Abstract
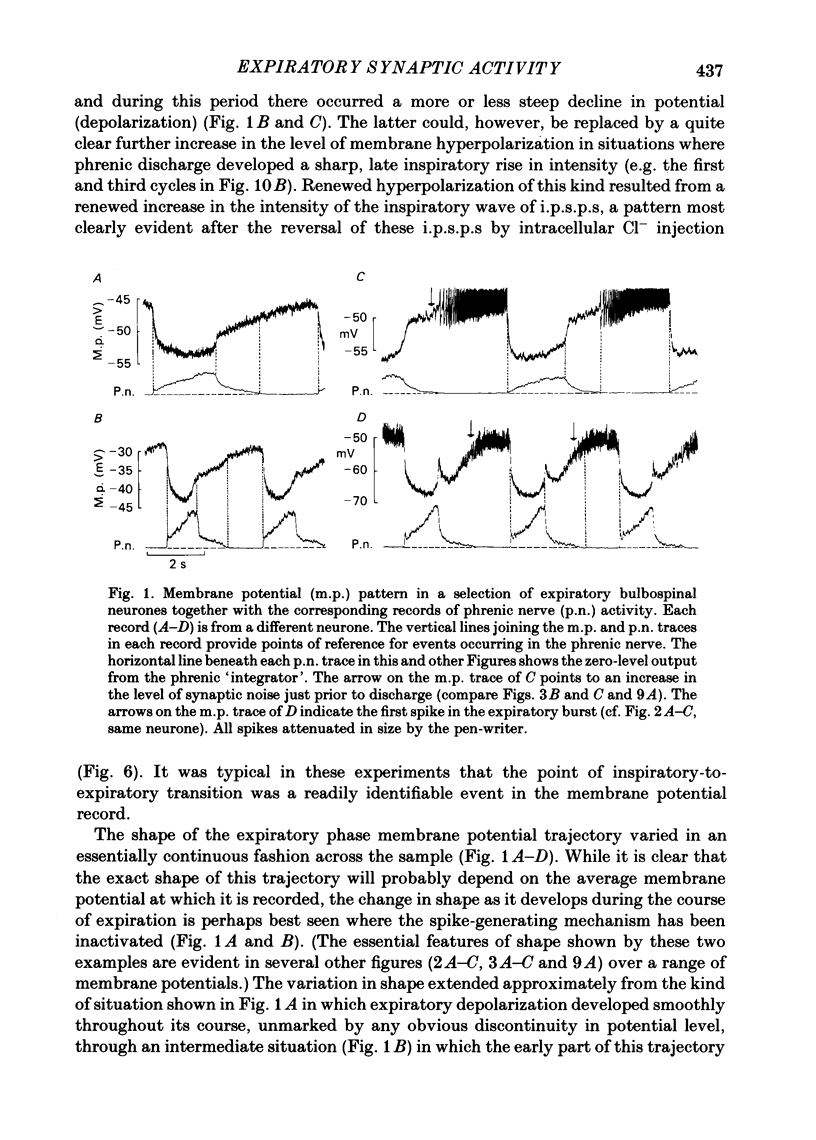
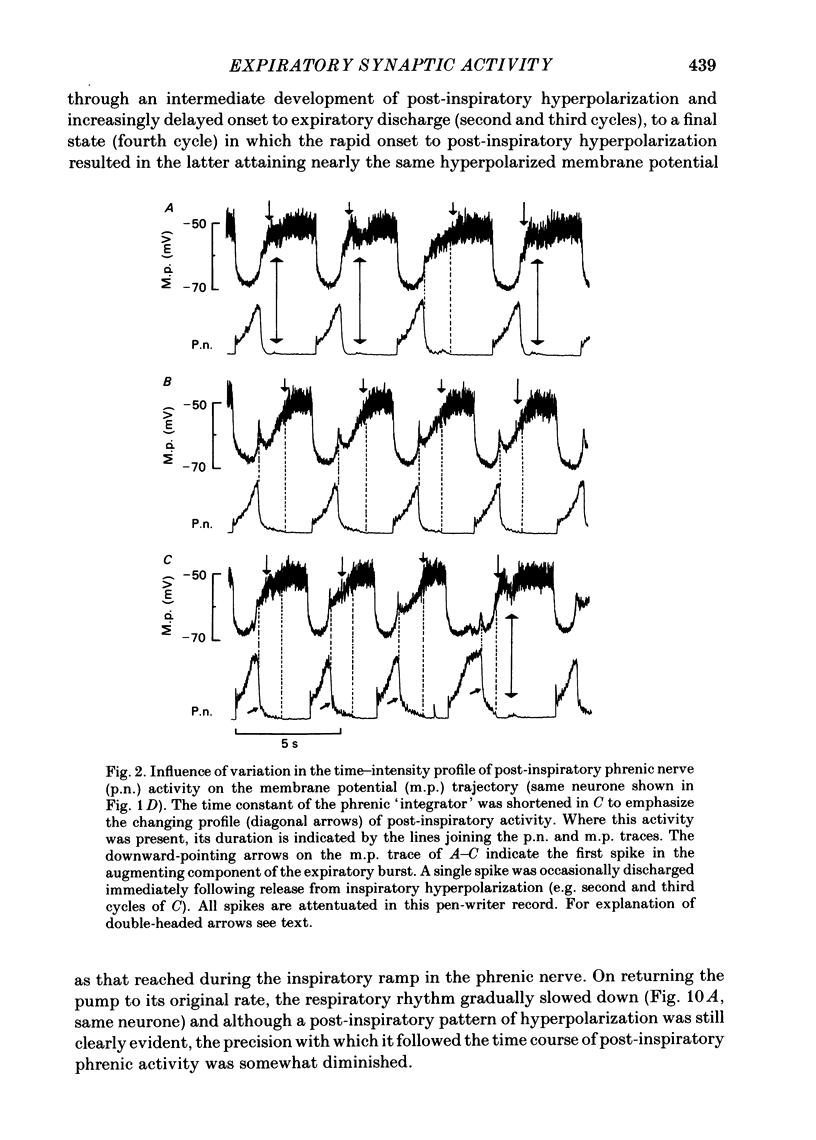
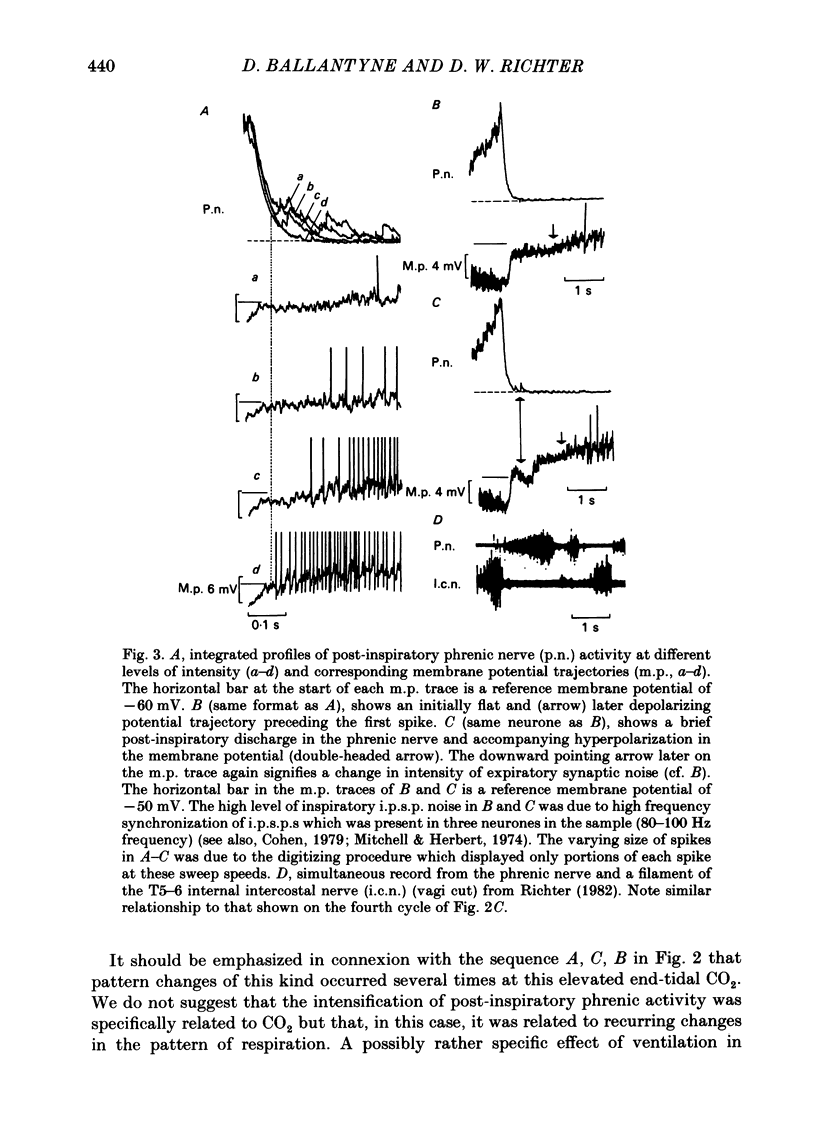
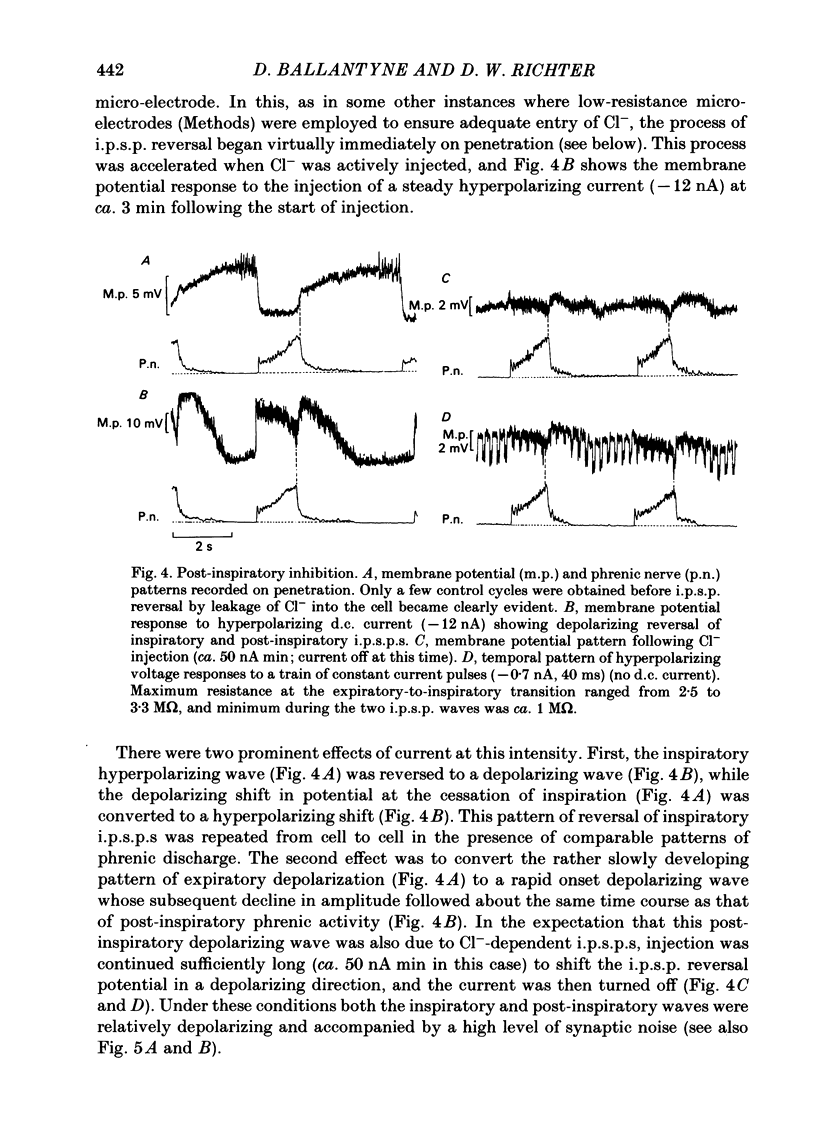
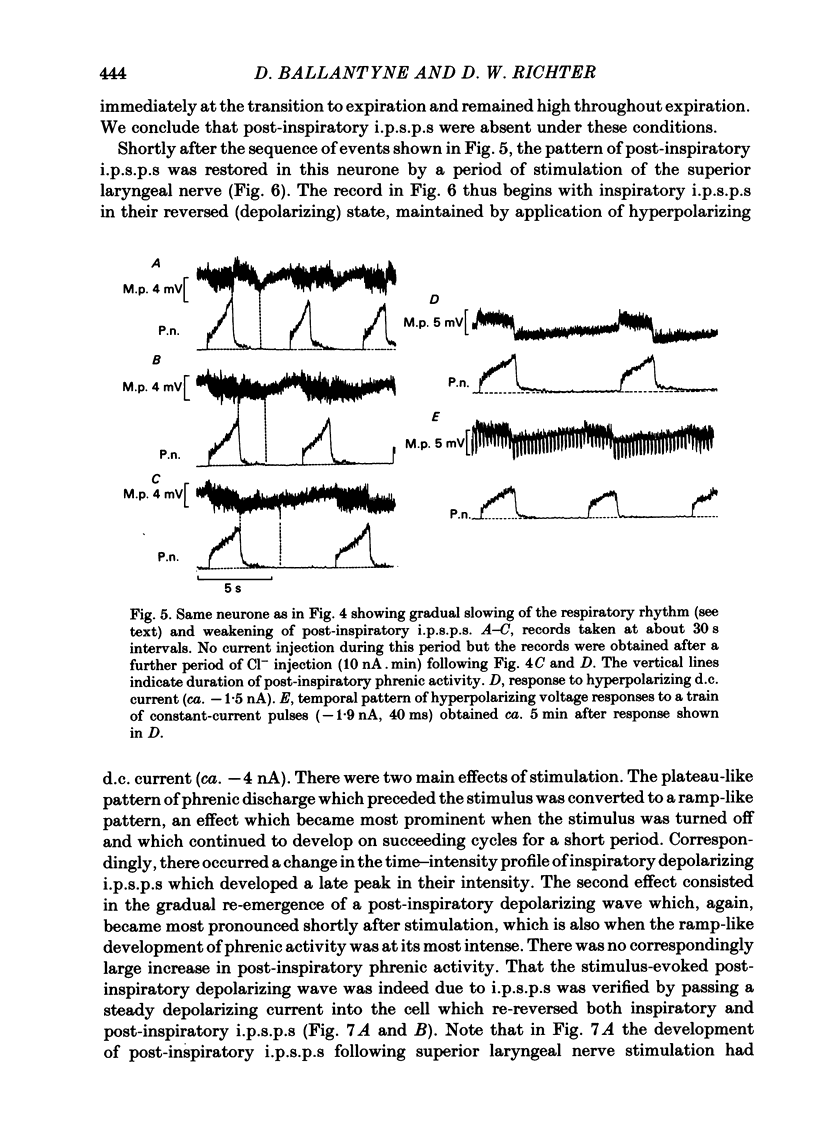
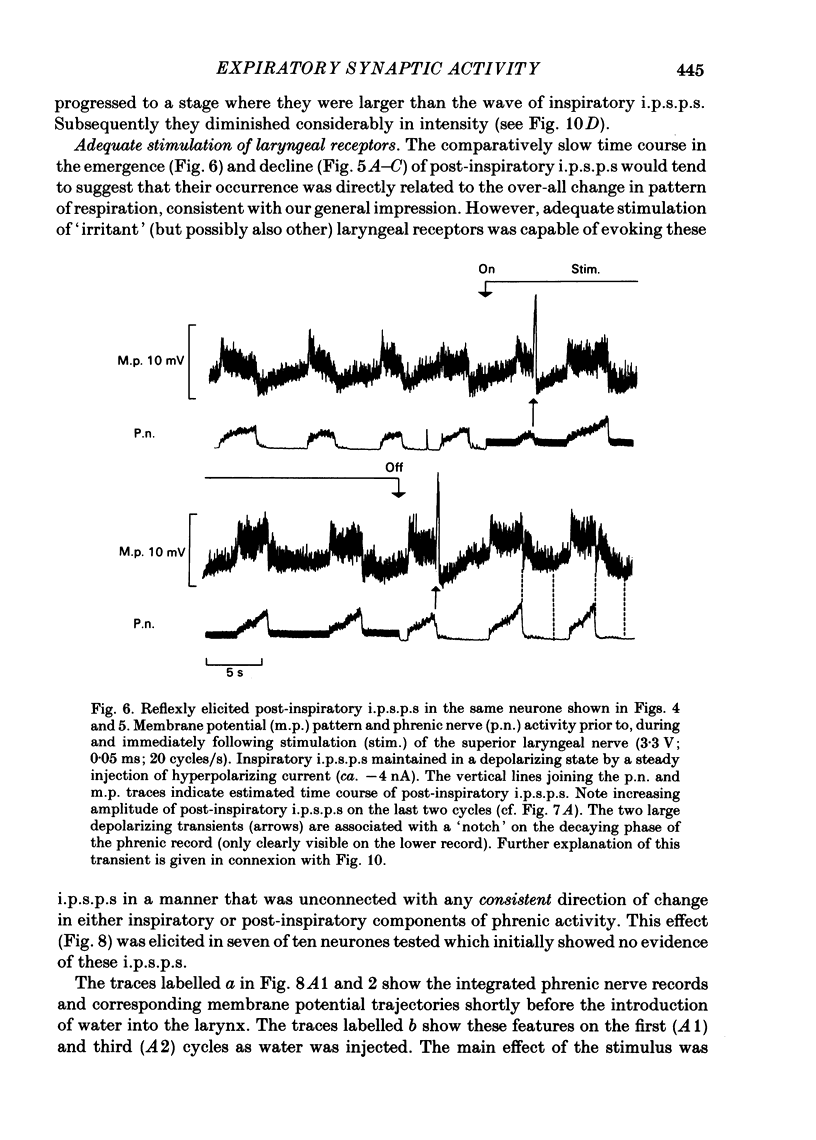
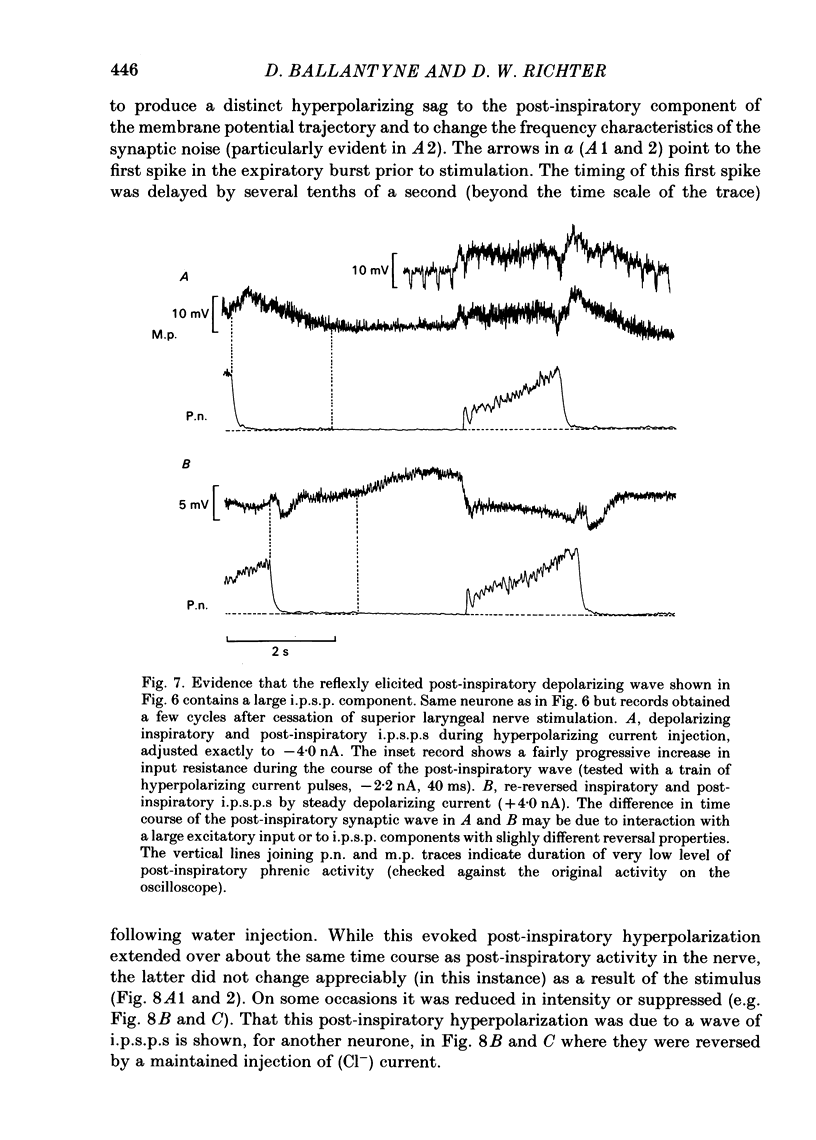
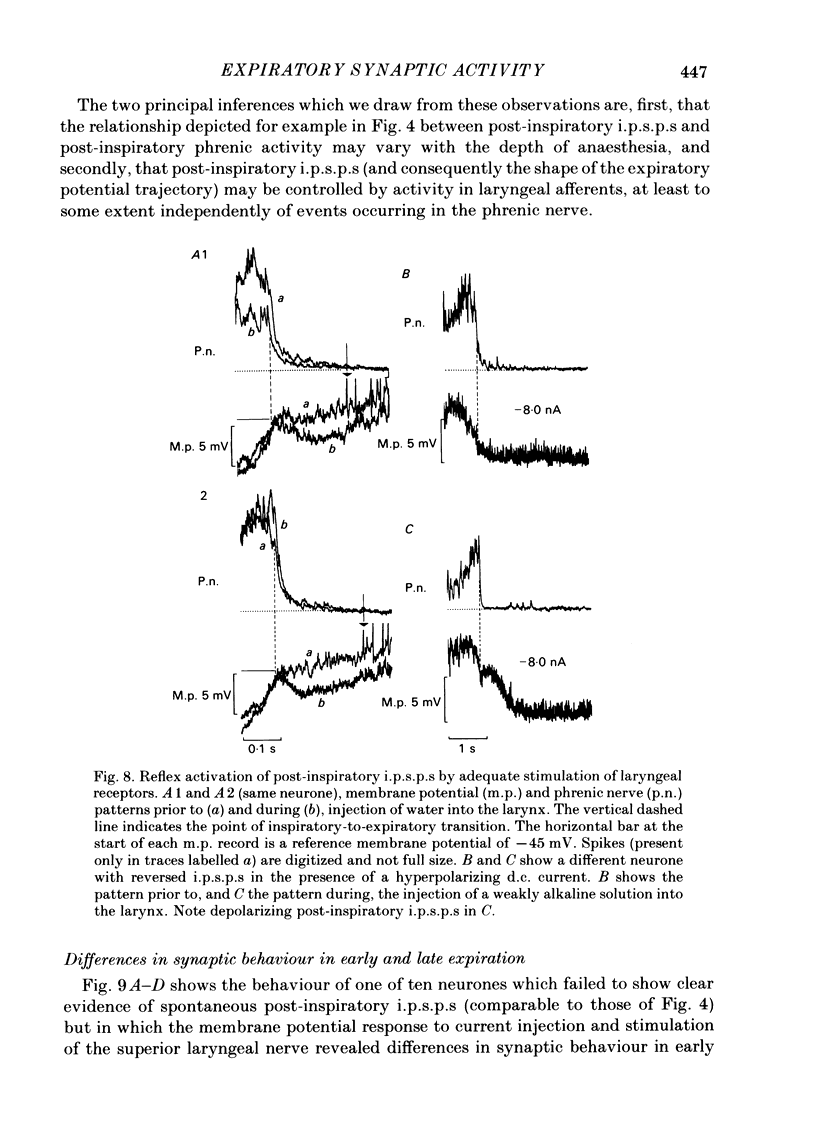
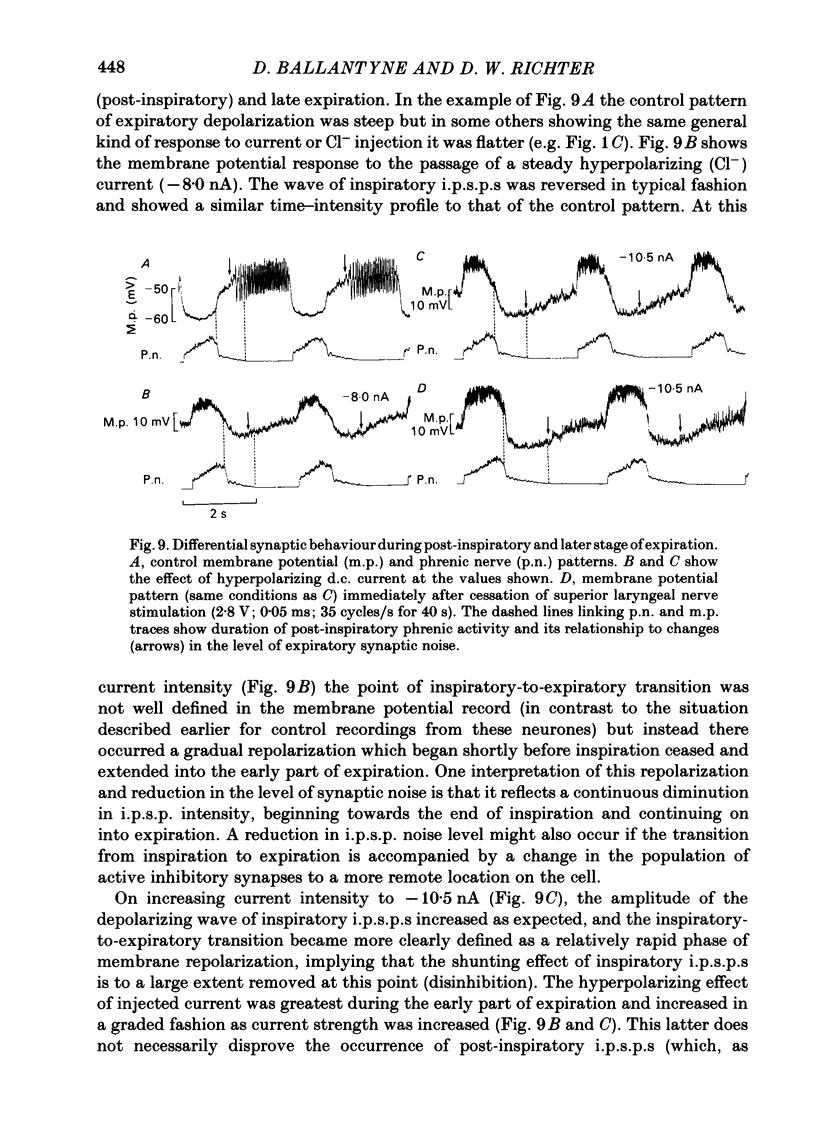
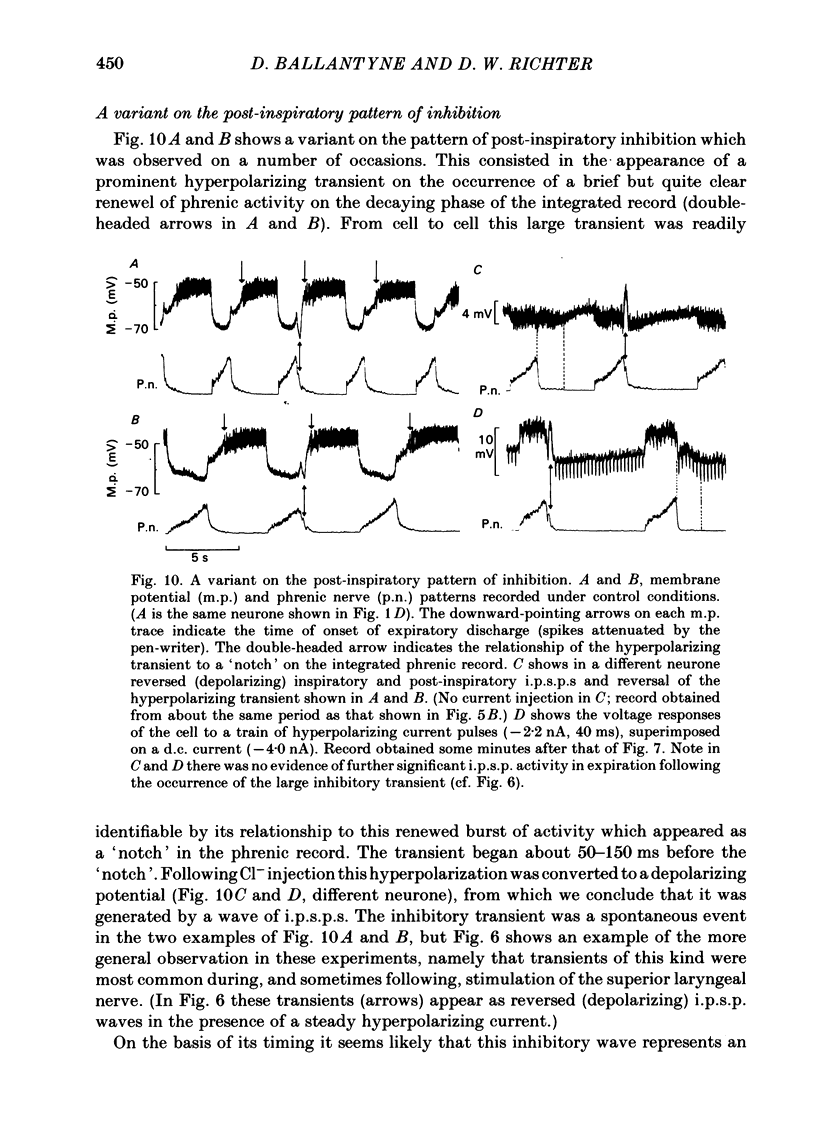
Intracellular recordings were made from caudal medullary expiratory neurones in pentobarbitone-anaesthetized, vagotomized and artificially ventilated cats. The sample consisted of thirty-three bulbospinal neurones and seven neurones which were not antidromically excited from either the spinal cord (C2-C3) or vagus nerve. Their rhythmic activity consisted of an alternating inspiratory hyperpolarization due to Cl(-)-dependent inhibitory post-synaptic potentials (i.p.s.p.s) (Mitchell & Herbert, 1974) and an expiratory depolarization. The precise shape of the expiratory depolarizing wave varied within a given neurone depending on the over-all pattern of respiration. This variation extended from a smoothly developing depolarization, continuous throughout its course, through an intermediate state in which depolarization proceeded in two stages with a definite transition between them, to a final state in which the early part of expiration was occupied by a distinct hyperpolarizing component to the membrane potential trajectory. Under conditions of a brisk phrenic nerve discharge, these variations in the shape of the membrane potential profile were related to the time course and intensity of post-inspiratory discharge in the nerve. However, other factors (depth of anaesthesia and stimulation of laryngeal receptors) could influence the time course of the membrane potential profile of expiratory neurones independently of post-inspiratory phrenic discharge. In five of fifteen neurones which were tested, early expiration was occupied by a rapidly developing, decrementing wave of Cl(-)-dependent i.p.s.p.s (post-inspiratory i.p.s.p.s). These i.p.s.p.s were present only under conditions of a strong phrenic rhythm (large amplitude, fairly rapid phrenic discharge). They became weaker and ultimately disappeared when the level of anaesthesia was deepened and the phrenic rhythm became slower. Under these conditions, the post-inspiratory wave of i.p.s.p.s could be restored by stimulation of the superior laryngeal nerve. Adequate stimulation of presumed 'irritant' laryngeal receptors elicited post-inspiratory i.p.s.p.s in seven of ten neurones tested which initially showed either no post-inspiratory i.p.s.p.s or possibly just a weak pattern. In ten of fifteen neurones tested, the responses to current injection revealed clear differences in membrane potential behaviour in early and late expiration, which became intensified following stimulation of the superior laryngeal nerve.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bainton C. R., Kirkwood P. A., Sears T. A. On the transmission of the stimulating effects of carbon dioxide to the muscles of respiration. J Physiol. 1978 Jul;280:249–272. doi: 10.1113/jphysiol.1978.sp012383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton C. R., Kirkwood P. A. The effect of carbon dioxide on the tonic and the rhythmic discharges of expiratory bulbospinal neurones. J Physiol. 1979 Nov;296:291–314. doi: 10.1113/jphysiol.1979.sp013006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantyne D., Richter D. W. Post-synaptic inhibition of bulbar inspiratory neurones in the cat. J Physiol. 1984 Mar;348:67–87. doi: 10.1113/jphysiol.1984.sp015100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger A. J. Phrenic motoneurons in the cat: subpopulations and nature of respiratory drive potentials. J Neurophysiol. 1979 Jan;42(1 Pt 1):76–90. doi: 10.1152/jn.1979.42.1.76. [DOI] [PubMed] [Google Scholar]
- Bianchi A. L. Localisation et étude des neurones respiratoires bulbaires. Mise en jeu antidromique par stimulation spinale ou vagale. J Physiol (Paris) 1971 Jan-Feb;63(1):5–40. [PubMed] [Google Scholar]
- Bryant H. L., Segundo J. P. Spike initiation by transmembrane current: a white-noise analysis. J Physiol. 1976 Sep;260(2):279–314. doi: 10.1113/jphysiol.1976.sp011516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. I. Neurogenesis of respiratory rhythm in the mammal. Physiol Rev. 1979 Oct;59(4):1105–1173. doi: 10.1152/physrev.1979.59.4.1105. [DOI] [PubMed] [Google Scholar]
- Dekin M. S., Getting P. A. Firing pattern of neurons in the nucleus tractus solitarius: modulation by membrane hyperpolarization. Brain Res. 1984 Dec 17;324(1):180–184. doi: 10.1016/0006-8993(84)90640-1. [DOI] [PubMed] [Google Scholar]
- Faber D. S., Korn H. Transmission at a central inhibitory synapse. I. Magnitude of unitary postsynaptic conductance change and kinetics of channel activation. J Neurophysiol. 1982 Sep;48(3):654–678. doi: 10.1152/jn.1982.48.3.654. [DOI] [PubMed] [Google Scholar]
- Fedorko L., Merrill E. G. Axonal projections from the rostral expiratory neurones of the Bötzinger complex to medulla and spinal cord in the cat. J Physiol. 1984 May;350:487–496. doi: 10.1113/jphysiol.1984.sp015214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman J. L., Cohen M. I. Relation between expiratory duration and rostral medullary expiratory neuronal discharge. Brain Res. 1978 Feb 3;141(1):172–178. doi: 10.1016/0006-8993(78)90627-3. [DOI] [PubMed] [Google Scholar]
- Fetz E. E., Gustafsson B. Relation between shapes of post-synaptic potentials and changes in firing probability of cat motoneurones. J Physiol. 1983 Aug;341:387–410. doi: 10.1113/jphysiol.1983.sp014812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt J. R. Intracellular activity of medullary respiratory neurons. Exp Neurol. 1974 Nov;45(2):298–313. doi: 10.1016/0014-4886(74)90120-4. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A. On the use and interpretation of cross-correlations measurements in the mammalian central nervous system. J Neurosci Methods. 1979 Aug;1(2):107–132. doi: 10.1016/0165-0270(79)90009-8. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. The synaptic connexions to intercostal motoneurones as revealed by the average common excitation potential. J Physiol. 1978 Feb;275:103–134. doi: 10.1113/jphysiol.1978.sp012180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A., Tuck D. L., Westgaard R. H. Variations in the time course of the synchronization of intercostal motoneurones in the cat. J Physiol. 1982 Jun;327:105–135. doi: 10.1113/jphysiol.1982.sp014223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox C. K., Poppele R. E. Correlation analysis of stimulus-evoked changes in excitability of spontaneously firing neurons. J Neurophysiol. 1977 May;40(3):616–625. doi: 10.1152/jn.1977.40.3.616. [DOI] [PubMed] [Google Scholar]
- Kreuter F., Richter D. W., Camerer H., Senekowitsch R. Morphological and electrical description of medullary respiratory neurons of the cat. Pflugers Arch. 1977 Nov 25;372(1):7–16. doi: 10.1007/BF00582200. [DOI] [PubMed] [Google Scholar]
- Lipski J., Trzebski A., Chodobska J., Kruk P. Effects of carotid chemoreceptor excitation on medullary expiratory neurons in cats. Respir Physiol. 1984 Sep;57(3):279–291. doi: 10.1016/0034-5687(84)90077-x. [DOI] [PubMed] [Google Scholar]
- Merrill E. G., Lipski J., Kubin L., Fedorko L. Origin of the expiratory inhibition of nucleus tractus solitarius inspiratory neurones. Brain Res. 1983 Mar 14;263(1):43–50. doi: 10.1016/0006-8993(83)91198-8. [DOI] [PubMed] [Google Scholar]
- Merrill E. G. The descending pathways from the lateral respiratory neurones in cats. J Physiol. 1971 Oct;218 (Suppl):82P–83P. [PubMed] [Google Scholar]
- Merrill E. G. The lateral respiratory neurones of the medulla: their associations with nucleus ambiguus, nucleus retroambigualis, the spinal accessory nucleus and the spinal cord. Brain Res. 1970 Nov 11;24(1):11–28. doi: 10.1016/0006-8993(70)90271-4. [DOI] [PubMed] [Google Scholar]
- Mitchell R. A., Herbert D. A. Synchronized high frequency synaptic potentials in medullary respiratory neurons. Brain Res. 1974 Jul 26;75(2):350–355. doi: 10.1016/0006-8993(74)90760-4. [DOI] [PubMed] [Google Scholar]
- Richter D. W., Camerer H., Meesmann M., Röhrig N. Studies on the synaptic interconnection between bulbar respiratory neurones of cats. Pflugers Arch. 1979 Jul;380(3):245–257. doi: 10.1007/BF00582903. [DOI] [PubMed] [Google Scholar]
- Richter D. W. Generation and maintenance of the respiratory rhythm. J Exp Biol. 1982 Oct;100:93–107. doi: 10.1242/jeb.100.1.93. [DOI] [PubMed] [Google Scholar]
- SALMOIRAGHI G. C., BURNS B. D. Localization and patterns of discharge of respiratory neurones in brain-stem of cat. J Neurophysiol. 1960 Jan;23:2–13. doi: 10.1152/jn.1960.23.1.2. [DOI] [PubMed] [Google Scholar]
- SEARS T. A. THE SLOW POTENTIALS OF THORACIC RESPIRATORY MOTONEURONES AND THEIR RELATION TO BREATHING. J Physiol. 1964 Dec;175:404–424. doi: 10.1113/jphysiol.1964.sp007524. [DOI] [PMC free article] [PubMed] [Google Scholar]


