been studied quite extensively. The Crf1 epitope induces memory CD4+ Th1 cells and elicits cross protection against lethal infection with both A. fumigatus and C. albicans (123). Recently, anti-Crf antibodies were found to neutralize the enzymatic activity of recombinant Crf1 protein, and when added to spores they led to delayed fungal growth in vitro. These studies demonstrate the therapeutic potential of targeting Crf cell wall proteins with anti-Crf antibodies (15). More recently, AF.KEX1, a recombinant vaccine candidate based on the A. fumigatus protease protein KexB, showed protection in immunocompromised mice that were treated with steroids or tacrolimus/hydrocortisone following challenge with wild-type Aspergillus. Vaccinated mice had prolonged survival rates and had reduced lung fungal burden compared to unvaccinated mice (97). However, despite these positive findings in animal models, no Aspergillus vaccine candidates have reached human trials.
Vaccine Candidates Against Endemic Mycoses
In addition to the major global fungal pathogens described above, endemic fungi exist in certain geographic regions and occupy specific niches. These endemic fungi can cause systemic infection in healthy human populations. Due to their ability to infect immunocompetent individuals, these fungi are attractive targets for vaccine development. Indeed, extensive vaccine research has targeted these endemic mycoses.
Coccidioidomycosis (Valley fever) is a fungal infection caused by inhalation of a Coccidioides species, mainly C. immitis and C. posadasii. These dimorphic fungi grow as a mycelium in the environment (e.g., soil) and produce arthroconidia that can be dispersed and inhaled by humans when the mycelium is fractured. Inside their mammalian host and at an elevated temperature of 37°C, arthroconidia develop into spherules with endospores. Coccidioidomycosis infections are geographically restricted to the southwestern desert regions of the United States and Mexico, where they are a significant public health burden. A vaccine for coccidioidomycosis is highly desired and appears feasible, as Coccidioides species are primary pathogens capable of infecting healthy individuals and secondary Coccidioides infections are very rare, suggesting long-lasting immune protection is possible. Indeed, multiple vaccine candidates have been reported in animal models. In the 1960s formalin-killed spherules or UV-irradiated live attenuated strains were used for Coccidioides vaccine development. Mice immunized with formalin-killed spherules were protected against challenge with inhaled Coccidioides (90). This is the only vaccine for this fungus that has been evaluated in human trials. Although it was deemed safe in humans, development stopped after a phase 3 trial did not demonstrate a clear reduction of incidence and disease severity in the vaccinated group (90). Live attenuated strains derived from either UV irradiation or a CPS1 gene deletion have also been tested and shown positive results in animal models (84). Nicely summarized in recent reviews (12, 59), live attenuated or single-antigen-component-based vaccine candidates have been tested in animal models, with encouraging results. Among these, antigen 2 has emerged as an attractive vaccine candidate. This proline-rich antigen is located under the surface of the spherule and is an effective vaccine against Coccidioides challenge in a murine inhalation model (56, 154). However, as Coccidioides cells contain multiple nuclei, making it a difficult system to study gene function, better understanding of the fungal biology and immunology will likely help in identifying a candidate and developing it into a suitable vaccine.
Blastomyces is an endemic fungus that causes blastomycosis, and the adhesion protein Bad1 is important for host-pathogen interactions. Mice had strong immune activation and increased survival when immunized with either a live attenuated bad1Δ mutant strain or a recombinant-Bad1-expressing yeast (144, 146). Histoplasmosis is another fungal infection, caused by the endemic fungus Histoplasma capsulatum. Histoplasmosis and blastomycosis are endemic in overlapping regions, with cases mostly concentrated along the Mississippi River valley in the east and Middle West of the United States. Early studies utilized cell wall extracts and fractions to confer protective immunity against otherwise lethal H. capsulatum challenge. Notably, a glycoprotein encoded by the heat shock gene HSP60 was identified in the 62-kDa fraction of the cell extract. Immunization with a recombinant Hsp60 (rHsp60) protein conferred strong protection against H. capsulatum challenge in a murine intranasal infection model. Immunized mice displayed priming of CD4+ Th1 effector responses against histoplasmosis (39). H antigen (27) and other antigens, as well as antibody-based passive immunization, have been tested for protection against histoplasmosis. The results are summarized in a recent review (107).
In conclusion, fungal vaccine development is the last frontier in vaccine development against infectious diseases and has gained significant interest in the past decade. A variety of fungal vaccine candidates, including both live attenuated and heat-killed whole-cell-based vaccines as well as recombinant antigen or subunit-based vaccines, have been reported for multiple fungal pathogens causing invasive mycoses (Table 1). However, very few of these vaccines have been evaluated in human clinical trials. It is hoped that some of these efforts will lead to an effective fungal vaccine for human clinical use in the near future.
FUTURE PERSPECTIVES
In addition to the previously described fungal vaccine candidates and platforms, several new platforms and technologies may help accelerate fungal vaccine research. The glucan-particle-based fungal vaccine delivery strategy has shown great potential (115, 116). Glucan particles are porous cell wall shells derived from the budding yeast S. cerevisiae. They contain primary β-1,3-glucan, which is recognized by host Dectin-1 receptors. One advantage of glucan particles is that they can be loaded with antigens, enabling delivery to phagocytes and eliciting robust and long-lasting antibody and Th1- and Th17-based protective T cell responses. Mice vaccinated with glucan particles carrying either crude antigen extracts or purified antigen proteins all showed strong protection against wild-type C. neoformans challenge (115). This glucan particle platform may also provide a screening opportunity to identify antigen candidates against different fungal infections.
Fungal extracellular vesicles (EVs) have been proposed as fungal vaccine candidates. EVs are lipid bilayer particles that are secreted by almost all living fungi (26, 103). In fungal pathogens like C. neoformans, EVs carry many immunogenic proteins, including mannoprotein MP88, chitin deacetylase Cda family proteins, and Vep proteins—all of which have been tested as vaccine candidates (101). Therefore, EVs may offer a novel approach. In a recent study, mice immunized with EVs isolated from Cryptococcus acapsular cap59Δ mutants produced antibodies against EV proteins and showed strong protection against wild-type H99 challenge (101). EVs isolated from Candida and Paracoccidioides also show positive immunogenicity and vaccine protection in animal models (73, 102). The potential advantage of an EV-based vaccine is that it is a natural mix of multiple antigens that provides strong immunity. EVs offer more stable conformational conditions for protein components that circulate in body fluids and show efficient association with antigen-presenting cells (55). However, there are potential obstacles for developing fungal-EV-based vaccines. EVs are a mixture of many components, some of which could be toxic or immunosuppressive. Additionally, given their heterogenicity, it may be challenging to produce EVs at a large scale. With continued refinement, EVs may provide a novel strategy for fungal vaccine development.
Nanoparticle technology has been proposed to enhance fungal vaccine efficacy (60). Nanoparticles can be made from different materials, including fungal cell surface carbon hydrate components, lipid-based particles, and metallic nanoparticles, and are effective fungal antigen carriers. Since mRNA was discovered to induce humoral immunity in 1995, mRNA vaccine technology has been proposed to develop a new generation of vaccines against cancer and infectious diseases (14). The recent approval of two COVID-19 mRNA vaccines has accelerated the use of this technology, offering hope for more mRNA-based vaccines in the future. Both COVID-19 mRNA vaccines use a lipid nanoparticle carrier to deliver SARS-CoV-2 spike protein mRNA. Similarly, lipid nanoparticles may be utilized to deliver mRNAs of fungal cell surface proteins.
The ultimate goal for fungal vaccine development may be a pan-fungal vaccine that can protect hosts against multiple fungal infections. Several strategies have been proposed for pan-fungal vaccine development: utilization of the β-glucan from S. cerevisiae, identification of monoclonal antibodies (IgG2, IgM) against conserved fungal antigens, etc. Although the goal remains challenging, the combination of improved technology platforms, a better understanding of fungal immunology (e.g., trained immunity) and disease mechanisms, and increased awareness of fungal infection may soon lead to new fungal vaccine breakthroughs and adoption of these vaccines into clinical care.
ACKNOWLEDGMENTS
We thank Samantha Avina for reading the manuscript and technique support. We thank Keyi Wang for assistance in the generation of figures. All figures were created with BioRender.com. This work is support by the NIH grants R01AI141368 to AR and CX, and R01AI125045 to JL. AR holds an Investigators in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund. The Xue lab is also supported by the NIH grants R01AI123315 and R21AI154318, and the Lodge lab is also supported by R01AI123407.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Bar E, Whitney PG, Moor K, Reis e Sousa C, LeibundGut-Landmann S. 2014. IL-17 regulates systemic fungal immunity by controlling the functional competence of NK cells. Immunity 40:117–27 [DOI] [PubMed] [Google Scholar]
- 2.Bistoni F, Vecchiarelli A, Cenci E, Puccetti P, Marconi P, Cassone A. 1986. Evidence for macrophage-mediated protection against lethal Candida albicans infection. Infect. Immun 51:668–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biswas PS. 2021. Vaccine-induced immunological memory in invasive fungal infections—a dream so close yet so far. Front. Immunol 12:671068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bongomin F, Gago S, Oladele RO, Denning DW. 2017. Global and multi-national prevalence of fungal diseases—estimate precision. J. Fungi 3:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourgeois C, Kuchler K. 2012. Fungal pathogens—a sweet and sour treat for Toll-like receptors. Front. Cell Infect. Microbiol 2:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchanan KL, Doyle HA. 2000. Requirement for CD4+ T lymphocytes in host resistance against Cryptococcus neoformans in the central nervous system of immunized mice. Infect. Immun 68:456–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caballero Van Dyke MC, Wormley FL Jr. 2018. A call to arms: quest for a cryptococcal vaccine. Trends Microbiol. 26:436–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caldera F, Mercer M, Samson SI, Pitt JM, Hayney MS. 2021. Influenza vaccination in immunocompromised populations: strategies to improve immunogenicity. Vaccine 39(Suppl. 1):A15–23 [DOI] [PubMed] [Google Scholar]
- 9.Casadevall A, Cleare W, Feldmesser M, Glatman-Freedman A, Goldman DL, et al. 1998. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob. Agents Chemother 42:1437–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casadevall A, Pirofski L. 2005. Insights into mechanisms of antibody-mediated immunity from studies with Cryptococcus neoformans. Curr. Mol. Med 5:421–33 [DOI] [PubMed] [Google Scholar]
- 11.Casadevall A, Pirofski LA. 2012. Immunoglobulins in defense, pathogenesis, and therapy of fungal diseases. Cell Host Microbe 11:447–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castro-Lopez N, Hung CY. 2017. Immune response to coccidioidomycosis and the development of a vaccine. Microorganisms 5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cenci E, Mencacci A, Bacci A, Bistoni F, Kurup VP, Romani L. 2000. T cell vaccination in mice with invasive pulmonary aspergillosis. J. Immunol 165:381–88 [DOI] [PubMed] [Google Scholar]
- 14.Chakraborty C, Sharma AR, Bhattacharya M, Lee SS. 2021. From COVID-19 to cancer mRNA vaccines: moving from bench to clinic in the vaccine landscape. Front. Immunol 12:679344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chauvin D, Hust M, Schutte M, Chesnay A, Parent C, et al. 2019. Targeting Aspergillus fumigatus Crf transglycosylases with neutralizing antibody is relevant but not sufficient to erase fungal burden in a neutropenic rat model. Front. Microbiol 10:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng G, Wozniak K, Wallig MA, Fidel PL Jr., Trupin SR, Hoyer LL. 2005. Comparison between Candida albicans agglutinin-like sequence gene expression patterns in human clinical specimens and models of vaginal candidiasis. Infect. Immun 73:1656–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, et al. 2014. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345:1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chow SK, Casadevall A. 2011. Evaluation of Cryptococcus neoformans galactoxylomannan-protein conjugate as vaccine candidate against murine cryptococcosis. Vaccine 29:1891–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chuck SL, Sande MA. 1989. Infections with Cryptococcus neoformans in the acquired immunodeficiency syndrome. N. Engl. J. Med 321:794–99 [DOI] [PubMed] [Google Scholar]
- 20.Colombo AC, Rella A, Normile T, Joffe LS, Tavares PM, et al. 2019. Cryptococcus neoformans glucuronoxylomannan and sterylglucoside are required for host protection in an animal vaccination model. mBio 10:e02909–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conti HR, Gaffen SL. 2015. IL-17-mediated immunity to the opportunistic fungal pathogen Candida albicans. J. Immunol 195:780–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crum-Cianflone NF, Wallace MR. 2014. Vaccination in HIV-infected adults. AIDS Patient Care STDS 28:397–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cutler JE, Deepe GS Jr., Klein BS. 2007. Advances in combating fungal diseases: vaccines on the threshold. Nat. Rev. Microbiol 5:13–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Bernardis F, Amacker M, Arancia S, Sandini S, Gremion C, et al. 2012. A virosomal vaccine against candidal vaginitis: immunogenicity, efficacy and safety profile in animal models. Vaccine 30:4490–98 [DOI] [PubMed] [Google Scholar]
- 25.de la Rua NM, Samuelson DR, Charles TP, Welsh DA, Shellito JE. 2016. CD4+ T-cell-independent secondary immune responses to Pneumocystis pneumonia. Front. Immunol 7:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deatherage BL, Cookson BT. 2012. Membrane vesicle release in bacteria, eukaryotes, and archaea: a conserved yet underappreciated aspect of microbial life. Infect. Immun 80:1948–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deepe GS Jr., Gibbons R. 2001. Protective efficacy of H antigen from Histoplasma capsulatum in a murine model of pulmonary histoplasmosis. Infect. Immun 69:3128–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Devi SJ. 1996. Preclinical efficacy of a glucuronoxylomannan-tetanus toxoid conjugate vaccine of Cryptococcus neoformans in a murine model. Vaccine 14:841–44 [DOI] [PubMed] [Google Scholar]
- 29.Diaz-Arevalo D, Ito JI, Kalkum M. 2012. Protective effector cells of the recombinant Asp f3 antiaspergillosis vaccine. Front. Microbiol 3:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Divangahi M, Aaby P, Khader SA, Barreiro LB, Bekkering S, et al. 2021. Trained immunity, tolerance, priming and differentiation: distinct immunological processes. Nat. Immunol 22:2–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Durkin M, Kohler S, Schnizlein-Bick C, LeMonte A, Connolly P, et al. 2001. Chronic infection and reactivation in a pulmonary challenge model of histoplasmosis. J. Infect. Dis 183:1822–24 [DOI] [PubMed] [Google Scholar]
- 32.Edwards JE Jr., Schwartz MM, Schmidt CS, Sobel JD, Nyirjesy P, et al. 2018. A fungal immunotherapeutic vaccine (NDV-3A) for treatment of recurrent vulvovaginal candidiasis—a phase 2 randomized, double-blind, placebo-controlled trial. Clin. Infect. Dis 66:1928–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El Chaer F, El Sahly HM. 2019. Vaccination in the adult patient infected with HIV: a review of vaccine efficacy and immunogenicity. Am. J. Med 132:437–46 [DOI] [PubMed] [Google Scholar]
- 34.Espinosa V, Rivera A. 2012. Cytokines and the regulation of fungus-specific CD4 T cell differentiation. Cytokine 58:100–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandez-Arenas E, Molero G, Nombela C, Diez-Orejas R, Gil C. 2004. Low virulent strains of Candida albicans: unravelling the antigens for a future vaccine. Proteomics 4:3007–20 [DOI] [PubMed] [Google Scholar]
- 36.Fierer J, Waters C, Walls L. 2006. Both CD4+ and CD8+ T cells can mediate vaccine-induced protection against Coccidioides immitis infection in mice. J. Infect. Dis 193:1323–31 [DOI] [PubMed] [Google Scholar]
- 37.Fleuridor R, Lees A, Pirofski L. 2001. A cryptococcal capsular polysaccharide mimotope prolongs the survival of mice with Cryptococcus neoformans infection. J. Immunol 166:1087–96 [DOI] [PubMed] [Google Scholar]
- 38.Glesby MJ. 1998. Immunizations during HIV infection. Curr. Opin. Infect. Dis 11:17–21 [DOI] [PubMed] [Google Scholar]
- 39.Gomez FJ, Allendoerfer R, Deepe GS Jr. 1995. Vaccination with recombinant heat shock protein 60 from Histoplasma capsulatum protects mice against pulmonary histoplasmosis. Infect. Immun 63:2587–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hardison SE, Brown GD. 2012. C-type lectin receptors orchestrate antifungal immunity. Nat. Immunol 13:817–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hatinguais R, Willment JA, Brown GD. 2020. PAMPs of the fungal cell wall and mammalian PRRs. Curr. Top. Microbiol. Immunol 425:187–223 [DOI] [PubMed] [Google Scholar]
- 42.Hawksworth DL. 1991. The fungal dimension of biodiversity: magnitude, significance, and conservation. Mycol. Res 95:641–55 [Google Scholar]
- 43.Hernandez-Santos N, Huppler AR, Peterson AC, Khader SA, McKenna KC, Gaffen SL. 2013. Th17 cells confer long-term adaptive immunity to oral mucosal Candida albicans infections. Mucosal. Immunol 6:900–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hill JO, Harmsen AG. 1991. Intrapulmonary growth and dissemination of an avirulent strain of Cryptococcus neoformans in mice depleted of CD4+ or CD8+ T cells. J. Exp. Med 173:755–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hole CR, Wager CML, Castro-Lopez N, Campuzano A, Cai H, et al. 2019. Induction of memory-like dendritic cell responses in vivo. Nat. Commun 10:2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoyer LL, Cota E. 2016. Candida albicans Agglutinin-Like Sequence (Als) family vignettes: a review of Als protein structure and function. Front. Microbiol 7:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoyer LL, Payne TL, Bell M, Myers AM, Scherer S. 1998. Candida albicans ALS3 and insights into the nature of the ALS gene family. Curr. Genet 33:451–59 [DOI] [PubMed] [Google Scholar]
- 48.Huang C, Nong SH, Mansour MK, Specht CA, Levitz SM. 2002. Purification and characterization of a second immunoreactive mannoprotein from Cryptococcus neoformans that stimulates T-cell responses. Infect. Immun 70:5485–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hube B, Sanglard D, Odds FC, Hess D, Monod M, et al. 1997. Disruption of each of the secreted aspartyl proteinase genes SAP1, SAP2, and SAP3 of Candida albicans attenuates virulence. Infect. Immun 65:3529–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huffnagle GB, Lipscomb MF, Lovchik JA, Hoag KA, Street NE. 1994. The role of CD4+ and CD8+ T cells in the protective inflammatory response to a pulmonary cryptococcal infection. J. Leukoc. Biol 55:35–42 [DOI] [PubMed] [Google Scholar]
- 51.Huffnagle GB, Yates JL, Lipscomb MF. 1991. Immunity to a pulmonary Cryptococcus neoformans infection requires both CD4+ and CD8+ T cells. J. Exp. Med 173:793–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hull CM, Heitman J. 2002. Genetics of Cryptococcus neoformans. Annu. Rev. Genet 36:557–615 [DOI] [PubMed] [Google Scholar]
- 53.Huppler AR, Conti HR, Hernández-Santos N, Darville T, Biswas PS, Gaffen SL. 2014. Role of neutrophils in IL-17-dependent immunity to mucosal candidiasis. J. Immunol 192:1745–52. Erratum. 2015.J. Immunol. 194:1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ibrahim AS, Spellberg BJ, Avenissian V, Fu Y, Filler SG, Edwards JE Jr. 2005. Vaccination with recombinant N-terminal domain of Als1p improves survival during murine disseminated candidiasis by enhancing cell-mediated, not humoral, immunity. Infect. Immun 73:999–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Joffe LS, Nimrichter L, Rodrigues ML, Del Poeta M. 2016. Potential roles of fungal extracellular vesicles during infection. mSphere 1:e00099–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johannesson H, Vidal P, Guarro J, Herr RA, Cole GT, Taylor JW. 2004. Positive directional selection in the proline-rich antigen (PRA) gene among the human pathogenic fungi Coccidioides immitis, C. posadasii and their closest relatives. Mol. Biol. Evol 21:1134–45 [DOI] [PubMed] [Google Scholar]
- 57.Kawakami K, Kohno S, Kadota J, Tohyama M, Teruya K, et al. 1995. T cell-dependent activation of macrophages and enhancement of their phagocytic activity in the lungs of mice inoculated with heat-killed Cryptococcus neoformans: involvement of IFN-gamma and its protective effect against cryptococcal infection. Microbiol. Immunol 39:135–43 [DOI] [PubMed] [Google Scholar]
- 58.Kelly MN, Zheng M, Ruan S, Kolls J, D’souza A, Shellito JE. 2013. Memory CD4+ T cells are required for optimal NK cell effector functions against the opportunistic fungal pathogen Pneumocystis murina. J. Immunol 190:285–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kirkland TN. 2016. The quest for a vaccine against coccidioidomycosis: a neglected disease of the Americas. J. Fungi 2:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kischkel B, Rossi SA, Santos SR, Nosanchuk JD, Travassos LR, Taborda CP. 2020. Therapies and vaccines based on nanoparticles for the treatment of systemic fungal infections. Front. Cell. Infect. Microbiol 10:463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Korting HC, Hube B, Oberbauer S, Januschke E, Hamm G, et al. 2003. Reduced expression of the hyphal-independent Candida albicans proteinase genes SAP1 and SAP3 in the efg1 mutant is associated with attenuated virulence during infection of oral epithelium. J. Med. Microbiol 52:623–32 [DOI] [PubMed] [Google Scholar]
- 62.Larsen RA, Pappas PG, Perfect J, Aberg JA, Casadevall A, et al. 2005. Phase I evaluation of the safety and pharmacokinetics of murine-derived anticryptococcal antibody 18B7 in subjects with treated cryptococcal meningitis. Antimicrob. Agents Chemother 49:952–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Levitz SM, Nong S, Mansour MK, Huang C, Specht CA. 2001. Molecular characterization of a mannoprotein with homology to chitin deacetylases that stimulates T cell responses to Cryptococcus neoformans. PNAS 98:10422–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levitz SM, Specht CA. 2006. The molecular basis for the immunogenicity of Cryptococcus neoformans mannoproteins. FEMS Yeast Res. 6:513–24 [DOI] [PubMed] [Google Scholar]
- 65.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, et al. 2006. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med 203:2271–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin JS, Yang CW, Wang DW, Wu-Hsieh BA. 2005. Dendritic cells cross-present exogenous fungal antigens to stimulate a protective CD8 T cell response in infection by Histoplasma capsulatum. J. Immunol 174:6282–91 [DOI] [PubMed] [Google Scholar]
- 67.Lindell DM, Moore TA, McDonald RA, Toews GB, Huffnagle GB. 2005. Generation of antifungal effector CD8+ T cells in the absence of CD4+ T cells during Cryptococcus neoformans infection. J. Immunol 174:7920–28 [DOI] [PubMed] [Google Scholar]
- 68.Liu F, Fan X, Auclair S, Ferguson M, Sun J, et al. 2016. Sequential dysfunction and progressive depletion of Candida albicans-specific CD4 T cell response in HIV-1 infection. PLOS Pathog. 12:e1005663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu TB, Xue C. 2014. Fbp1-mediated ubiquitin-proteasome pathway controls Cryptococcus neoformans virulence by regulating fungal intracellular growth in macrophages. Infect. Immun 82:557–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu Y, Filler SG. 2011. Candida albicans Als3, a multifunctional adhesin and invasin. Eukaryot. Cell 10:168–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Low A, Gavriilidis G, Larke N, BL MR, Drouin O, et al. 2016. Incidence of opportunistic infections and the impact of antiretroviral therapy among HIV-infected adults in low- and middle-income countries: a systematic review and meta-analysis. Clin. Infect. Dis 62:1595–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mansour MK, Yauch LE, Rottman JB, Levitz SM. 2004. Protective efficacy of antigenic fractions in mouse models of cryptococcosis. Infect. Immun 72:1746–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marina CL, Burgel PH, Agostinho DP, Zamith-Miranda D, Las-Casas LO, et al. 2020. Nutritional conditions modulate C. neoformans extracellular vesicles’ capacity to elicit host immune response. Microorganisms 8:1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martinez-Lopez R, Nombela C, Diez-Orejas R, Monteoliva L, Gil C. 2008. Immunoproteomic analysis of the protective response obtained from vaccination with Candida albicans ecm33 cell wall mutant in mice. Proteomics 8:2651–64 [DOI] [PubMed] [Google Scholar]
- 75.Masso-Silva J, Espinosa V, Liu TB, Wang Y, Xue C, Rivera A. 2018. The F-Box protein Fbp1 shapes the immunogenic potential of Cryptococcus neoformans. mBio 9:e01828–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Masur H, Ognibene FP, Yarchoan R, Shelhamer JH, Baird BF, et al. 1989. CD4 counts as predictors of opportunistic pneumonias in human immunodeficiency virus (HIV) infection. Ann. Intern. Med 111:223–31 [DOI] [PubMed] [Google Scholar]
- 77.Matthews RC, Burnie JP, Tabaqchali S. 1987. Isolation of immunodominant antigens from sera of patients with systemic candidiasis and characterization of serological response to Candida albicans. J. Clin. Microbiol 25:230–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McDermott AJ, Klein BS. 2018. Helper T-cell responses and pulmonary fungal infections. Immunology 155:155–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Medici NP, Del Poeta M. 2015. New insights on the development of fungal vaccines: from immunity to recent challenges. Mem. Inst. Oswaldo Cruz 110:966–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miyagi K, Kawakami K, Kinjo Y, Uezu K, Kinjo T, et al. 2005. CpG oligodeoxynucleotides promote the host protective response against infection with Cryptococcus neoformans through induction of interferon-gamma production by CD4+ T cells. Clin. Exp. Immunol 140:220–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mizutani S, Endo M, Ino-Ue T, Kurasawa M, Uno Y, et al. 2000. CD4+-T-cell-mediated resistance to systemic murine candidiasis induced by a membrane fraction of Candida albicans. Antimicrob. Agents Chemother 44:2653–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nami S, Mohammadi R, Vakili M, Khezripour K, Mirzaei H, Morovati H. 2019. Fungal vaccines, mechanism of actions and immunology: a comprehensive review. Biomed. Pharmacother 109:333–44 [DOI] [PubMed] [Google Scholar]
- 83.Nanjappa SG, Heninger E, Wuthrich M, Gasper DJ, Klein BS. 2012. Tc17 cells mediate vaccine immunity against lethal fungal pneumonia in immune deficient hosts lacking CD4+ T cells. PLOS Pathog. 8:e1002771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Narra HP, Shubitz LF, Mandel MA, Trinh HT, Griffin K, et al. 2016. A Coccidioides posadasii CPS1 deletion mutant is avirulent and protects mice from lethal infection. Infect. Immun 84:3007–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Netea MG, Dominguez-Andres J, Barreiro LB, Chavakis T, Divangahi M, et al. 2020. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol 20:375–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, et al. 2016. Trained immunity: a program of innate immune memory in health and disease. Science 352:aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Normile TG, Rella A, Del Poeta M. 2021. Cryptococcus neoformans Δsgl1 vaccination requires either CD4+ or CD8+ T cells for complete host protection. Front. Cell. Infect. Microbiol 11:739027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oliveira LVN, Wang R, Specht CA, Levitz SM. 2021. Vaccines for human fungal diseases: close but still a long way to go. npj Vaccines 6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pachl J, Svoboda P, Jacobs F, Vandewoude K, van der Hoven B, et al. 2006. A randomized, blinded, multicenter trial of lipid-associated amphotericin B alone versus in combination with an antibody-based inhibitor of heat shock protein 90 in patients with invasive candidiasis. Clin. Infect. Dis 42:1404–13 [DOI] [PubMed] [Google Scholar]
- 90.Pappagianis D. 1993. Evaluation of the protective efficacy of the killed Coccidioides immitis spherule vaccine in humans: the Valley Fever Vaccine Study Group. Am. Rev. Respir. Dis 148:656–60 [DOI] [PubMed] [Google Scholar]
- 91.Phair J, Munoz A, Detels R, Kaslow R, Rinaldo C, Saah A. 1990. The risk of Pneumocystis carinii pneumonia among men infected with human immunodeficiency virus type 1: Multicenter AIDS Cohort Study Group. N. Engl. J. Med 322:161–65 [DOI] [PubMed] [Google Scholar]
- 92.Phan QT, Myers CL, Fu Y, Sheppard DC, Yeaman MR, et al. 2007. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLOS Biol. 5:e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pietrella D, Rachini A, Torosantucci A, Chiani P, Brown AJ, et al. 2010. A beta-glucan-conjugate vaccine and anti-beta-glucan antibodies are effective against murine vaginal candidiasis as assessed by a novel in vivo imaging technique. Vaccine 28:1717–25 [DOI] [PubMed] [Google Scholar]
- 94.Pilon VA, Echols RM, Celo JS, Elmendorf SL. 1987. Disseminated Pneumocystis carinii infection in AIDS. N. Engl. J. Med 316:1410–11 [DOI] [PubMed] [Google Scholar]
- 95.Plotkin S, Plotkin S. 2013. A short history of vaccination. In Vaccines, ed. Plotkin S, O’renstein W, Offit P, pp. 1–13. Cambridge, MA: Elsevier [Google Scholar]
- 96.Rachini A, Pietrella D, Lupo P, Torosantucci A, Chiani P, et al. 2007. An anti-beta-glucan monoclonal antibody inhibits growth and capsule formation of Cryptococcus neoformans in vitro and exerts therapeutic, anticryptococcal activity in vivo. Infect. Immun 75:5085–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rayens E, Rabacal W, Kang SE, Celia BN, Momany M, Norris KA. 2021. Vaccine-induced protection in two murine models of invasive pulmonary aspergillosis. Front. Immunol 12:670578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rella A, Mor V, Farnoud AM, Singh A, Shamseddine AA, et al. 2015. Role of Sterylglucosidase 1 (Sgl1) on the pathogenicity of Cryptococcus neoformans: potential applications for vaccine development. Front. Microbiol 6:836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Richie DL, Ghannoum MA, Isham N, Thompson KV, Ryder NS. 2012. Nonspecific effect of Mycograb on amphotericin B MIC. Antimicrob. Agents Chemother 56:3963–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rivera A, Hohl TM. 2015. Calnexin bridges the gap toward a pan-fungal vaccine. Cell Host Microbe 17:421–23 [DOI] [PubMed] [Google Scholar]
- 101.Rizzo J, Chaze T, Miranda K, Roberson RW, Gorgette O, et al. 2020. Characterization of extracellular vesicles produced by Aspergillus fumigatus protoplasts. mSphere 5:e00476–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rizzo J, Rodrigues ML, Janbon G. 2020. Extracellular vesicles in fungi: past, present, and future perspectives. Front. Cell. Infect. Microbiol 10:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rodrigues ML, Nimrichter L, Oliveira DL, Frases S, Miranda K, et al. 2007. Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Eukaryot Cell 6:48–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rodrigues ML, Shi L, Barreto-Bergter E, Nimrichter L, Farias SE, et al. 2007. Monoclonal antibody to fungal glucosylceramide protects mice against lethal Cryptococcus neoformans infection. Clin. Vaccine Immunol 14:1372–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Romani L. 2004. Immunity to fungal infections. Nat. Rev. Immunol 4:1–23 [DOI] [PubMed] [Google Scholar]
- 106.Rosas AL, Nosanchuk JD, Casadevall A. 2001. Passive immunization with melanin-binding monoclonal antibodies prolongs survival of mice with lethal Cryptococcus neoformans infection. Infect. Immun 69:3410–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Roth MT, Zamith-Miranda D, Nosanchuk JD. 2019. Immunization strategies for the control of histoplasmosis. Curr. Trop. Med. Rep 6:35–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Saville SP, Lazzell AL, Chaturvedi AK, Monteagudo C, Lopez-Ribot JL. 2009. Efficacy of a genetically engineered Candida albicans tet-NRG1 strain as an experimental live attenuated vaccine against hematogenously disseminated candidiasis. Clin. Vaccine Immunol 16:430–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schnizlein-Bick C, Durkin M, Kohler S, Connolly P, LeMonte A, et al. 2003. Effects of CD4 and CD8 T lymphocyte depletion on the course of histoplasmosis following pulmonary challenge. Med. Mycol 41:189–97 [DOI] [PubMed] [Google Scholar]
- 110.Shellito J, Suzara VV, Blumenfeld W, Beck JM, Steger HJ, Ermak TH. 1990. A new model of Pneumocystis carinii infection in mice selectively depleted of helper T lymphocytes. J. Clin. Investig 85:1686–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Singh VR, Smith DK, Lawerence J, Kelly PC, Thomas AR, et al. 1996. Coccidioidomycosis in patients infected with human immunodeficiency virus: review of 91 cases at a single institution. Clin. Infect. Dis 23:563–68 [DOI] [PubMed] [Google Scholar]
- 112.Siqueira IM, Wuthrich M, Li M, Wang H, Las-Casas LO, et al. 2020. Early immune response against Fonsecaea pedrosoi requires Dectin-2-mediated Th17 activity, whereas Th1 response, aided by Treg cells, is crucial for fungal clearance in later stage of experimental chromoblastomycosis. PLOS Negl. Trop. Dis 14:e0008386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sparber F, LeibundGut-Landmann S. 2019. Interleukin-17 in antifungal immunity. Pathogens 8:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Speakman EA, Dambuza IM, Salazar F, Brown GD. 2020. T cell antifungal immunity and the role of C-type lectin receptors. Trends Immunol. 41:61–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Specht CA, Lee CK, Huang H, Hester MM, Liu J, et al. 2017. Vaccination with recombinant Cryptococcus proteins in glucan particles protects mice against cryptococcosis in a manner dependent upon mouse strain and cryptococcal species. mBio 8:e01872–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Specht CA, Lee CK, Huang H, Tipper DJ, Shen ZT, et al. 2015. Protection against experimental cryptococcosis following vaccination with glucan particles containing Cryptococcus alkaline extracts. mBio 6:e01905–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Specht CA, Nong S, Dan JM, Lee CK, Levitz SM. 2007. Contribution of glycosylation to T cell responses stimulated by recombinant Cryptococcus neoformans mannoprotein. J. Infect. Dis 196:796–800 [DOI] [PubMed] [Google Scholar]
- 118.Spellberg B. 2011. Vaccines for invasive fungal infections. F1000 Med. Rep 3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Spellberg B, Ibrahim AS, Yeaman MR, Lin L, Fu Y, et al. 2008. The antifungal vaccine derived from the recombinant N terminus of Als3p protects mice against the bacterium Staphylococcus aureus. Infect. Immun 76:4574–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Spellberg BJ, Ibrahim AS, Avanesian V, Fu Y, Myers C, et al. 2006. Efficacy of the anti-Candida rAls3p-N or rAls1p-N vaccines against disseminated and mucosal candidiasis. J. Infect. Dis 194:256–60 [DOI] [PubMed] [Google Scholar]
- 121.Spellberg BJ, Ibrahim AS, Avenissian V, Filler SG, Myers CL, et al. 2005. The anti-Candida albicans vaccine composed of the recombinant N terminus of Als1p reduces fungal burden and improves survival in both immunocompetent and immunocompromised mice. Infect. Immun 73:6191–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Stott KE, Loyse A, Jarvis JN, Alufandika M, Harrison TS, et al. 2021. Cryptococcal meningoencephalitis: time for action. Lancet Infect. Dis 21:e259–71 [DOI] [PubMed] [Google Scholar]
- 123.Stuehler C, Khanna N, Bozza S, Zelante T, Moretti S, et al. 2011. Cross-protective TH1 immunity against Aspergillus fumigatus and Candida albicans. Blood 117:5881–91 [DOI] [PubMed] [Google Scholar]
- 124.Sudbery PE. 2011. Growth of Candida albicans hyphae. Nat. Rev. Microbiol 9:737–48 [DOI] [PubMed] [Google Scholar]
- 125.Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, et al. 2007. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat. Immunol 8:31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Thomas DP, Viudes A, Monteagudo C, Lazzell AL, Saville SP, Lopez-Ribot JL. 2006. A proteomic-based approach for the identification of Candida albicans protein components present in a subunit vaccine that protects against disseminated candidiasis. Proteomics 6:6033–41 [DOI] [PubMed] [Google Scholar]
- 127.Torosantucci A, Bromuro C, Chiani P, De Bernardis F, Berti F, et al. 2005. A novel glyco-conjugate vaccine against fungal pathogens. J. Exp. Med 202:597–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Travassos LR, Taborda CP. 2017. Linear epitopes of Paracoccidioides brasiliensis and other fungal agents of human systemic mycoses as vaccine candidates. Front. Immunol 8:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tso GHW, Reales-Calderon JA, Pavelka N. 2018. The elusive anti-Candida vaccine: lessons from the past and opportunities for the future. Front. Immunol 9:897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ueno K, Urai M, Sadamoto S, Shinozaki M, Takatsuka S, et al. 2019. A dendritic cell-based systemic vaccine induces long-lived lung-resident memory Th17 cells and ameliorates pulmonary mycosis. Mucosal. Immunol 12:265–76 [DOI] [PubMed] [Google Scholar]
- 131.Uicker WC, McCracken JP, Buchanan KL. 2006. Role of CD4+ T cells in a protective immune response against Cryptococcus neoformans in the central nervous system. Med. Mycol 44:1–11 [DOI] [PubMed] [Google Scholar]
- 132.Ulrich S, Ebel F. 2020. Monoclonal antibodies as tools to combat fungal infections. J. Fungi 6:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Upadhya R, Baker LG, Lam WC, Specht CA, Donlin MJ, Lodge JK. 2018. Cryptococcus neoformans Cda1 and its chitin deacetylase activity are required for fungal pathogenesis. mBio 9:e02087–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Upadhya R, Lam WC, Maybruck B, Specht CA, Levitz SM, Lodge JK. 2016. Induction of protective immunity to cryptococcal infection in mice by a heat-killed, chitosan-deficient strain of Cryptococcus neoformans. mBio 7:e01433–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.van de Veerdonk FL, Netea MG. 2010. T-cell subsets and antifungal host defenses. Curr Fungal. Infect. Rep 4:238–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vermitsky JP, Earhart KD, Smith WL, Homayouni R, Edlind TD, Rogers PD. 2006. Pdr1 regulates multidrug resistance in Candida glabrata: gene disruption and genome-wide expression studies. Mol. Microbiol 61:704–22 [DOI] [PubMed] [Google Scholar]
- 137.Wang H, Lee TJ, Fites SJ, Merkhofer R, Zarnowski R, et al. 2017. Ligation of Dectin-2 with a novel microbial ligand promotes adjuvant activity for vaccination. PLOS Pathog. 13:e1006568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang Y, Wang K, Masso-Silva JA, Rivera A, Xue C. 2019. A heat-killed Cryptococcus mutant strain induces host protection against multiple invasive mycoses in a murine vaccine model. mBio 10:e02145–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Whibley N, Gaffen SL. 2014. Brothers in arms: Th17 and Treg responses in Candida albicans immunity. PLOS Pathog. 10:e1004456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis 39:309–17 [DOI] [PubMed] [Google Scholar]
- 141.Wormley FL Jr., Perfect JR, Steele C, Cox GM. 2007. Protection against cryptococcosis by using a murine gamma interferon-producing Cryptococcus neoformans strain. Infect. Immun 75:1453–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wozniak KL, Young ML, Wormley FL Jr. 2011. Protective immunity against experimental pulmonary cryptococcosis in T cell-depleted mice. Clin. Vaccine Immunol 18:717–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wuthrich M, Brandhorst TT, Sullivan TD, Filutowicz H, Sterkel A, et al. 2015. Calnexin induces expansion of antigen-specific CD4+ T cells that confer immunity to fungal ascomycetes via conserved epitopes. Cell. Host Microbe 17:452–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wuthrich M, Chang WL, Klein BS. 1998. Immunogenicity and protective efficacy of the WI-1 adhesin of Blastomyces dermatitidis. Infect. Immun 66:5443–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wuthrich M, Deepe GS Jr., Klein B. 2012. Adaptive immunity to fungi. Annu. Rev. Immunol 30:115–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wuthrich M, Filutowicz HI, Klein BS. 2000. Mutation of the WI-1 gene yields an attenuated Blastomyces dermatitidis strain that induces host resistance. J. Clin. Investig 106:1381–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wuthrich M, Gern B, Hung CY, Ersland K, Rocco N, et al. 2011. Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. J. Clin. Invesigt 121:554–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xin H, Dziadek S, Bundle DR, Cutler JE. 2008. Synthetic glycopeptide vaccines combining beta-mannan and peptide epitopes induce protection against candidiasis. PNAS 105:13526–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yang YL, Wang CW, Chen CT, Wang MH, Hsiao CF, Lo HJ. 2009. Non-lethal Candida albicans cph1/cph1 efg1/efg1 mutant partially protects mice from systemic infections by lethal wild-type cells. Mycol. Res 113:388–90 [DOI] [PubMed] [Google Scholar]
- 150.Zhai B, Wozniak KL, Masso-Silva J, Upadhyay S, Hole C, et al. 2015. Development of protective inflammation and cell-mediated immunity against Cryptococcus neoformans after exposure to hyphal mutants. mBio 6:e01433–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zheng M, Ramsay AJ, Robichaux MB, Kliment C, Crowe C, et al. 2005. CD4+ T cell-independent DNA vaccination against opportunistic infections. J. Clin. Investig 115:3536–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zheng M, Shellito JE, Marrero L, Zhong Q, Julian S, et al. 2001. CD4+ T cell-independent vaccination against Pneumocystis carinii in mice. J. Clin. Investig 108:1469–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhou P, Seder RA. 1998. CD40 ligand is not essential for induction of type 1 cytokine responses or protective immunity after primary or secondary infection with Histoplasma capsulatum. J. Exp. Med 187:1315–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhu Y, Yang C, Magee DM, Cox RA. 1996. Molecular cloning and characterization of Coccidioides immitis antigen 2 cDNA. Infect. Immun 64:2695–99 [DOI] [PMC free article] [PubMed] [Google Scholar]


