Abstract
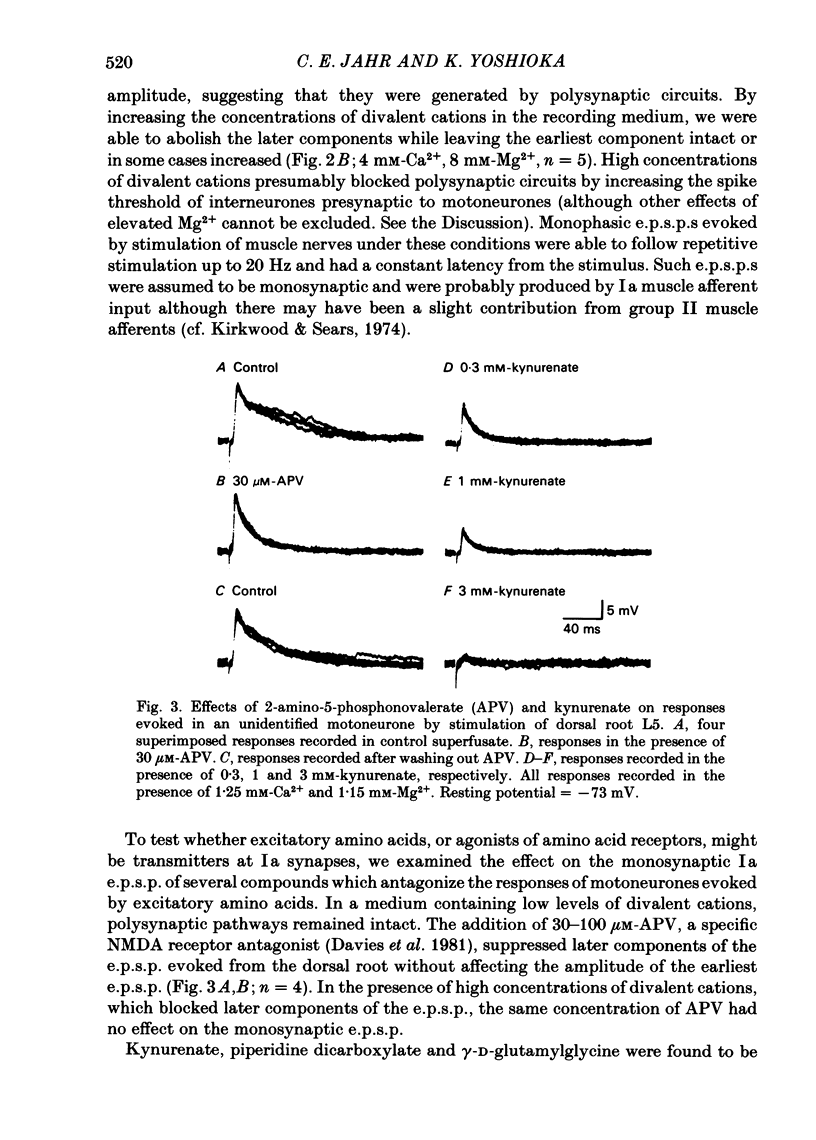
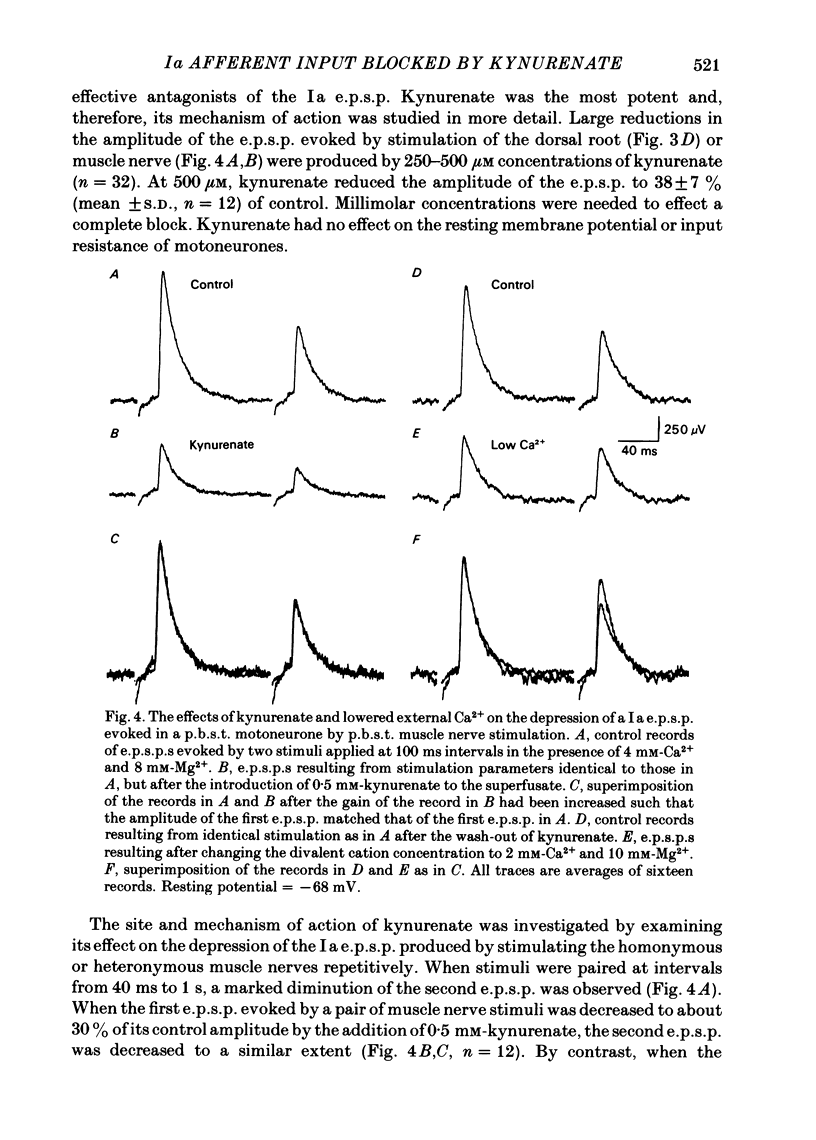
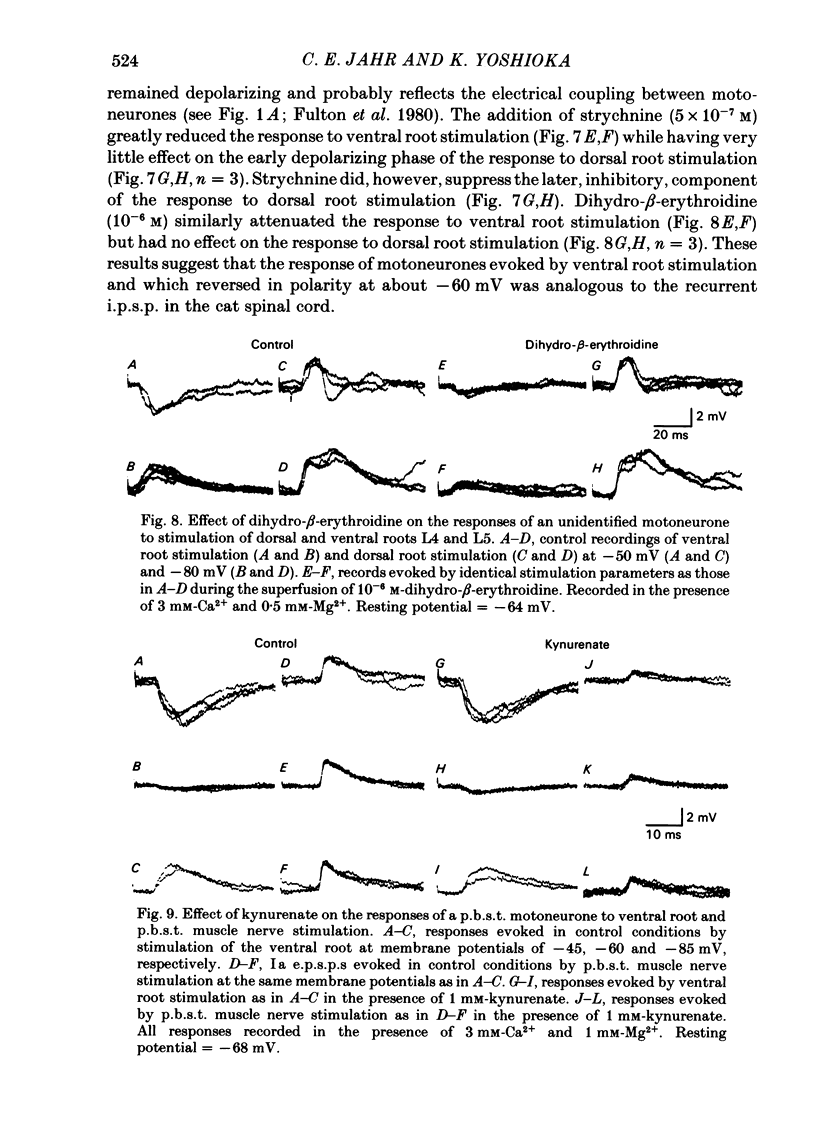
Intracellular recordings from motoneurones in in vitro preparations of new-born rat spinal cord were used to study the sensitivity of the Ia excitatory post-synaptic potential (e.p.s.p.) to antagonists of excitatory amino acids, in order to test whether group Ia primary afferents release L-glutamate, or a similar compound, as a neurotransmitter. The Ia e.p.s.p. was isolated for study by using low intensity stimulation of individual muscle nerves and by the addition to the superfusate of high concentrations of divalent cations which suppressed polysynaptic inputs to the motoneurones. The pattern of convergence of group Ia afferents from homonymous, heteronymous and antagonist muscle nerves onto motoneurones in the new-born rat was similar to that reported in the adult cat spinal cord. Homonymous muscle nerve stimulation evoked the largest amplitude Ia e.p.s.p.s while heteronymous muscle nerve stimulation elicited smaller e.p.s.p.s or had no effect. Stimulation of antagonist muscle nerves resulted in inhibitory post-synaptic potentials (i.p.s.p.). Superfusion of the specific N-methyl-D-aspartate (NMDA) receptor antagonist, 2-amino-5-phosphonovalerate, did not inhibit the Ia e.p.s.p. but did suppress later, polysynaptic components of the response evoked from dorsal roots. Kynurenate was a potent inhibitor of the Ia e.p.s.p. The site of action of kynurenate was examined by observing its effect on synaptic depression and was found to be consistent with a post-synaptic mechanism. Kynurenate selectively blocked the depolarization of motoneurones elicited by L-glutamate and had no effect on the depolarization evoked by carbachol. The selectivity of action of kynurenate was further examined by comparing its effect on the recurrent i.p.s.p. evoked by ventral root stimulation with its effect on the Ia e.p.s.p. The recurrent i.p.s.p. was antagonized by strychnine and dihydro-beta-erythroidine while kynurenate, at a concentration which greatly reduced the Ia e.p.s.p., had no effect. These results suggest that stimulation of group Ia primary afferents evokes the release of L-glutamate, or a similar compound, which activates non-NMDA excitatory amino acid receptors on motoneurones which, in turn, mediate the Ia e.p.s.p.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ault B., Evans R. H., Francis A. A., Oakes D. J., Watkins J. C. Selective depression of excitatory amino acid induced depolarizations by magnesium ions in isolated spinal cord preparations. J Physiol. 1980 Oct;307:413–428. doi: 10.1113/jphysiol.1980.sp013443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROCK L. G., COOMBS J. S., ECCLES J. C. The recording of potentials from motoneurones with an intracellular electrode. J Physiol. 1952 Aug;117(4):431–460. doi: 10.1113/jphysiol.1952.sp004759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., ECCLES J. C., FATT P. Excitatory synaptic action in motoneurones. J Physiol. 1955 Nov 28;130(2):374–395. doi: 10.1113/jphysiol.1955.sp005413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS D. R., ECCLES J. C. Synaptic action during and after repetitive stimulation. J Physiol. 1960 Feb;150:374–398. doi: 10.1113/jphysiol.1960.sp006393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D. R., Hösli L., Johnston G. A., Johnston I. H. The hyperpolarization of spinal motoneurones by glycine and related amino acids. Exp Brain Res. 1968;5(3):235–258. doi: 10.1007/BF00238666. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Ryall R. W. The excitation of Renshaw cells by cholinomimetics. Exp Brain Res. 1966;2(1):49–65. doi: 10.1007/BF00234360. [DOI] [PubMed] [Google Scholar]
- Davies J., Francis A. A., Jones A. W., Watkins J. C. 2-Amino-5-phosphonovalerate (2APV), a potent and selective antagonist of amino acid-induced and synaptic excitation. Neurosci Lett. 1981 Jan 1;21(1):77–81. doi: 10.1016/0304-3940(81)90061-6. [DOI] [PubMed] [Google Scholar]
- Davies J., Watkins J. C. Role of excitatory amino acid receptors in mono- and polysynaptic excitation in the cat spinal cord. Exp Brain Res. 1983;49(2):280–290. doi: 10.1007/BF00238587. [DOI] [PubMed] [Google Scholar]
- Dodd J., Solter D., Jessell T. M. Monoclonal antibodies against carbohydrate differentiation antigens identify subsets of primary sensory neurones. Nature. 1984 Oct 4;311(5985):469–472. doi: 10.1038/311469a0. [DOI] [PubMed] [Google Scholar]
- Duggan A. W., Johnston G. A. Glutamate and related amino acids in cat spinal roots, dorsal root ganglia and peripheral nerves. J Neurochem. 1970 Aug;17(8):1205–1208. doi: 10.1111/j.1471-4159.1970.tb03369.x. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol. 1957 Jun 18;137(1):22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., FATT P., KOKETSU K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. J Physiol. 1954 Dec 10;126(3):524–562. doi: 10.1113/jphysiol.1954.sp005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg I., Marshall K. C. Reversal potential for Ia excitatory post synaptic potentials in spinal motoneurones of cats. Neuroscience. 1979;4(11):1583–1591. doi: 10.1016/0306-4522(79)90021-6. [DOI] [PubMed] [Google Scholar]
- Evans R. H. The effects of amino acids and antagonists on the isolated hemisected spinal cord of the immature rat. Br J Pharmacol. 1978 Feb;62(2):171–176. doi: 10.1111/j.1476-5381.1978.tb08442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagg G. E., Foster A. C. Amino acid neurotransmitters and their pathways in the mammalian central nervous system. Neuroscience. 1983 Aug;9(4):701–719. doi: 10.1016/0306-4522(83)90263-4. [DOI] [PubMed] [Google Scholar]
- Finkel A. S., Redman S. J. The synaptic current evoked in cat spinal motoneurones by impulses in single group 1a axons. J Physiol. 1983 Sep;342:615–632. doi: 10.1113/jphysiol.1983.sp014872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster A. C., Fagg G. E. Acidic amino acid binding sites in mammalian neuronal membranes: their characteristics and relationship to synaptic receptors. Brain Res. 1984 May;319(2):103–164. doi: 10.1016/0165-0173(84)90020-1. [DOI] [PubMed] [Google Scholar]
- Fulton B. P., Miledi R., Takahashi T. Electrical synapses between motoneurons in the spinal cord of the newborn rat. Proc R Soc Lond B Biol Sci. 1980 Jun 23;208(1170):115–120. doi: 10.1098/rspb.1980.0045. [DOI] [PubMed] [Google Scholar]
- Ganong A. H., Lanthorn T. H., Cotman C. W. Kynurenic acid inhibits synaptic and acidic amino acid-induced responses in the rat hippocampus and spinal cord. Brain Res. 1983 Aug 22;273(1):170–174. doi: 10.1016/0006-8993(83)91108-3. [DOI] [PubMed] [Google Scholar]
- Graham L. T., Jr, Shank R. P., Werman R., Aprison M. H. Distribution of some synaptic transmitter suspects in cat spinal cord: glutamic acid, aspartic acid, gamma-aminobutyric acid, glycine and glutamine. J Neurochem. 1967 Apr;14(4):465–472. doi: 10.1111/j.1471-4159.1967.tb09545.x. [DOI] [PubMed] [Google Scholar]
- Jahr C. E., Jessell T. M. ATP excites a subpopulation of rat dorsal horn neurones. Nature. 1983 Aug 25;304(5928):730–733. doi: 10.1038/304730a0. [DOI] [PubMed] [Google Scholar]
- Jahr C. E., Jessell T. M. Synaptic transmission between dorsal root ganglion and dorsal horn neurons in culture: antagonism of monosynaptic excitatory postsynaptic potentials and glutamate excitation by kynurenate. J Neurosci. 1985 Aug;5(8):2281–2289. doi: 10.1523/JNEUROSCI.05-08-02281.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E., Roberts W. J. Synaptic actions of single interneurones mediating reciprocal Ia inhibition of motoneurones. J Physiol. 1972 May;222(3):623–642. doi: 10.1113/jphysiol.1972.sp009818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell T. M., Dodd J. Structure and expression of differentiation antigens on functional subclasses of primary sensory neurons. Philos Trans R Soc Lond B Biol Sci. 1985 Feb 19;308(1136):271–281. doi: 10.1098/rstb.1985.0027. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. Monosynaptic excitation of motoneurones from secondary endings of muscle spindles. Nature. 1974 Nov 15;252(5480):243–244. doi: 10.1038/252243a0. [DOI] [PubMed] [Google Scholar]
- Macdonald R. L., Pun R. Y., Neale E. A., Nelson P. G. Synaptic interactions between mammalian central neurons in cell culture. I. Reversal potential for excitatory postsynaptic potentials. J Neurophysiol. 1983 Jun;49(6):1428–1441. doi: 10.1152/jn.1983.49.6.1428. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. Mixed-agonist action of excitatory amino acids on mouse spinal cord neurones under voltage clamp. J Physiol. 1984 Sep;354:29–53. doi: 10.1113/jphysiol.1984.sp015360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- OTSUKA M., ENDO M., NONOMURA Y. Presynaptic nature of neuromuscular depression. Jpn J Physiol. 1962 Dec 15;12:573–584. doi: 10.2170/jjphysiol.12.573. [DOI] [PubMed] [Google Scholar]
- Otsuka M., Konishi S. Electrophysiology of mammalian spinal cord in vitro. Nature. 1974 Dec 20;252(5485):733–734. doi: 10.1038/252733a0. [DOI] [PubMed] [Google Scholar]
- Ransom B. R., Bullock P. N., Nelson P. G. Mouse spinal cord in cell culture. III. Neuronal chemosensitivity and its relationship to synaptic activity. J Neurophysiol. 1977 Sep;40(5):1163–1177. doi: 10.1152/jn.1977.40.5.1163. [DOI] [PubMed] [Google Scholar]
- Redman S. Junctional mechanisms at group Ia synapses. Prog Neurobiol. 1979;12(1):33–83. doi: 10.1016/0301-0082(79)90010-8. [DOI] [PubMed] [Google Scholar]
- Roberts P. J., Keen P., Mitchell J. F. The distribution and axonal transport of free amino acids and related compounds in the dorsal sensory neuron of the rat, as determined by the dansyl reaction. J Neurochem. 1973 Jul;21(1):199–209. doi: 10.1111/j.1471-4159.1973.tb04239.x. [DOI] [PubMed] [Google Scholar]
- Roberts P. J. The release of amino acids with proposed neurotransmitter function from the cuneate and gracile nuclei of the rat in vivo. Brain Res. 1974 Mar 8;67(3):419–428. doi: 10.1016/0006-8993(74)90491-0. [DOI] [PubMed] [Google Scholar]
- Salt T. E., Hill R. G. Neurotransmitter candidates of somatosensory primary afferent fibres. Neuroscience. 1983 Dec;10(4):1083–1103. doi: 10.1016/0306-4522(83)90101-x. [DOI] [PubMed] [Google Scholar]
- Shapovalov A. I., Shiriaev B. I., Tamarova Z. A. Synaptic activity in motoneurons of the immature cat spinal cord in vitro. Effects of manganese and tetrodotoxin. Brain Res. 1979 Jan 19;160(3):524–528. doi: 10.1016/0006-8993(79)91080-1. [DOI] [PubMed] [Google Scholar]
- THIES R. E. NEUROMUSCULAR DEPRESSION AND THE APPARENT DEPLETION OF TRANSMITTER IN MAMMALIAN MUSCLE. J Neurophysiol. 1965 May;28:428–442. doi: 10.1152/jn.1965.28.3.427. [DOI] [PubMed] [Google Scholar]
- Takahashi T. Inhibitory miniature synaptic potentials in rat motoneurons. Proc R Soc Lond B Biol Sci. 1984 Mar 22;221(1222):103–109. doi: 10.1098/rspb.1984.0025. [DOI] [PubMed] [Google Scholar]
- Walmsley B., Tracey D. J. An intracellular study of Renshaw cells. Brain Res. 1981 Oct 26;223(1):170–175. doi: 10.1016/0006-8993(81)90818-0. [DOI] [PubMed] [Google Scholar]
- Watkins J. C., Evans R. H. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]


