Abstract
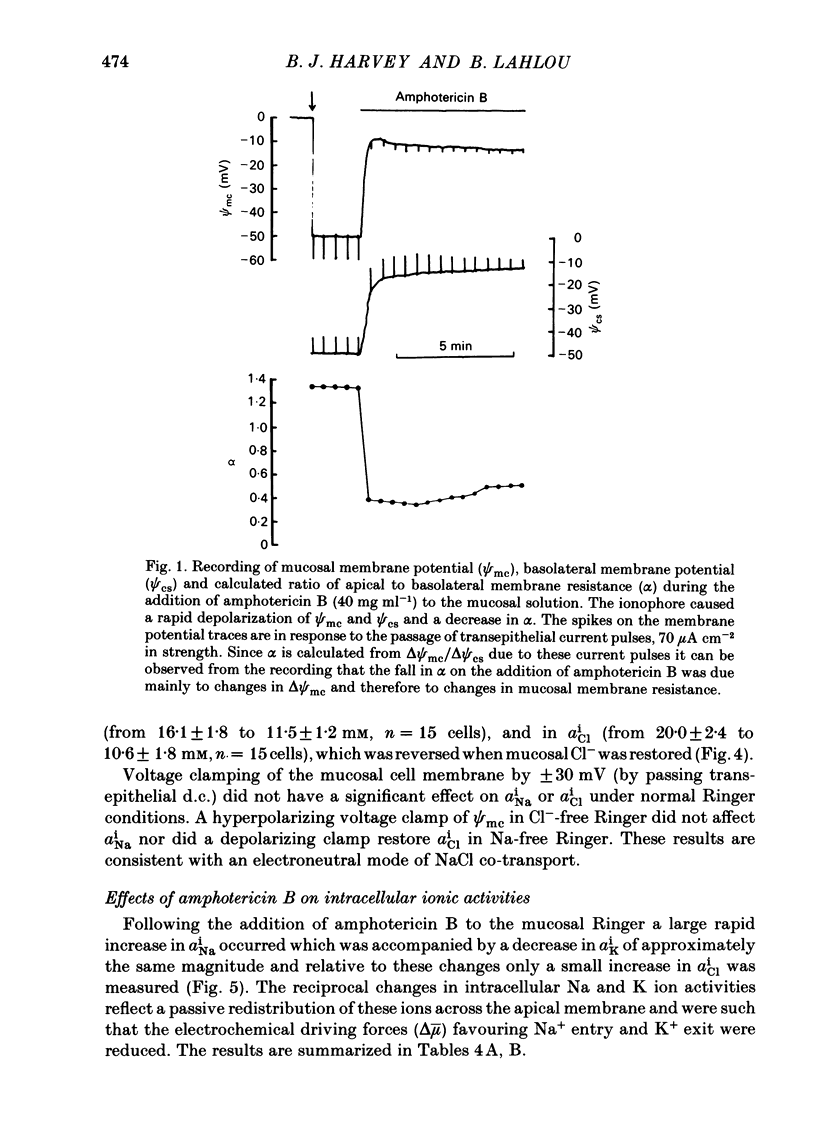
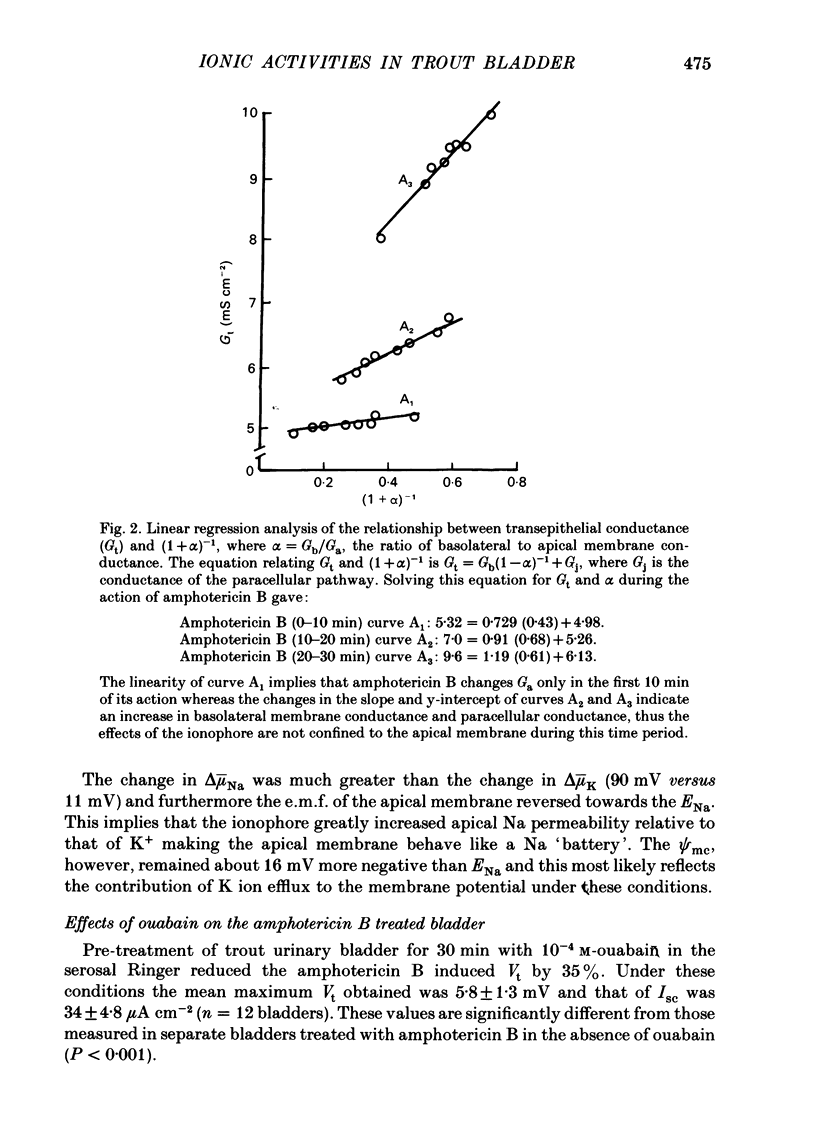
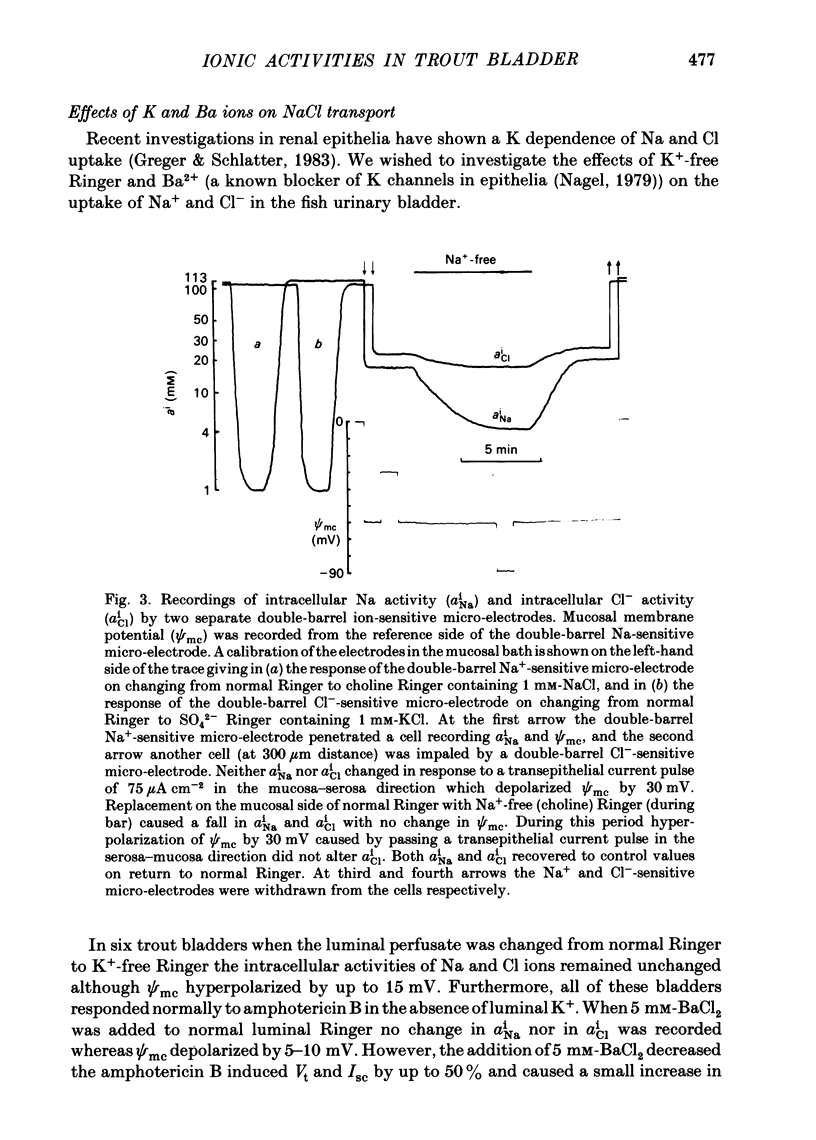
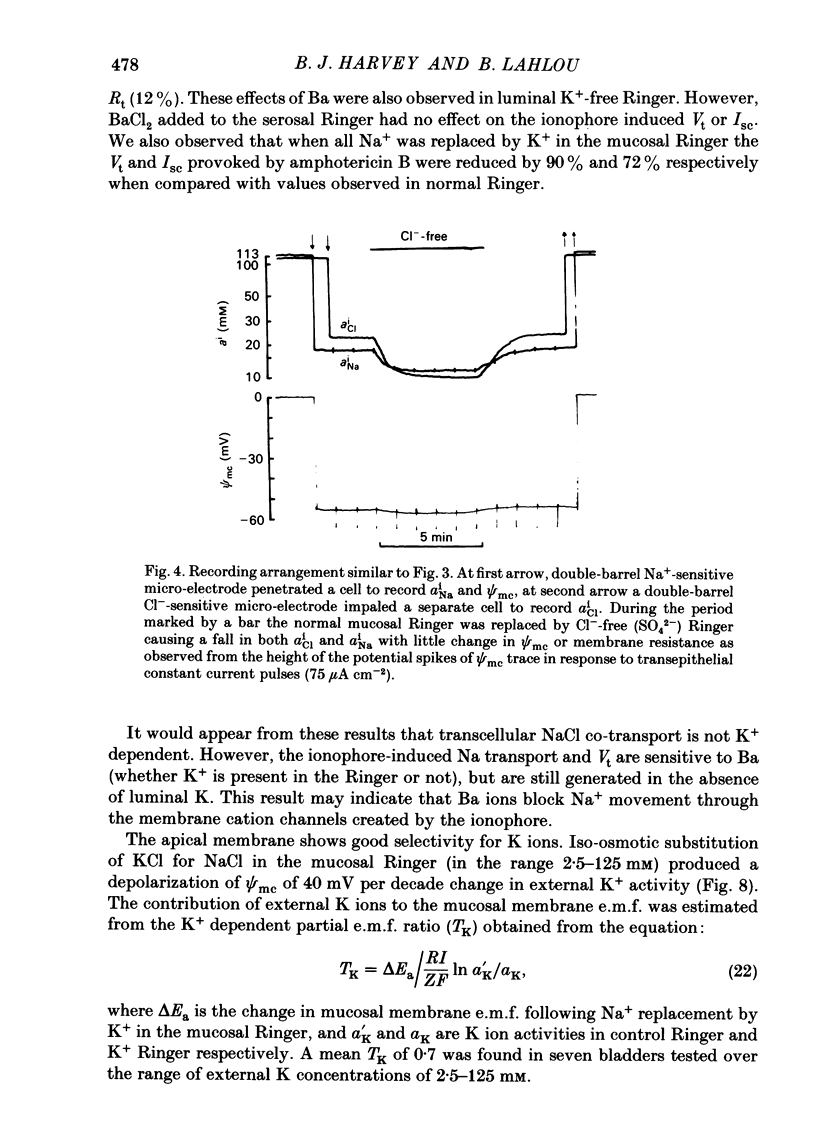
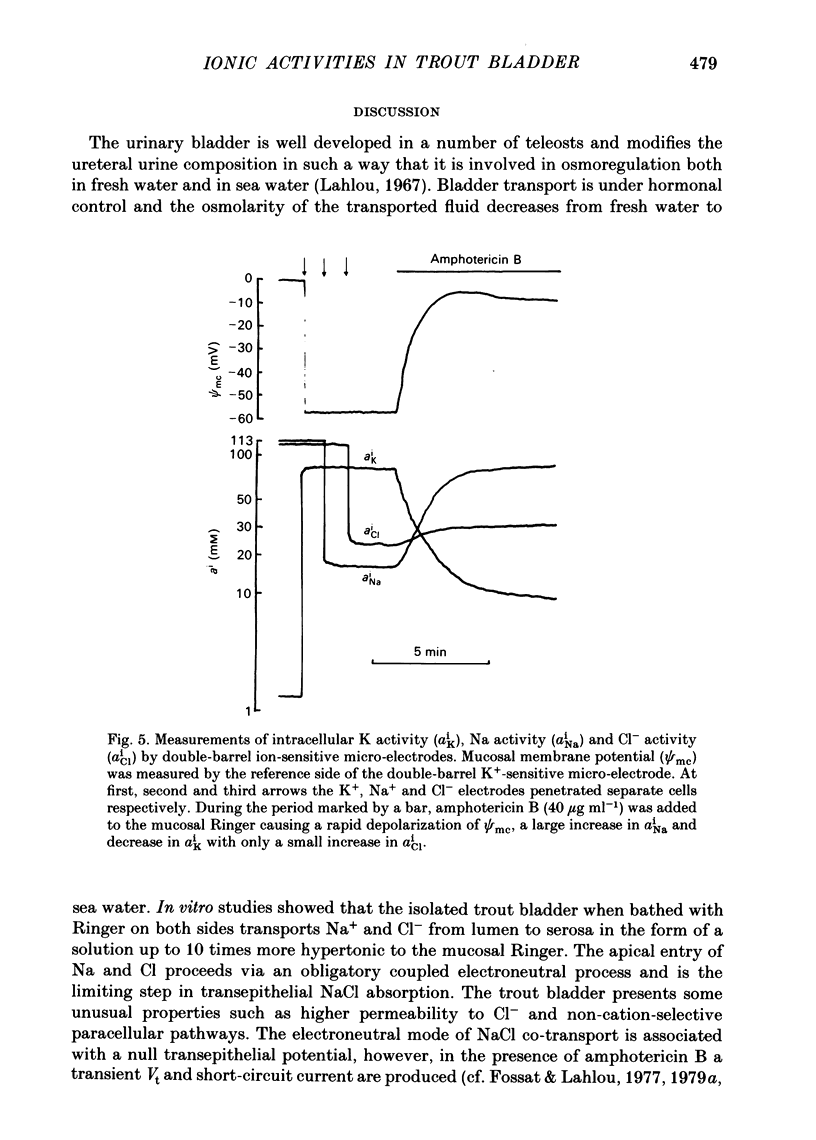
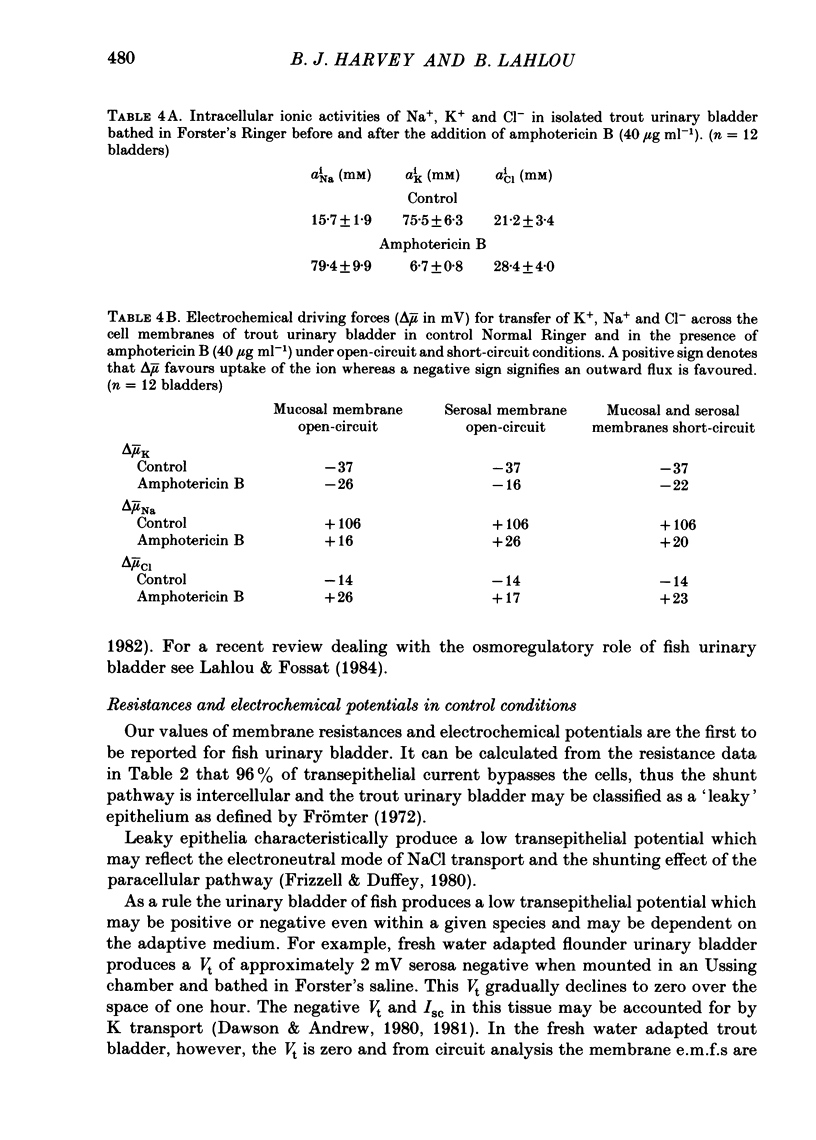
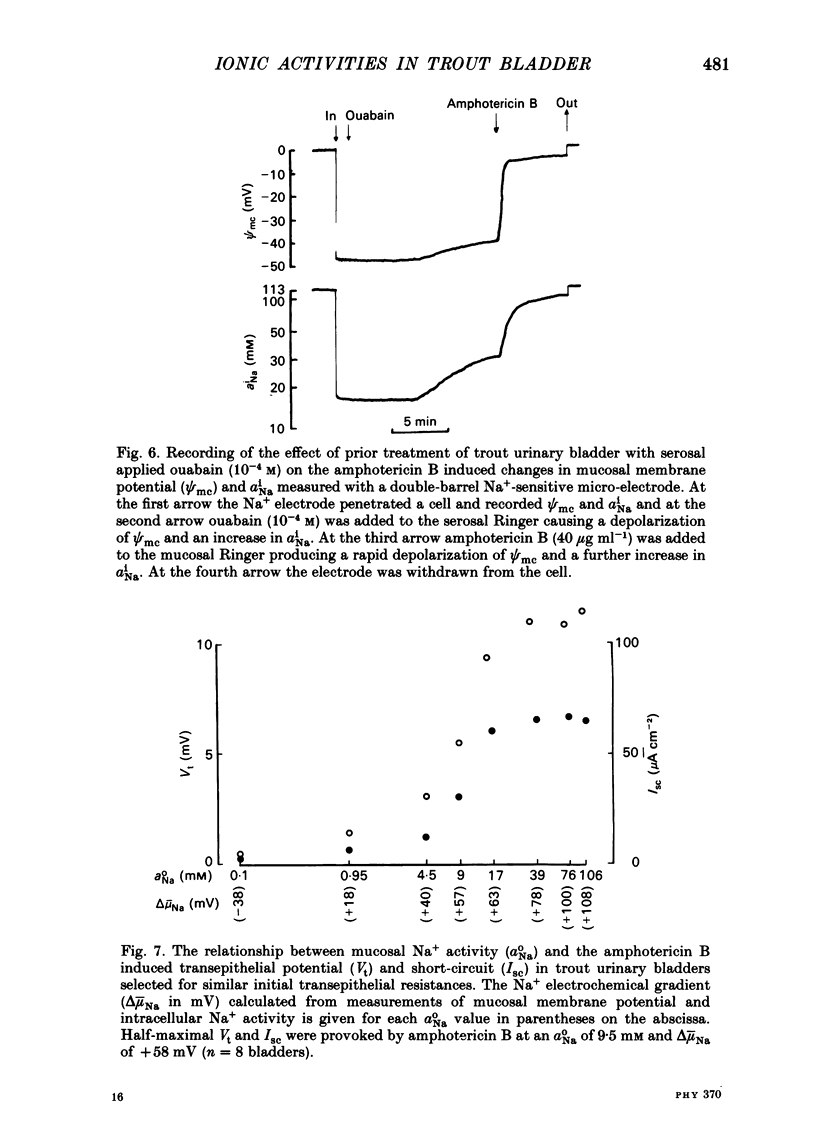
Intracellular micro-electrode techniques were used to measure the electrical resistances of the cell membranes and the shunt pathway and intracellular ionic activities in trout urinary bladder when the tissue was incubated in Ringer solution and in the presence of the polyene antibiotic ionophore amphotericin B. In control conditions the transepithelial potential was zero and the intracellular potential was -56 mV. The intracellular ionic activities measured with single- and double-barrel ion-sensitive micro-electrodes for the first time in a fish bladder (aiNa = 16 mM, aiK = 87 mM, and aiCl = 21 mM) indicate an active accumulation of K and Cl ions and an active extrusion of Na ions by the cell. The maintenance of intracellular Cl activity above its equilibrium value depended on the presence of Na ions in the mucosal medium, but was independent of the presence of K ions. Flat cable analysis yielded values for transepithelial, apical, basolateral and shunt resistances of 197, 2790, 1986 and 205 omega cm-2 respectively. Equivalent circuit analysis using amphotericin B yielded similar values for shunt resistance. The paracellular pathway accounts for 96% of transepithelial current flow and this epithelium may be classified as 'leaky'. The cells are electrically coupled with a space constant of 354 micron. Amphotericin B when added to the mucosal solution induced an immediate serosa positive transepithelial potential of about 9 mV and a short-circuit current of 64 microA cm-2. The Vt was ouabain sensitive and dependent on mucosal Na concentration. The origin of the antibiotic induced transepithelial potential was an increase in the sum of the cell membrane electromotive forces. The apical membrane potential depolarized to -7 mV and its resistance fell to 433 omega cm-2. During the first 10 min of exposure aiNa increased to 80 mM and aiK decreased to 7 mM with only a small change in aiCl. The changes in cellular Na+ and K+ activities were in accordance with their passive redistribution down their electrochemical gradients.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cremaschi D., Hénin S., Meyer G., Bacciola T. Does amphotericin B unmask an electrogenic Na+ pump in rabbit gallbladder? Shift of gallbladders with negative to gallbladders with positive transepithelial p.d.'s. J Membr Biol. 1977 Jun 3;34(1):55–71. doi: 10.1007/BF01870293. [DOI] [PubMed] [Google Scholar]
- Cremaschi D., Hénin S. Na+ and Cl- transepithelial routes in rabbit gallbladder: tracer analysis of the transports. Pflugers Arch. 1975 Dec 19;361(1):33–41. doi: 10.1007/BF00587337. [DOI] [PubMed] [Google Scholar]
- DIAMOND J. M. The mechanism of water transport by the gall-bladder. J Physiol. 1962 May;161:503–527. doi: 10.1113/jphysiol.1962.sp006900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossat B., Lahlou B., Bornancin M. Involvement of Na-K-ATPase in sodium transport by fish urinary bladder. Experientia. 1974 Apr 15;30(4):376–377. doi: 10.1007/BF01921673. [DOI] [PubMed] [Google Scholar]
- Fossat B., Lahlou B. Failure of 2,4,6-triaminopyrimidine (TAP) to block sodium pathways in a "leaky" epithelium: the urinary bladder of the trout. Pflugers Arch. 1979 Apr 30;379(3):287–290. doi: 10.1007/BF00581433. [DOI] [PubMed] [Google Scholar]
- Fossat B., Lahlou B. Ion flux changes induced by voltage clamping or by amphotericin B in the isolated urinary bladder of the trout. J Physiol. 1982 Apr;325:111–123. doi: 10.1113/jphysiol.1982.sp014139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossat B., Lahlou B. Osmotic and solute permeabilities of isolated urinary bladder of the trout. Am J Physiol. 1977 Dec;233(6):F525–F531. doi: 10.1152/ajprenal.1977.233.6.F525. [DOI] [PubMed] [Google Scholar]
- Fossat B., Lahlou B. The mechanism of coupled transport of sodium and chloride in isolated urinary bladder of the trout. J Physiol. 1979 Sep;294:211–222. doi: 10.1113/jphysiol.1979.sp012926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frizzell R. A., Duffey M. E. Chloride activities in epithelia. Fed Proc. 1980 Sep;39(11):2860–2864. [PubMed] [Google Scholar]
- Frömter E. The route of passive ion movement through the epithelium of Necturus gallbladder. J Membr Biol. 1972;8(3):259–301. doi: 10.1007/BF01868106. [DOI] [PubMed] [Google Scholar]
- Graf J., Giebisch G. Intracellular sodium activity and sodium transport in necturus gallbladder epithelium. J Membr Biol. 1979 Jun 7;47(4):327–355. doi: 10.1007/BF01869743. [DOI] [PubMed] [Google Scholar]
- Greger R., Schlatter E. Presence of luminal K+, a prerequisite for active NaCl transport in the cortical thick ascending limb of Henle's loop of rabbit kidney. Pflugers Arch. 1981 Nov;392(1):92–94. doi: 10.1007/BF00584588. [DOI] [PubMed] [Google Scholar]
- Greger R., Schlatter E. Properties of the lumen membrane of the cortical thick ascending limb of Henle's loop of rabbit kidney. Pflugers Arch. 1983 Mar;396(4):315–324. doi: 10.1007/BF01063937. [DOI] [PubMed] [Google Scholar]
- Harvey B. J., Kernan R. P. Intracellular ion activities in frog skin in relation to external sodium and effects of amiloride and/or ouabain. J Physiol. 1984 Apr;349:501–517. doi: 10.1113/jphysiol.1984.sp015170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey B. J., Kernan R. P. Sodium-selective micro-electrode study of apical permeability in frog skin: effects of sodium, amiloride and ouabain. J Physiol. 1984 Nov;356:359–374. doi: 10.1113/jphysiol.1984.sp015470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hénin S., Cremaschi D. Reply to: On the use of amphotericin B as a probe to determine cell membrane resistances in gallbladder epithelium. J Membr Biol. 1978 Jun 22;41(1):96–100. [PubMed] [Google Scholar]
- Lahlou B., Fossat B. Mécanisme du transport de l'eau et du sel à travers la vessie urinaire d'un poisson téléostéen en eau douce, la truite arc-en-ciel. C R Acad Sci Hebd Seances Acad Sci D. 1971 Nov 29;273(22):2108–2110. [PubMed] [Google Scholar]
- Lewis S. A., Eaton D. C., Clausen C., Diamond J. M. Nystatin as a probe for investigating the electrical properties of a tight epithelium. J Gen Physiol. 1977 Oct;70(4):427–440. doi: 10.1085/jgp.70.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore E. W. Cation measurements in biological materials. Ann N Y Acad Sci. 1968 Feb 1;148(1):93–109. doi: 10.1111/j.1749-6632.1968.tb20343.x. [DOI] [PubMed] [Google Scholar]
- Nagel W. Inhibition of potassium conductance by barium in frog skin epithelium. Biochim Biophys Acta. 1979 Apr 4;552(2):346–357. doi: 10.1016/0005-2736(79)90289-x. [DOI] [PubMed] [Google Scholar]
- Nellans H. N., Frizzell R. A., Schultz S. G. Brush-border processes and transepithelial Na and Cl transport by rabbit ileum. Am J Physiol. 1974 May;226(5):1131–1141. doi: 10.1152/ajplegacy.1974.226.5.1131. [DOI] [PubMed] [Google Scholar]
- Nellans H. N., Frizzell R. A., Schultz S. G. Coupled sodium-chloride influx across the brush border of rabbit ileum. Am J Physiol. 1973 Aug;225(2):467–475. doi: 10.1152/ajplegacy.1973.225.2.467. [DOI] [PubMed] [Google Scholar]
- Renfro J. L. Interdependence of Active Na+ and Cl- transport by the isolated urinary bladder of the teleost, Pseudopleuronectes americanus. J Exp Zool. 1977 Mar;199(3):383–390. doi: 10.1002/jez.1401990311. [DOI] [PubMed] [Google Scholar]
- Renfro J. L., Miller D. S., Karnaky K. J., Jr, Kinter W. B. Na-K-ATPase localization in teleost urinary bladder by [3H]ouabain autoradiography. Am J Physiol. 1976 Dec;231(6):1735–1743. doi: 10.1152/ajplegacy.1976.231.6.1735. [DOI] [PubMed] [Google Scholar]
- Reuss L. Effects of amphotericin b on the electrical properties of Necturus gallbladder: intracellular microelectrode studies. J Membr Biol. 1978 Jun 22;41(1):65–86. doi: 10.1007/BF01873340. [DOI] [PubMed] [Google Scholar]
- Reuss L., Finn A. L. Electrical properties of the cellular transepithelial pathway in Necturus gallbladder. I. Circuit analysis and steady-state effects of mucosal solution ionic substitutions. J Membr Biol. 1975 Dec 4;25(1-2):115–139. doi: 10.1007/BF01868571. [DOI] [PubMed] [Google Scholar]
- Reuss L., Gatzy J. T., Finn A. L. Dual effects of amphotericin B on ion permeation in toad urinary bladder epithelium. Am J Physiol. 1978 Nov;235(5):F507–F514. doi: 10.1152/ajprenal.1978.235.5.F507. [DOI] [PubMed] [Google Scholar]
- Reuss L. Mechanisms of sodium and chloride transport by gallbladder epithelium. Fed Proc. 1979 Dec;38(13):2733–2738. [PubMed] [Google Scholar]
- Reuss L. Mechanisms of the mucosa-negative transepithelial potential produced by amphotericin B in gallbladder epithelium. Fed Proc. 1981 Jun;40(8):2206–2212. [PubMed] [Google Scholar]
- Reuss L., Weinman S. A., Grady T. P. Intracellular K+ activity and its relation to basolateral membrane ion transport in Necturus gallbladder epithelium. J Gen Physiol. 1980 Jul;76(1):33–52. doi: 10.1085/jgp.76.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose R. C., Nahrwold D. L. Electrolyte transport by gallbladders of rabbit and guinea pig: effect of amphotericin B and evidence of rheogenic Na transport. J Membr Biol. 1976 Oct 20;29(1-2):1–22. doi: 10.1007/BF01868949. [DOI] [PubMed] [Google Scholar]
- Schultz S. G. Electrical potential differences and electromotive forces in epithelial tissues. J Gen Physiol. 1972 Jun;59(6):794–798. doi: 10.1085/jgp.59.6.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba H. Heaviside's "Bessel cable" as an electric model for flat simple epithelial cells with low resistive junctional membranes. J Theor Biol. 1971 Jan;30(1):59–68. doi: 10.1016/0022-5193(71)90036-1. [DOI] [PubMed] [Google Scholar]
- Wills N. K., Lewis S. A., Eaton D. C. Active and passive properties of rabbit descending colon: a microelectrode and nystatin study. J Membr Biol. 1979 Mar 28;45(1-2):81–108. doi: 10.1007/BF01869296. [DOI] [PubMed] [Google Scholar]


