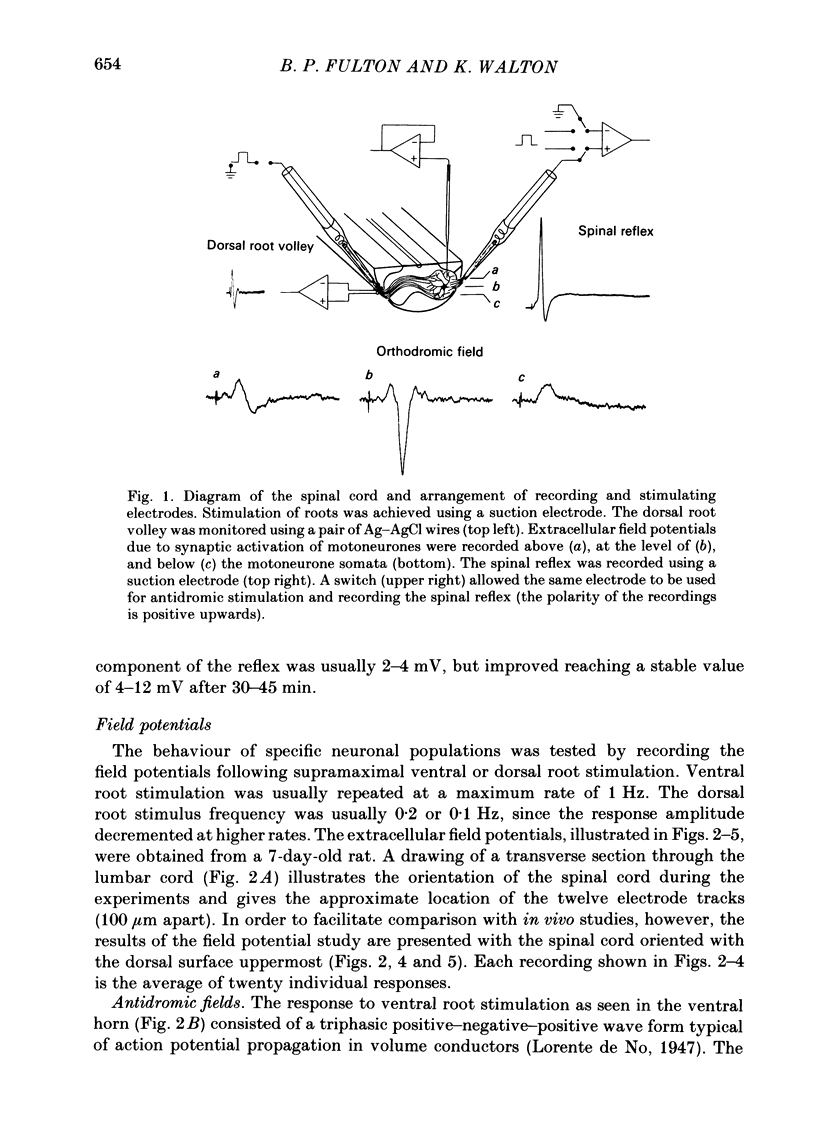
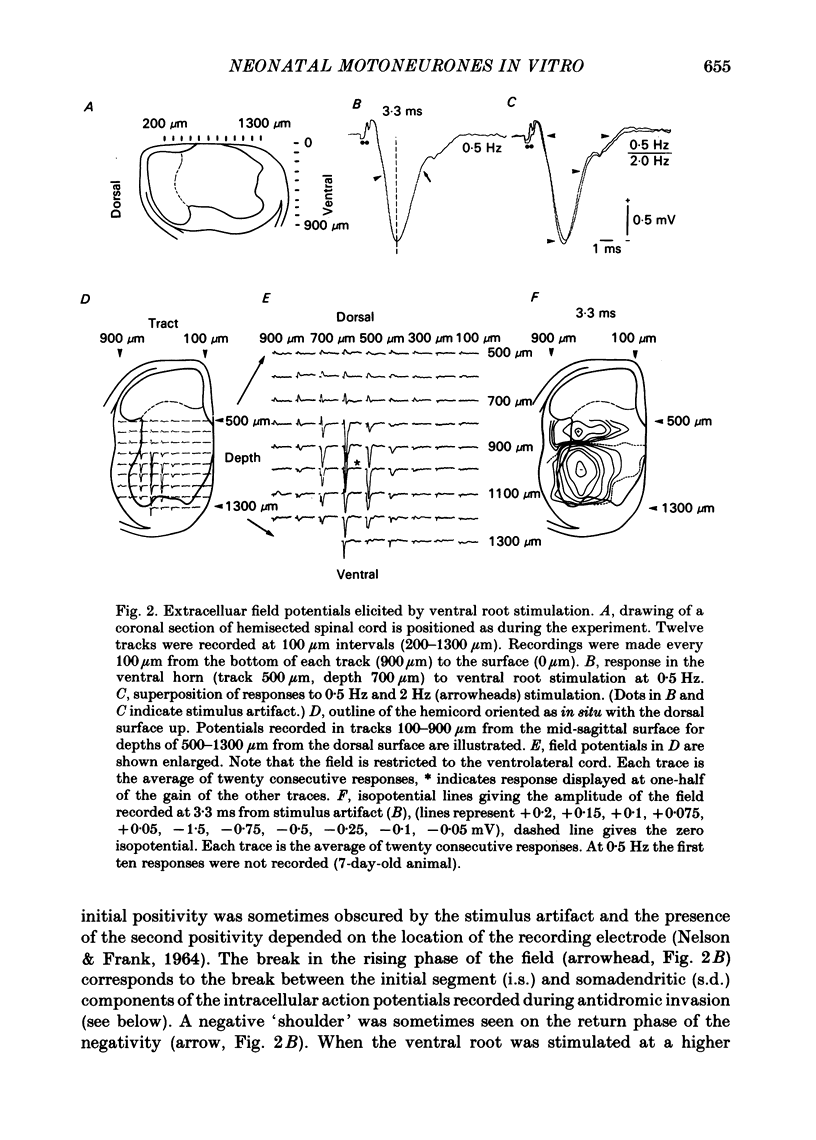
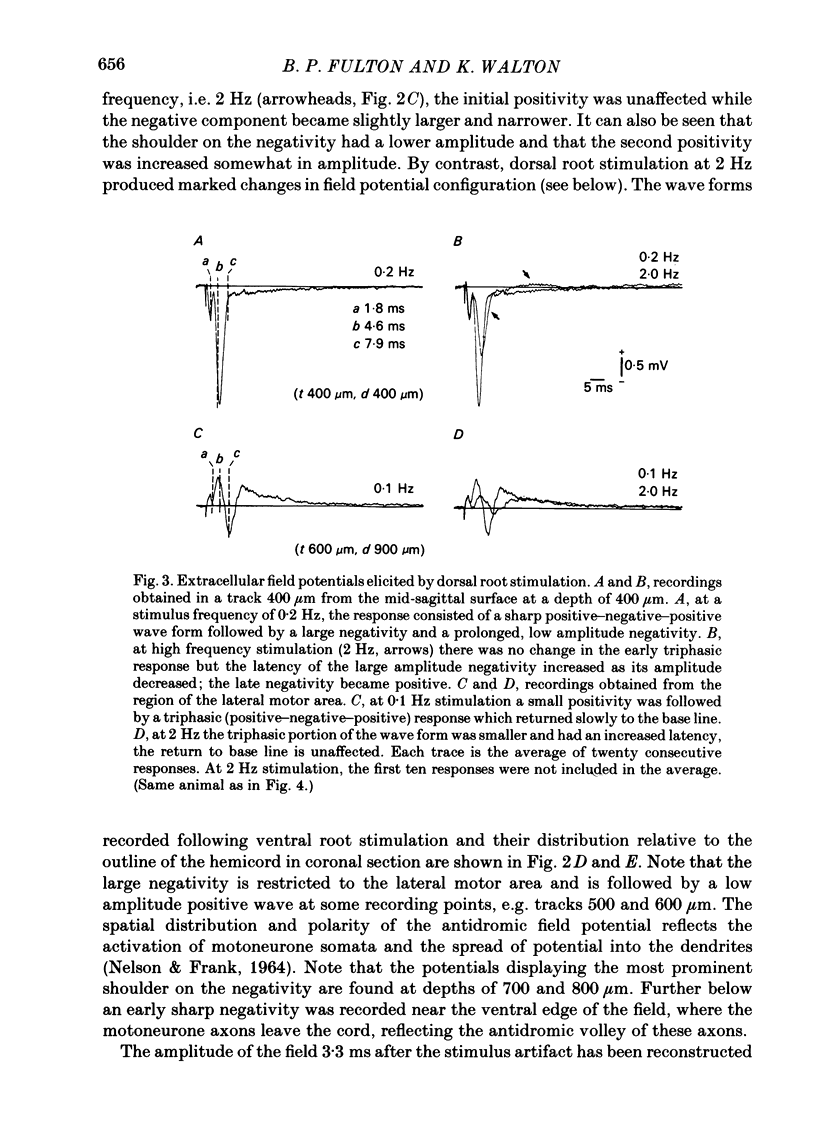
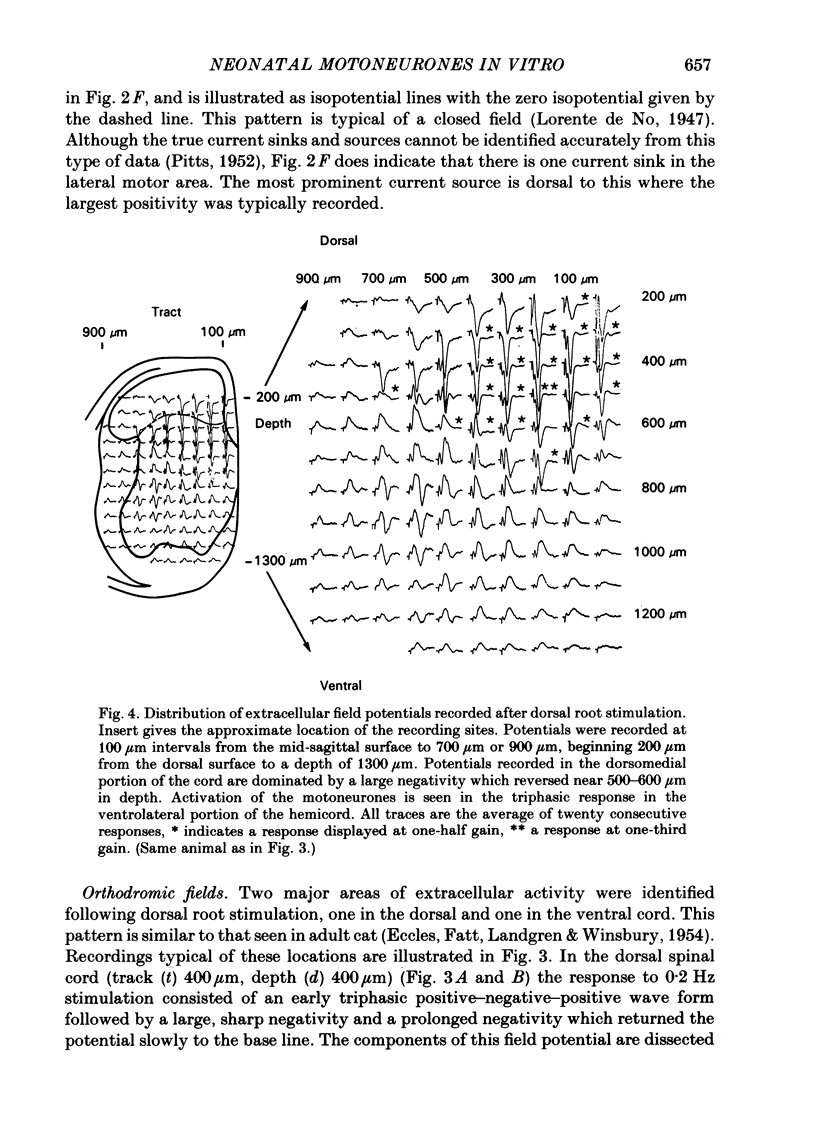
Abstract
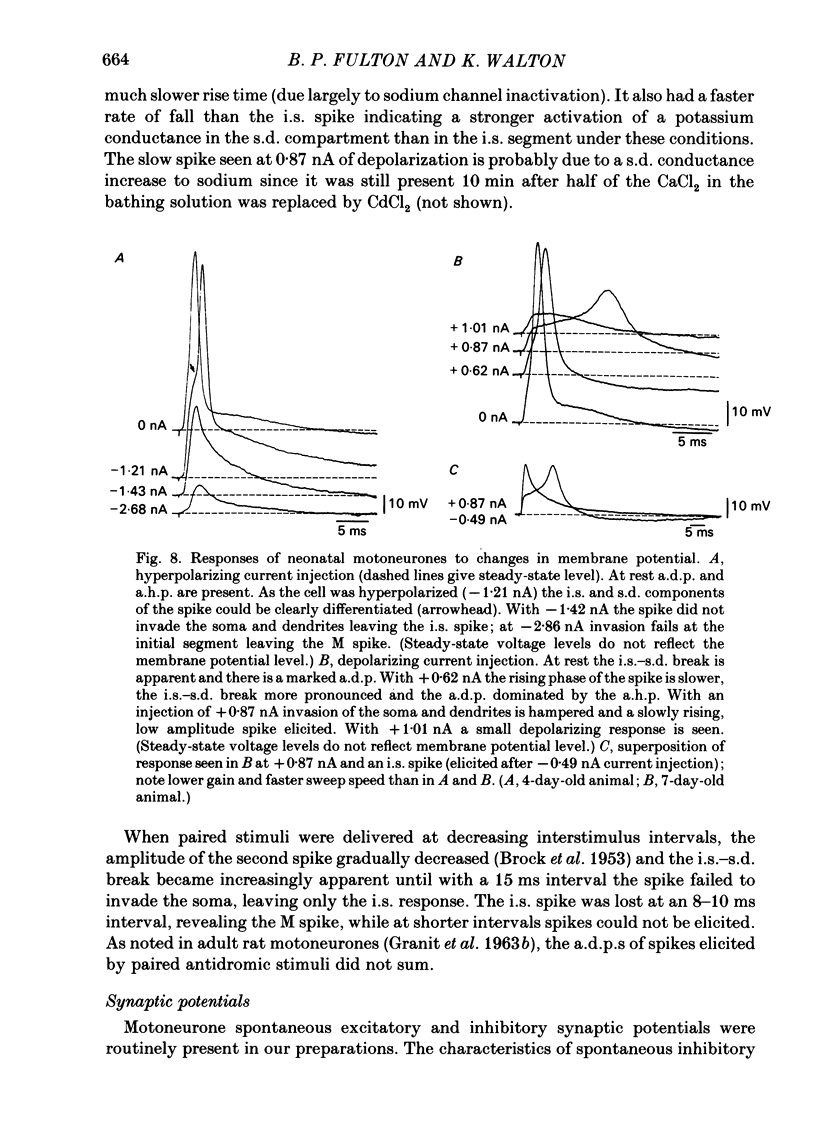
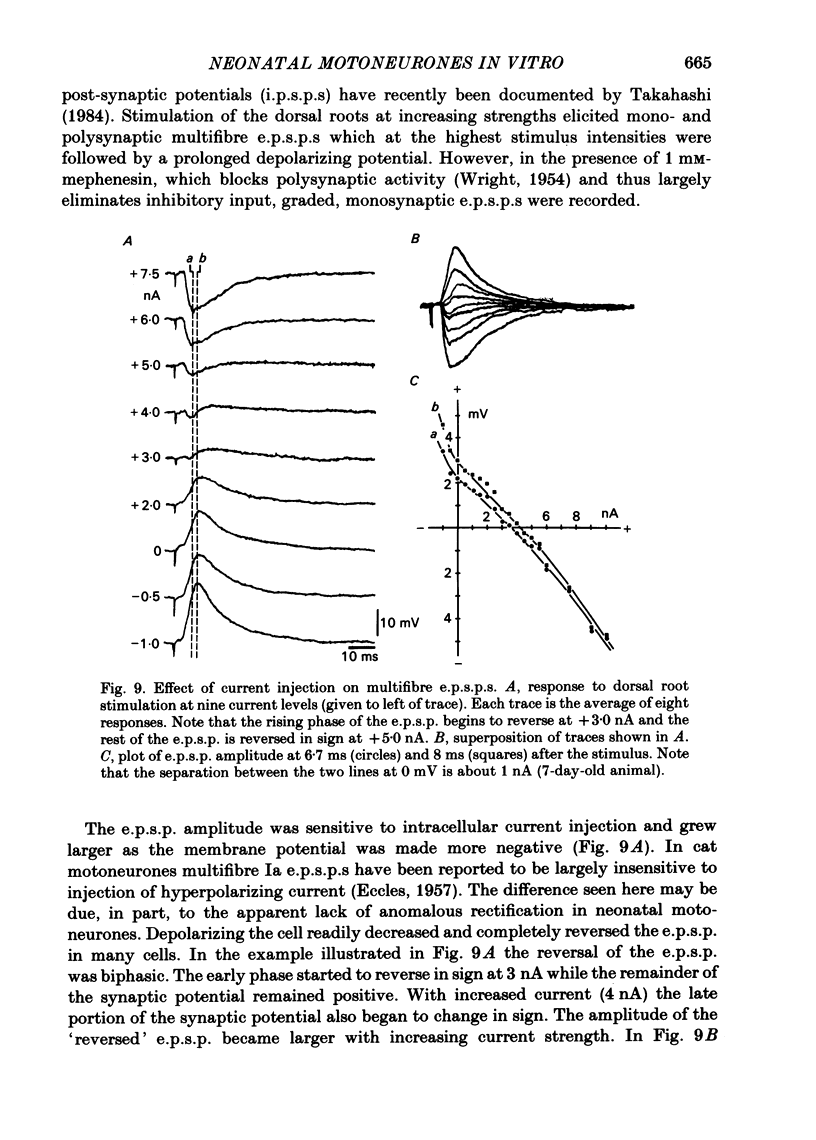
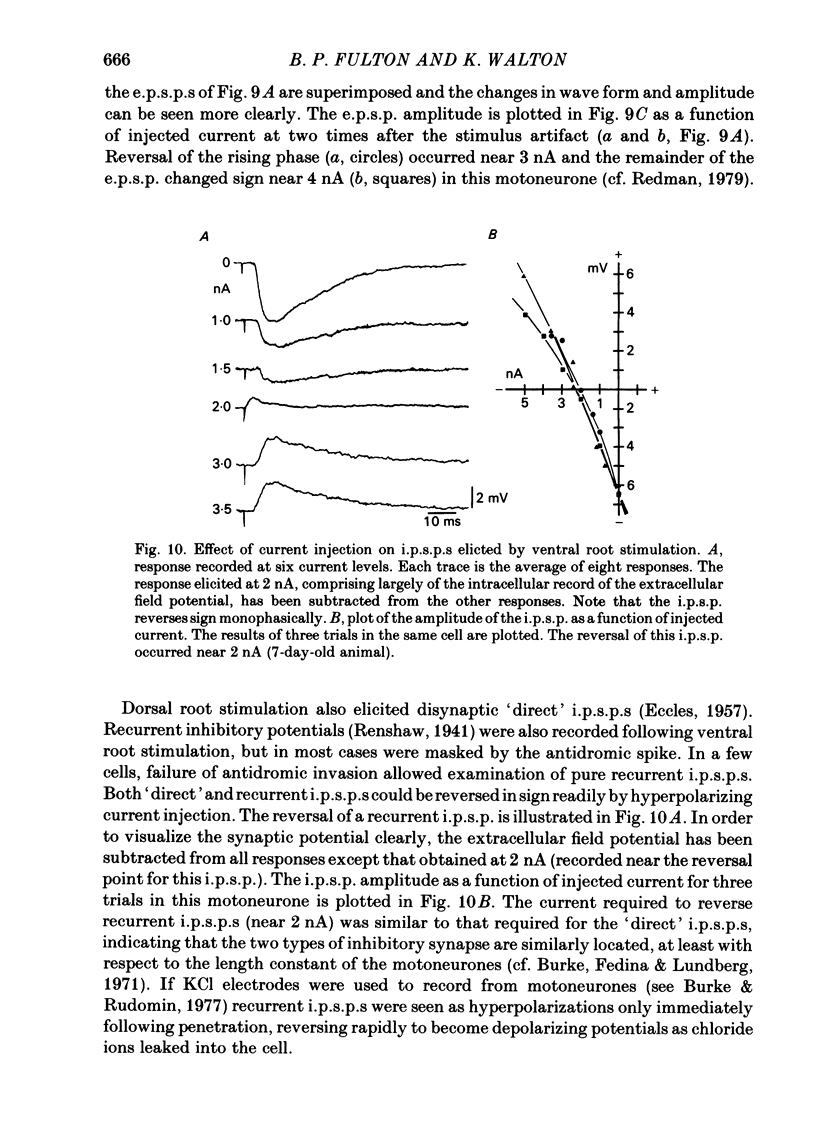
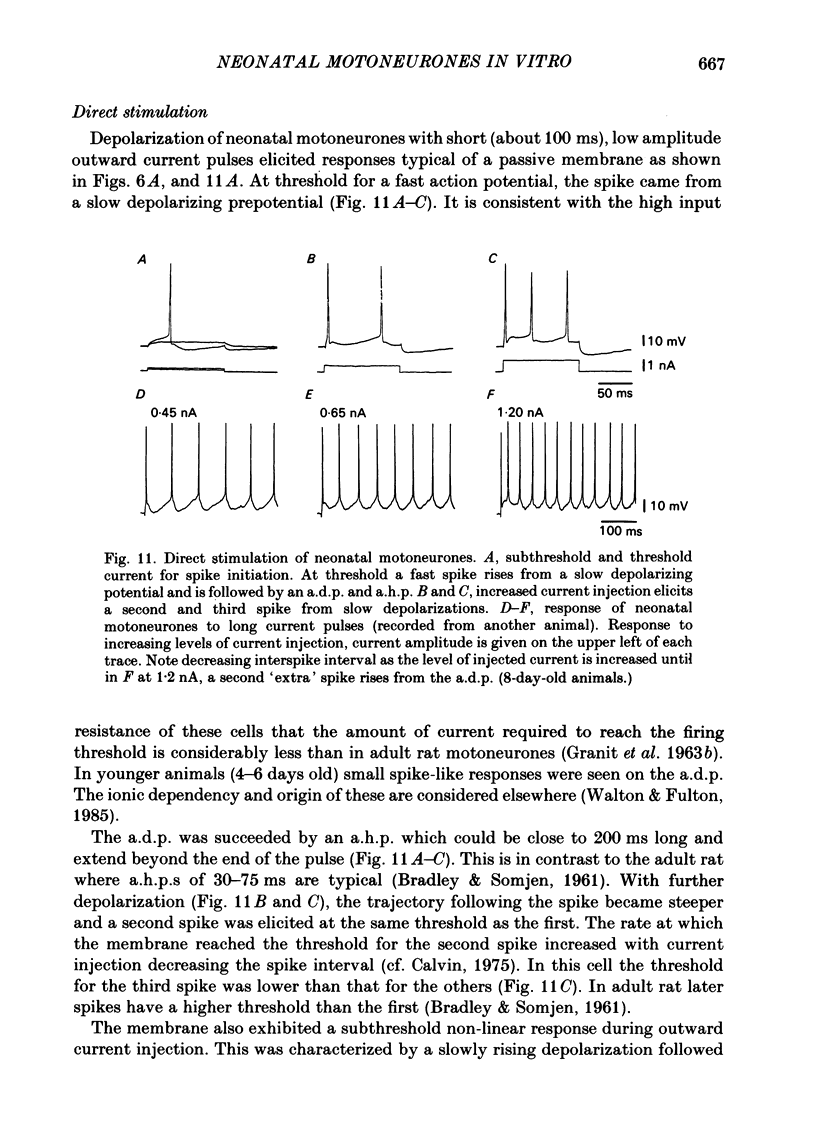
The electroresponsive properties of neonatal lumbar spinal motoneurones were studied using isolated, hemisected spinal cords from neonatal rats aged 3-12 days. The extracellular and intracellular responses to electrical stimulation of the ventral and dorsal root were studied as well as the intracellular response to current injection. Field potentials recorded in the lateral motor area following electrical stimulation of lumbar ventral roots had a triphasic positive-negative-positive wave form. The negative component did not return to the base line smoothly but exhibited a 'shoulder' where the negativity increased in duration. Following electrical stimulation of the dorsal root, presynaptic field potentials were recorded upon activation of the afferent axons as well as following synaptic activation of interneurones and motoneurones. The input resistances of neonatal motoneurones determined from the slope of current-voltage plots were high compared with the adult. The resistance decreased with age with a mean of 18.1 M omega for animals 3-5 days old, 8.8 M omega for animals 6-8 days old and 5.4 M omega for animals 9-11 days old. Values for the membrane time constant were similar to those in the adult with a mean of 4.5 ms. Action potentials elicited by ventral or dorsal root stimulation or by intracellular current injection were marked by a pronounced after-depolarization (a.d.p.) and an after-hyperpolarization (a.h.p.). The amplitude of the a.h.p. varied with that of the a.d.p. The amplitude of excitatory post-synaptic potentials (e.p.s.p.s) elicited by electrical stimulation of the dorsal root was affected by intracellular current injection. Two types of e.p.s.p.s were distinguished: those with a biphasic reversal (early phase first) and those in which the early phase was unaffected by inward current injection while the later phase was reversed. Unlike in the adult, the reversals could be achieved with low current levels and the amplitude of both types of e.p.s.p. was increased by inward current injection. Inhibitory post-synaptic potentials (i.p.s.p.s) were elicited by dorsal or ventral root stimulation. The amplitude of these i.p.s.p.s was diminished and reversed in sign with inward current injection and their amplitude was enhanced with outward current injection. Activation of neonatal motoneurones with long current pulses revealed that there is one steady-state firing range.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


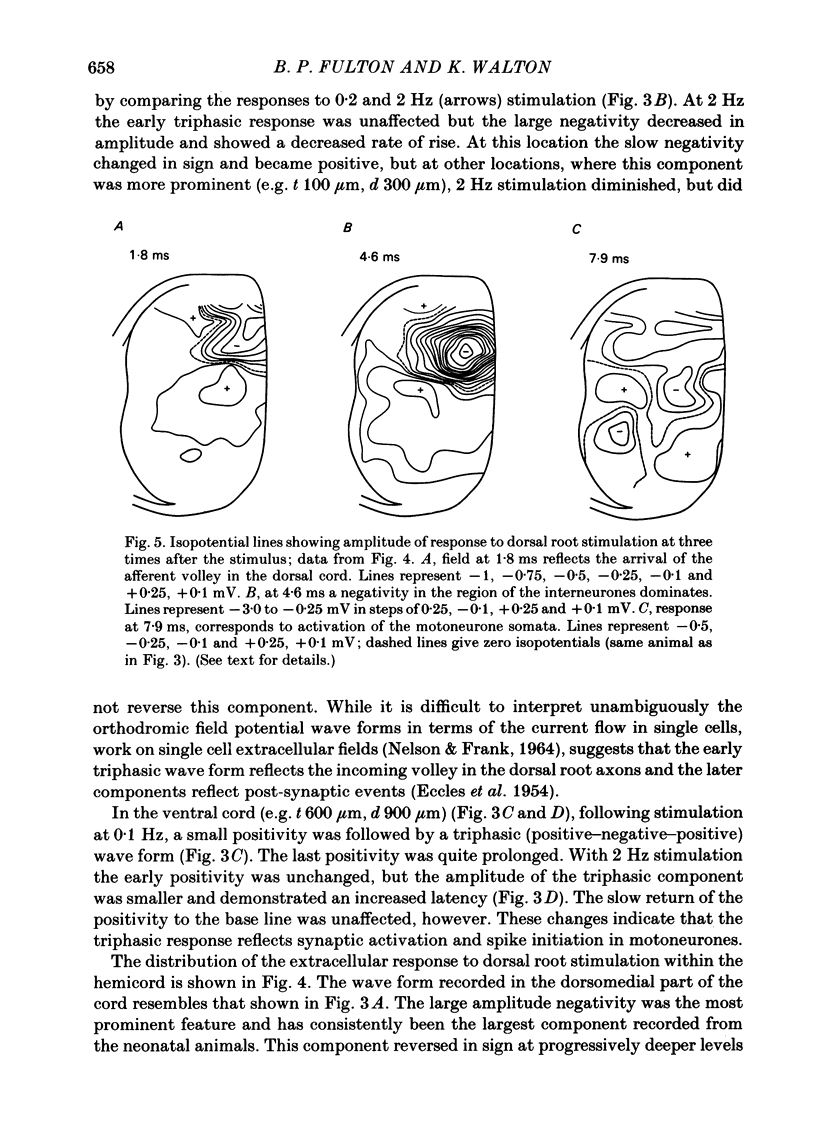

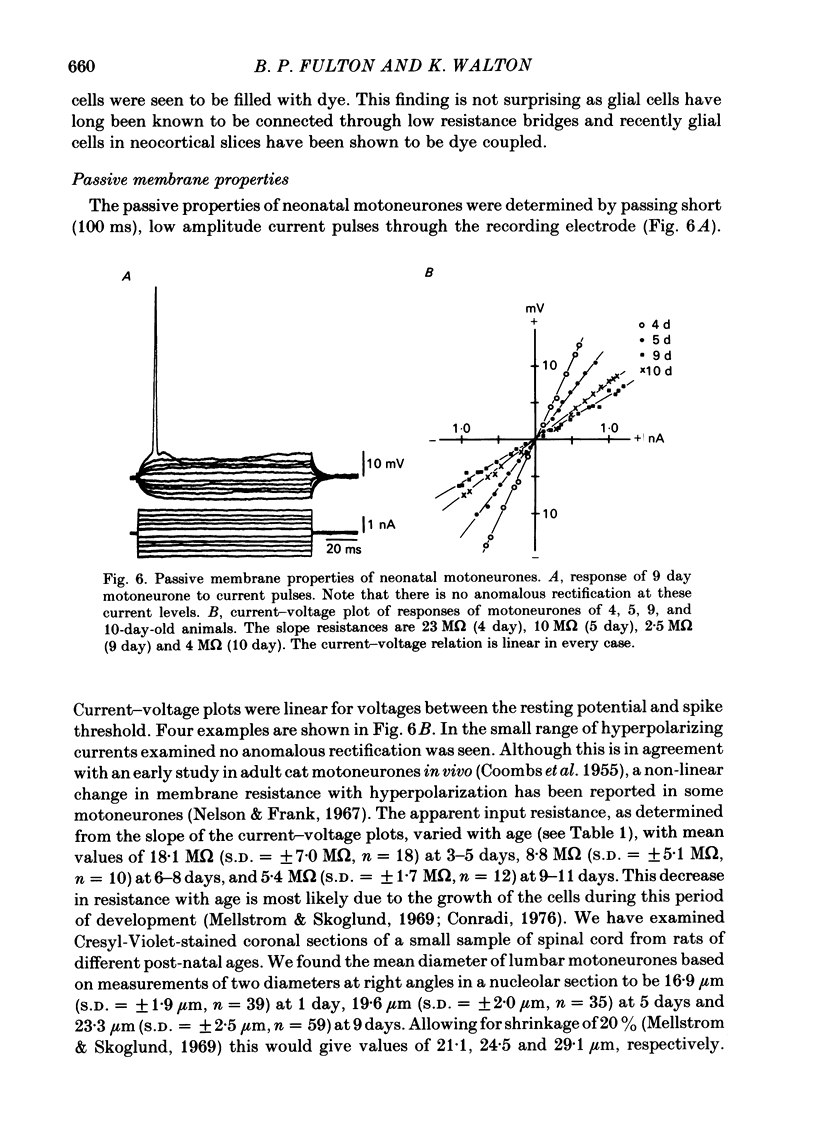
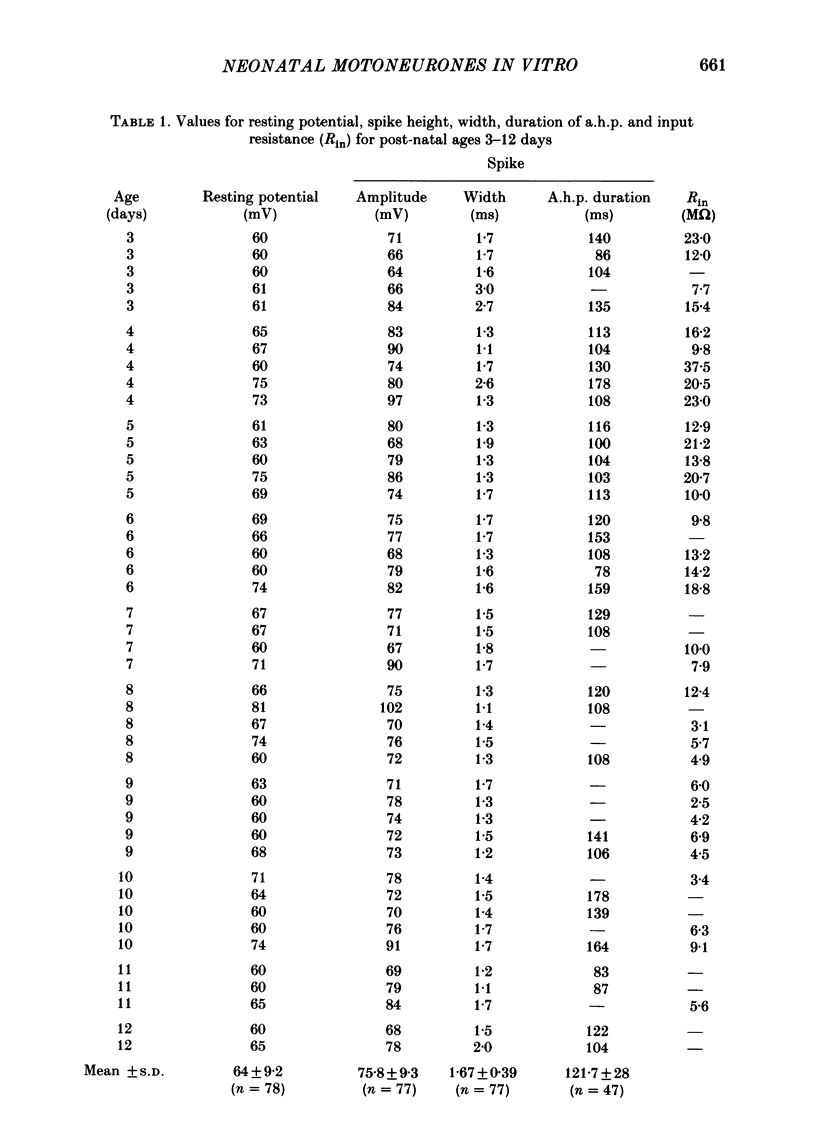
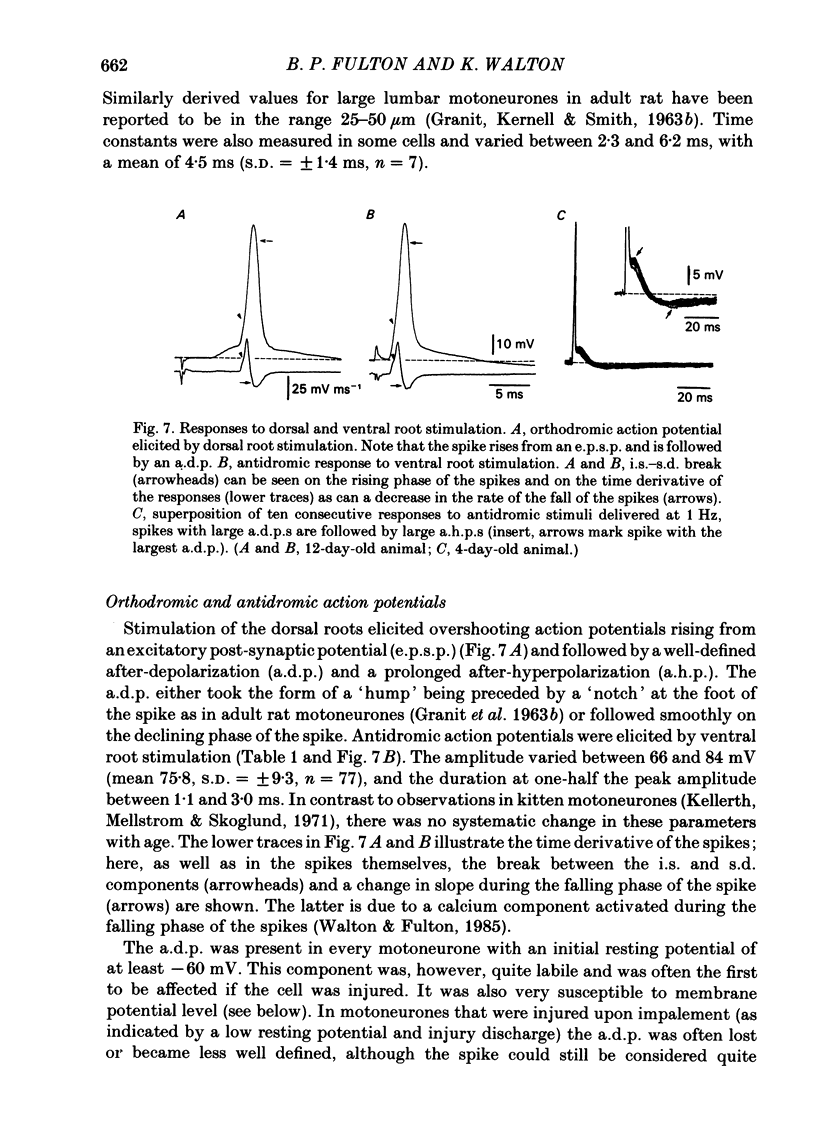










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROCK L. G., COOMBS J. S., ECCLES J. C. Intracellular recording from antidromically activated motoneurones. J Physiol. 1953 Dec 29;122(3):429–461. doi: 10.1113/jphysiol.1953.sp005013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROCK L. G., COOMBS J. S., ECCLES J. C. The recording of potentials from motoneurones with an intracellular electrode. J Physiol. 1952 Aug;117(4):431–460. doi: 10.1113/jphysiol.1952.sp004759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley K., Somjen G. G. Accommodation in motoneurones of the rat and the cat. J Physiol. 1961 Apr;156(1):75–92. doi: 10.1113/jphysiol.1961.sp006659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Fedina L., Lundberg A. Spatial synaptic distribution of recurrent and group Ia inhibitory systems in cat spinal motoneurones. J Physiol. 1971 Apr;214(2):305–326. doi: 10.1113/jphysiol.1971.sp009434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLOSE R. DYNAMIC PROPERTIES OF FAST AND SLOW SKELETAL MUSCLES OF THE RAT DURING DEVELOPMENT. J Physiol. 1964 Sep;173:74–95. doi: 10.1113/jphysiol.1964.sp007444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., ECCLES J. C., FATT P. The electrical properties of the motoneurone membrane. J Physiol. 1955 Nov 28;130(2):291–325. doi: 10.1113/jphysiol.1955.sp005411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvin W. H. Generation of spike trains in CNS neurons. Brain Res. 1975 Jan 24;84(1):1–22. doi: 10.1016/0006-8993(75)90796-9. [DOI] [PubMed] [Google Scholar]
- Connor K. M., Ferrington D. G., Rowe M. J. Tactile sensory coding during development: signaling capacities of neurons in kitten dorsal column nuclei. J Neurophysiol. 1984 Jul;52(1):86–98. doi: 10.1152/jn.1984.52.1.86. [DOI] [PubMed] [Google Scholar]
- Dennis M. J., Ziskind-Conhaim L., Harris A. J. Development of neuromuscular junctions in rat embryos. Dev Biol. 1981 Jan 30;81(2):266–279. doi: 10.1016/0012-1606(81)90290-6. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The action potentials of the alpha motoneurones supplying fast and slow muscles. J Physiol. 1958 Jul 14;142(2):275–291. doi: 10.1113/jphysiol.1958.sp006015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. Types of neurone in and around the intermediate nucleus of the lumbosacral cord. J Physiol. 1960 Nov;154:89–114. doi: 10.1113/jphysiol.1960.sp006566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., FATT P., LANDGREN S., WINSBURY G. J. Spinal cord potentials generated by volleys in the large muscle afferents. J Physiol. 1954 Sep 28;125(3):590–606. doi: 10.1113/jphysiol.1954.sp005183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrington D. G., Rowe M. J. Functional capacities of tactile afferent fibres in neonatal kittens. J Physiol. 1980 Oct;307:335–353. doi: 10.1113/jphysiol.1980.sp013438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R., KERNELL D., SHORTESS G. K. QUANTITATIVE ASPECTS OF REPETITIVE FIRING OF MAMMALIAN MOTONEURONES, CAUSED BY INJECTED CURRENTS. J Physiol. 1963 Oct;168:911–931. doi: 10.1113/jphysiol.1963.sp007230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R., KERNELL D., SMITH R. S. DELAYED DEPOLARIZATION AND THE REPETITIVE RESPONSE TO INTRACELLULAR STIMULATION OF MAMMALIAN MOTONEURONES. J Physiol. 1963 Oct;168:890–910. doi: 10.1113/jphysiol.1963.sp007229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert M., Stelzner D. J. The development of descending and dorsal root connections in the lumbosacral spinal cord of the postnatal rat. J Comp Neurol. 1979 Apr 15;184(4):821–838. doi: 10.1002/cne.901840413. [DOI] [PubMed] [Google Scholar]
- Granit R., Kernell D., Lamarre Y. Synaptic stimulation superimposed on motoneurones firing in the 'secondary range' to injected current. J Physiol. 1966 Nov;187(2):401–415. doi: 10.1113/jphysiol.1966.sp008098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B., Pinter M. J. Relations among passive electrical properties of lumbar alpha-motoneurones of the cat. J Physiol. 1984 Nov;356:401–431. doi: 10.1113/jphysiol.1984.sp015473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENNEMAN E., SOMJEN G., CARPENTER D. O. FUNCTIONAL SIGNIFICANCE OF CELL SIZE IN SPINAL MOTONEURONS. J Neurophysiol. 1965 May;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- Harada Y., Takahashi T. The calcium component of the action potential in spinal motoneurones of the rat. J Physiol. 1983 Feb;335:89–100. doi: 10.1113/jphysiol.1983.sp014521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer C. B., Llinás R. Control of rhythmic firing in normal and axotomized cat spinal motoneurons. J Neurophysiol. 1977 May;40(3):480–488. doi: 10.1152/jn.1977.40.3.480. [DOI] [PubMed] [Google Scholar]
- Huizar P., Kuno M., Miyata Y. Differentiation of motoneurones and skeletal muscles in kittens. J Physiol. 1975 Nov;252(2):465–479. doi: 10.1113/jphysiol.1975.sp011152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERNELL D. THE DELAYED DEPOLARIZATION IN CAT AND RAT MOTONEURONES. Prog Brain Res. 1964;12:42–55. doi: 10.1016/s0079-6123(08)60616-0. [DOI] [PubMed] [Google Scholar]
- KOLMODIN G. M., SKOGLUND C. R. Slow membrane potential changes accompanying excitation and inhibition in spinal moto- and interneurons in the cat during natural activation. Acta Physiol Scand. 1958 Oct 28;44(1):11–54. doi: 10.1111/j.1748-1716.1958.tb01607.x. [DOI] [PubMed] [Google Scholar]
- Kellerth J. O., Mellström A., Skoglund S. Postnatal excitability changes of kitten motoneurones. Acta Physiol Scand. 1971 Sep;83(1):31–41. doi: 10.1111/j.1748-1716.1971.tb05048.x. [DOI] [PubMed] [Google Scholar]
- Kernell D. Input resistance, electrical excitability, and size of ventral horn cells in cat spinal cord. Science. 1966 Jun 17;152(3729):1637–1640. doi: 10.1126/science.152.3729.1637. [DOI] [PubMed] [Google Scholar]
- Kernell D., Zwaagstra B. Input conductance axonal conduction velocity and cell size among hindlimb motoneurones of the cat. Brain Res. 1981 Jan 12;204(2):311–326. doi: 10.1016/0006-8993(81)90591-6. [DOI] [PubMed] [Google Scholar]
- Lannou J., Precht W., Cazin L. The postnatal development of functional properties of central vestibular neurons in the rat. Brain Res. 1979 Oct 19;175(2):219–232. doi: 10.1016/0006-8993(79)91002-3. [DOI] [PubMed] [Google Scholar]
- Letinsky M. S. Physiological properties of developing frog tadpole nerve-muscle junctions during repetitive stimulation. Dev Biol. 1974 Sep;40(1):154–161. doi: 10.1016/0012-1606(74)90115-8. [DOI] [PubMed] [Google Scholar]
- Llinás R., Nicholson C. Reversal properties of climbing fiber potential in cat Purkinje cells: an example of a distributed synapse. J Neurophysiol. 1976 Mar;39(2):311–323. doi: 10.1152/jn.1976.39.2.311. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980 Aug;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKA K. I. ELECTROPHYSIOLOGY OF THE FETAL SPINAL CORD. I. ACTION POTENTIALS OF THE MOTONEURON. J Gen Physiol. 1964 May;47:1003–1022. doi: 10.1085/jgp.47.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NELSON P. G., FRANK K. EXTRACELLULAR POTENTIAL FIELDS OF SINGLE SPINAL MOTONEURONS. J Neurophysiol. 1964 Sep;27:913–927. doi: 10.1152/jn.1964.27.5.913. [DOI] [PubMed] [Google Scholar]
- Nelson P. G., Burke R. E. Delayed depolarization in cat spinal motoneurons. Exp Neurol. 1967 Jan;17(1):16–26. doi: 10.1016/0014-4886(67)90118-5. [DOI] [PubMed] [Google Scholar]
- Nelson P. G., Frank K. Anomalous rectification in cat spinal motoneurons and effect of polarizing currents on excitatory postsynaptic potential. J Neurophysiol. 1967 Sep;30(5):1097–1113. doi: 10.1152/jn.1967.30.5.1097. [DOI] [PubMed] [Google Scholar]
- Otsuka M., Konishi S. Electrophysiology of mammalian spinal cord in vitro. Nature. 1974 Dec 20;252(5485):733–734. doi: 10.1038/252733a0. [DOI] [PubMed] [Google Scholar]
- REXED B. A cytoarchitectonic atlas of the spinal cord in the cat. J Comp Neurol. 1954 Apr;100(2):297–379. doi: 10.1002/cne.901000205. [DOI] [PubMed] [Google Scholar]
- Redman S. Junctional mechanisms at group Ia synapses. Prog Neurobiol. 1979;12(1):33–83. doi: 10.1016/0301-0082(79)90010-8. [DOI] [PubMed] [Google Scholar]
- SPRAGUE J. M., HONGCHIEN H. A. THE TERMINAL FIELDS OF DORSAL ROOT FIBERS IN THE LUMBOSACRAL SPINAL CORD OF THE CAT, AND THE DENDRITIC ORGANIZATION OF THE MOTOR NUCLEI. Prog Brain Res. 1964;11:120–154. doi: 10.1016/s0079-6123(08)64046-7. [DOI] [PubMed] [Google Scholar]
- Saito K. Development of spinal reflexes in the rat fetus studied in vitro. J Physiol. 1979 Sep;294:581–594. doi: 10.1113/jphysiol.1979.sp012947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzner D. J. The normal postnatal development of synaptic end-feet in the lumbosacral spinal cord and of responses in the hind limbs of the albino rat. Exp Neurol. 1971 Jun;31(3):337–357. doi: 10.1016/0014-4886(71)90237-8. [DOI] [PubMed] [Google Scholar]
- Takahashi T. Inhibitory miniature synaptic potentials in rat motoneurons. Proc R Soc Lond B Biol Sci. 1984 Mar 22;221(1222):103–109. doi: 10.1098/rspb.1984.0025. [DOI] [PubMed] [Google Scholar]
- Takahashi T. Intracellular recording from visually identified motoneurons in rat spinal cord slices. Proc R Soc Lond B Biol Sci. 1978 Jul 26;202(1148):417–421. doi: 10.1098/rspb.1978.0076. [DOI] [PubMed] [Google Scholar]
- WRIGHT E. B. Effect of mephenesin and other depressants on spinal cord transmission in frog and cat. Am J Physiol. 1954 Nov;179(2):390–401. doi: 10.1152/ajplegacy.1954.179.2.390. [DOI] [PubMed] [Google Scholar]
- Walton K., Fulton B. Hydrogen peroxide as a source of molecular oxygen for in vitro mammalian CNS preparations. Brain Res. 1983 Nov 14;278(1-2):387–393. doi: 10.1016/0006-8993(83)90280-9. [DOI] [PubMed] [Google Scholar]



