Abstract
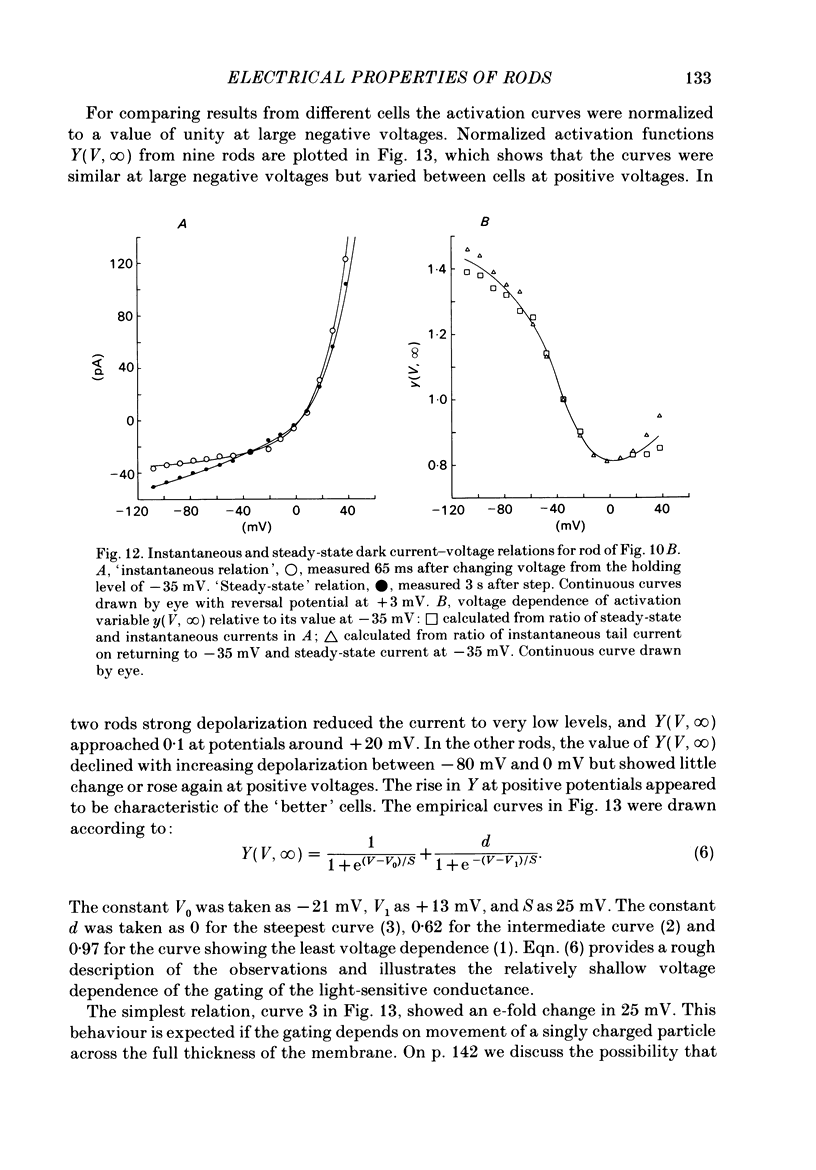
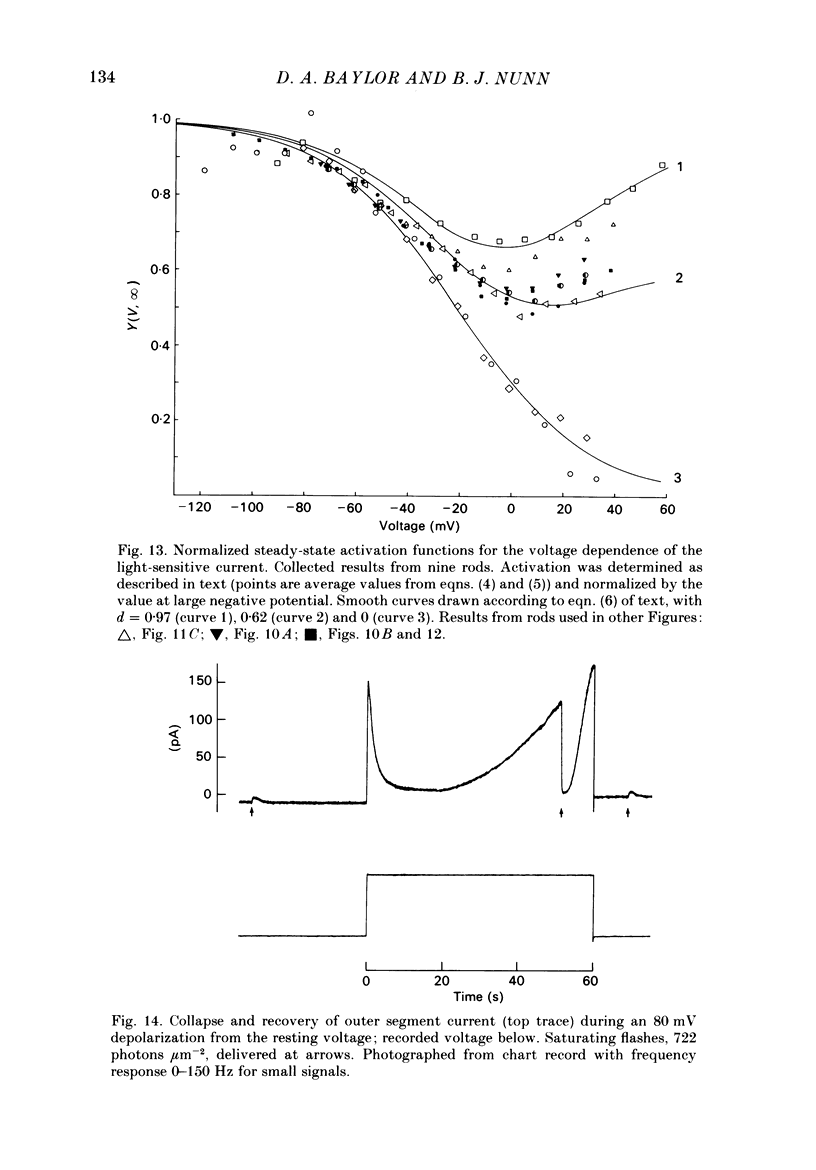
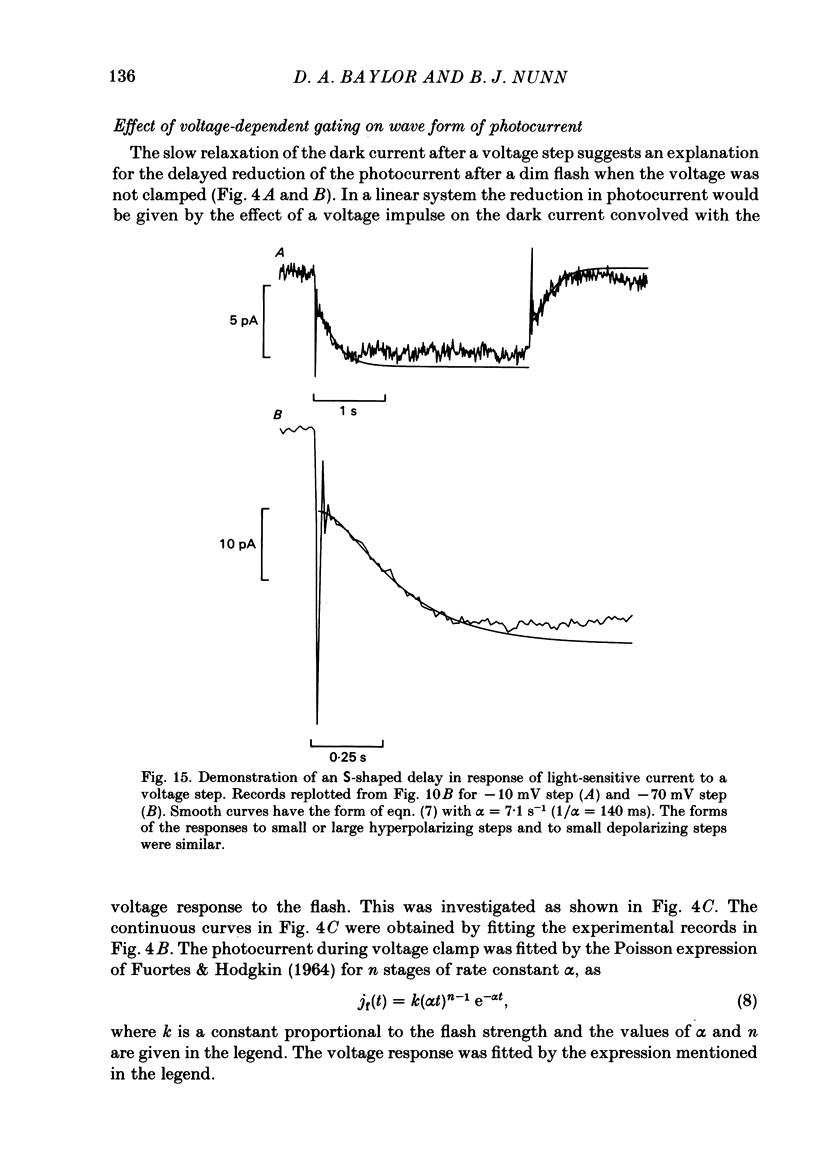
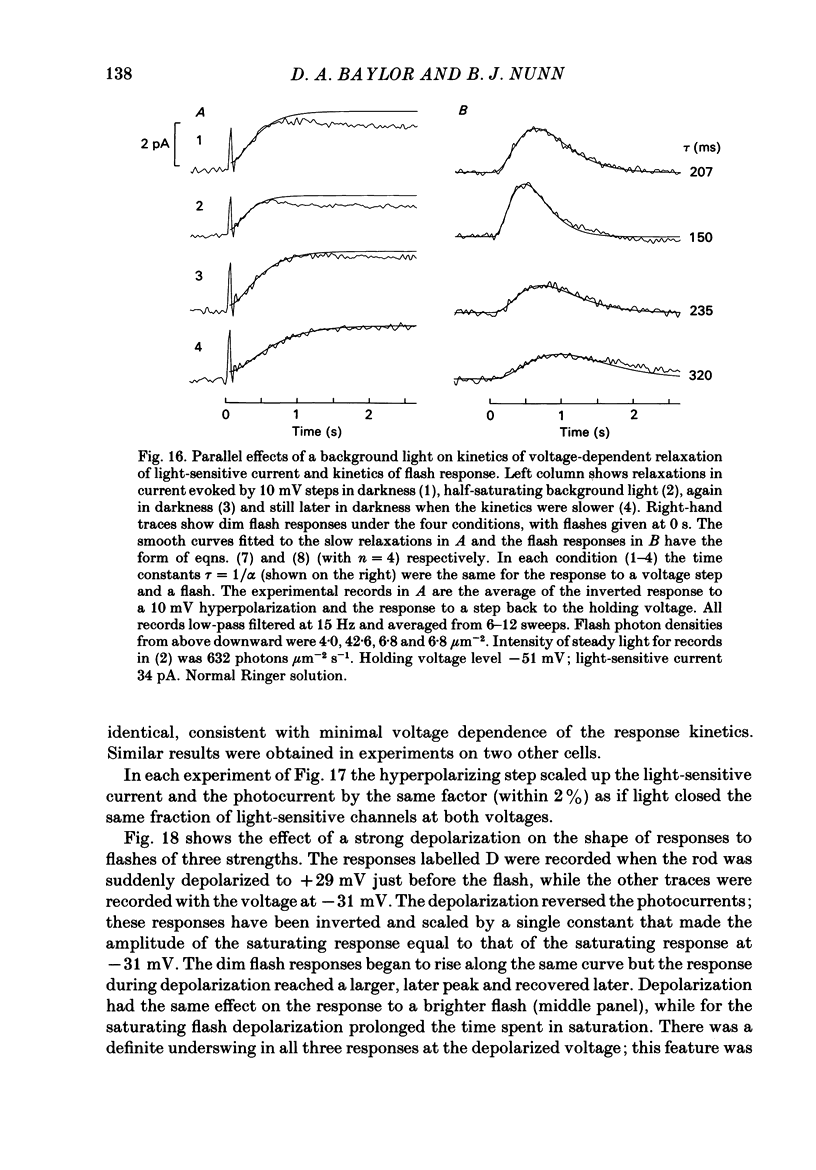
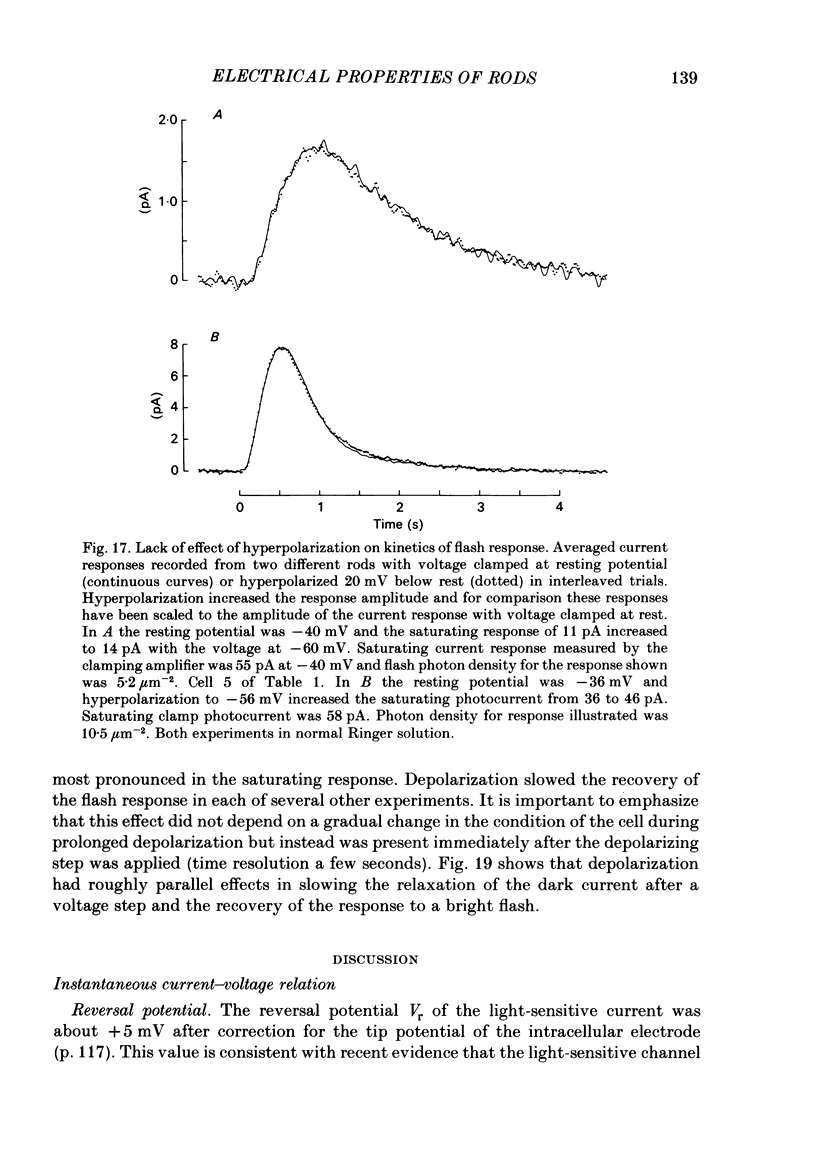
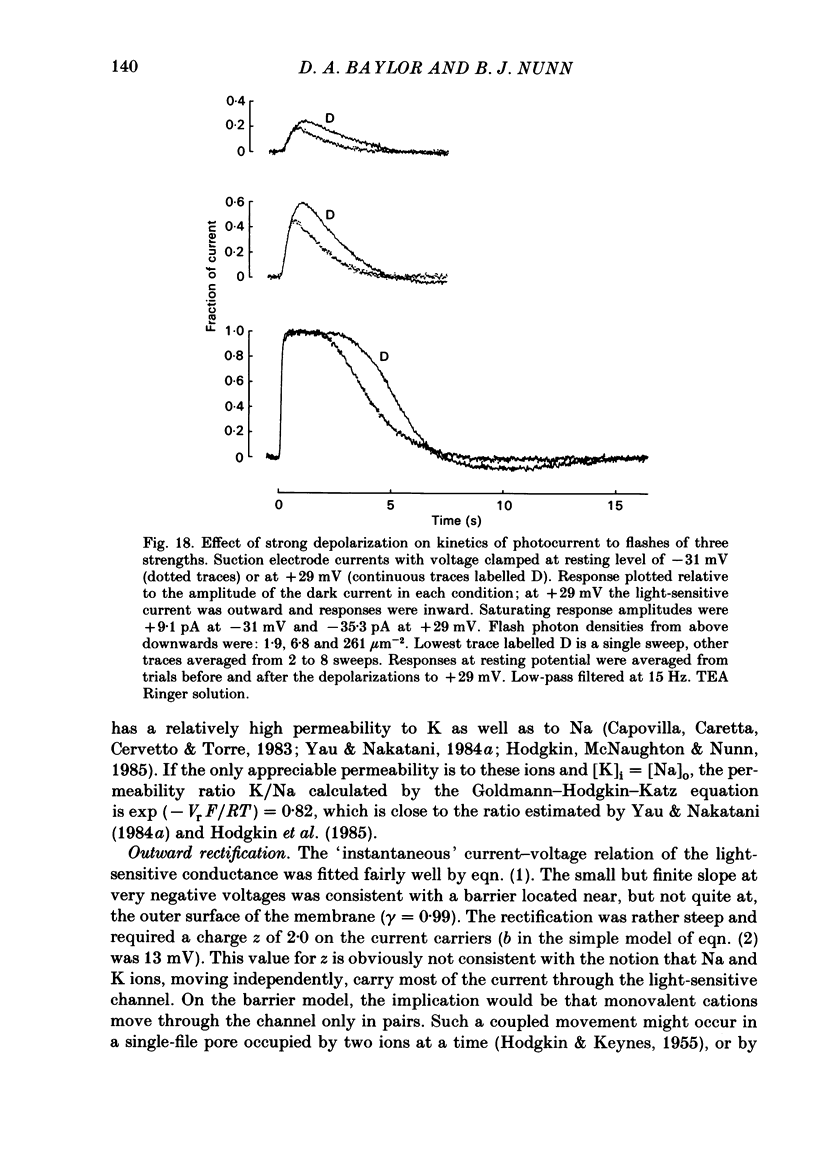
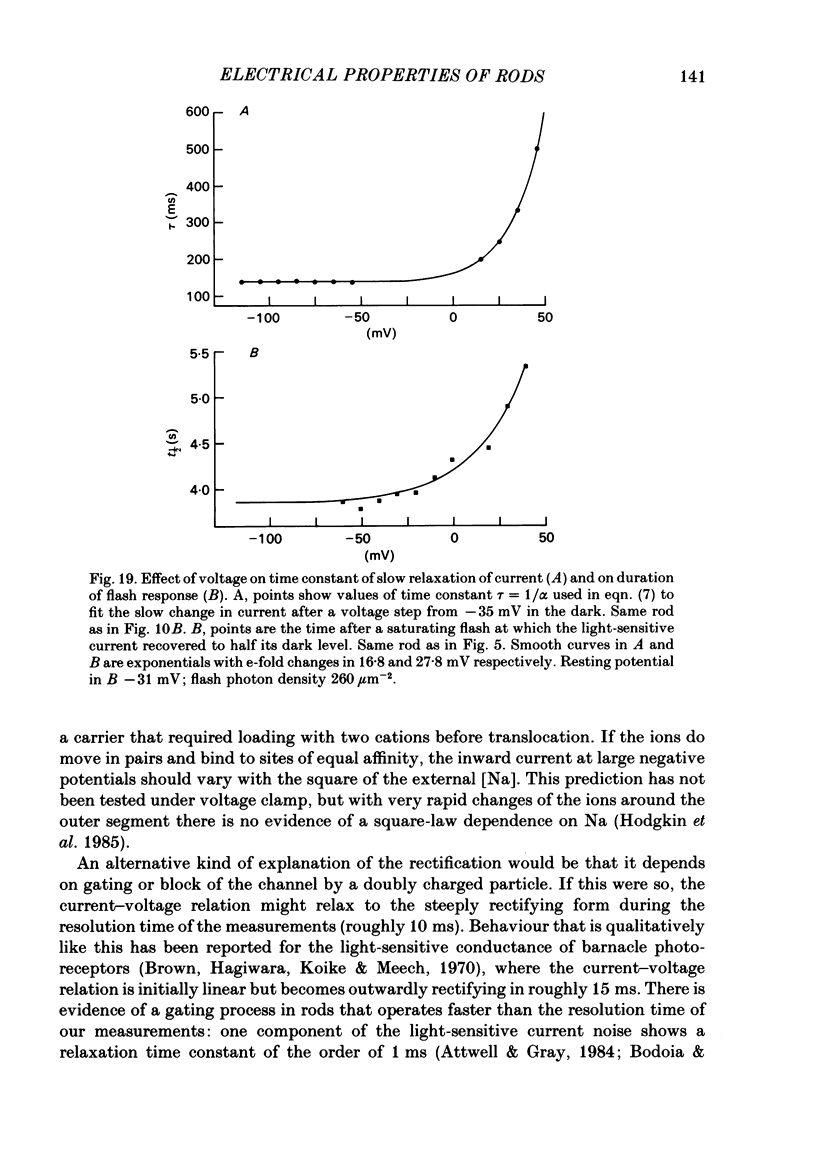
The light-sensitive conductance of isolated rods from the retina of the tiger salamander was studied using a voltage-clamp method. The membrane current of the outer segment was collected with a suction electrode while the internal voltage was measured and controlled with a pair of intracellular electrodes. Saturating light blocked the outer segment current at all potentials, the residual conductance usually becoming less than 20 pS. This suggests that light-sensitive channels comprise the main ionic conductance in the surface membrane of the outer segment. Current-voltage relations determined 10-40 ms after changing the voltage showed outward-going rectification, the outward current increasing e-fold for a depolarization of 11-14 mV. The reversal potential of the light-sensitive current was estimated as 5 +/- 4 mV. This is consistent with other evidence indicating that the channel is not exclusively permeable to Na. Applying steady light, lowering external Ca, or changing the intracellular voltage to a new steady level scaled the light-sensitive current without altering the reversal potential or the form of the rectification. This suggests that all three manipulations change the number of channels in the conducting state without changing the ionic concentration gradients or the mechanism of permeation through an 'open' channel. Hyperpolarizing voltage steps slowly increased the light-sensitive current and depolarizing steps reduced it. A gating variable Y expressing the fractional activation of the light-sensitive conductance in the steady state was derived from the ratio of the instantaneous and steady-state currents. Y declined at voltages positive to -100 mV and usually reached a minimum near 0 mV, with a secondary rise positive to 0 mV. Around the dark voltage Y changed e-fold in roughly 25 mV. The voltage-dependent gating in (6). appeared to involve two delays similar in magnitude to those of the four principal delays in the rod's response to a dim flash. Steady background light shortened the time-scale of gating and flash responses to a similar degree. Clamping the voltage at the dark level had little effect on the photocurrent evoked by a flash. The small, delayed effect actually observed is explained by the slow voltage-dependent gating of the light-sensitive conductance. Hyperpolarization had little effect on the kinetics of the response to a flash, but depolarization slowed the response, causing it to reach a larger, later peak. Depolarization also prolonged the blockage of the light-sensitive current after a saturating flash.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF

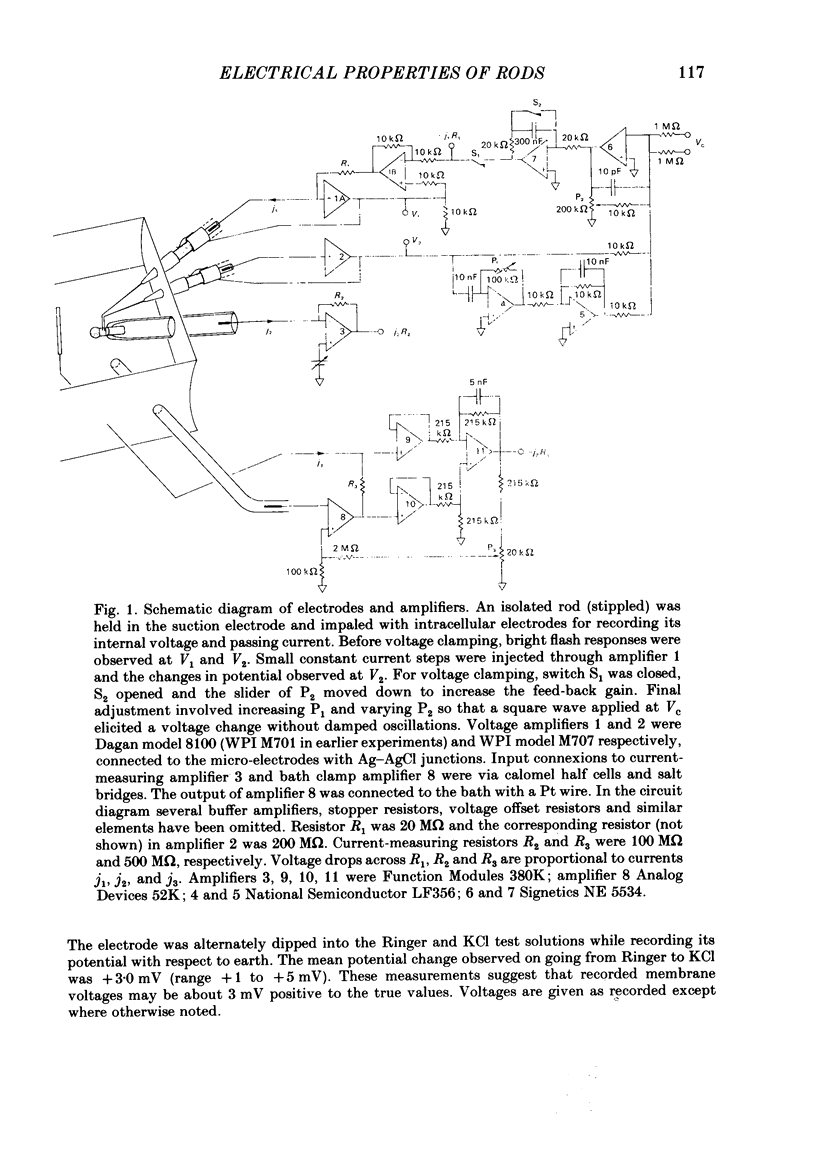
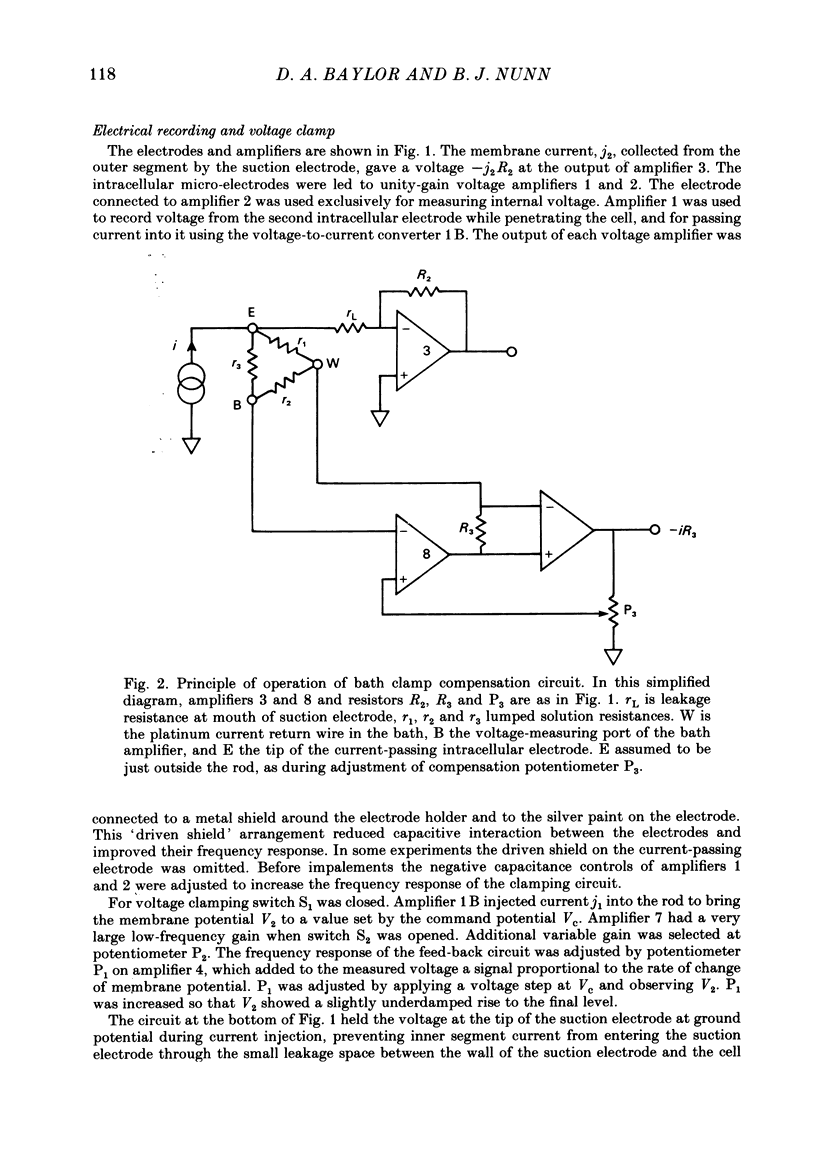


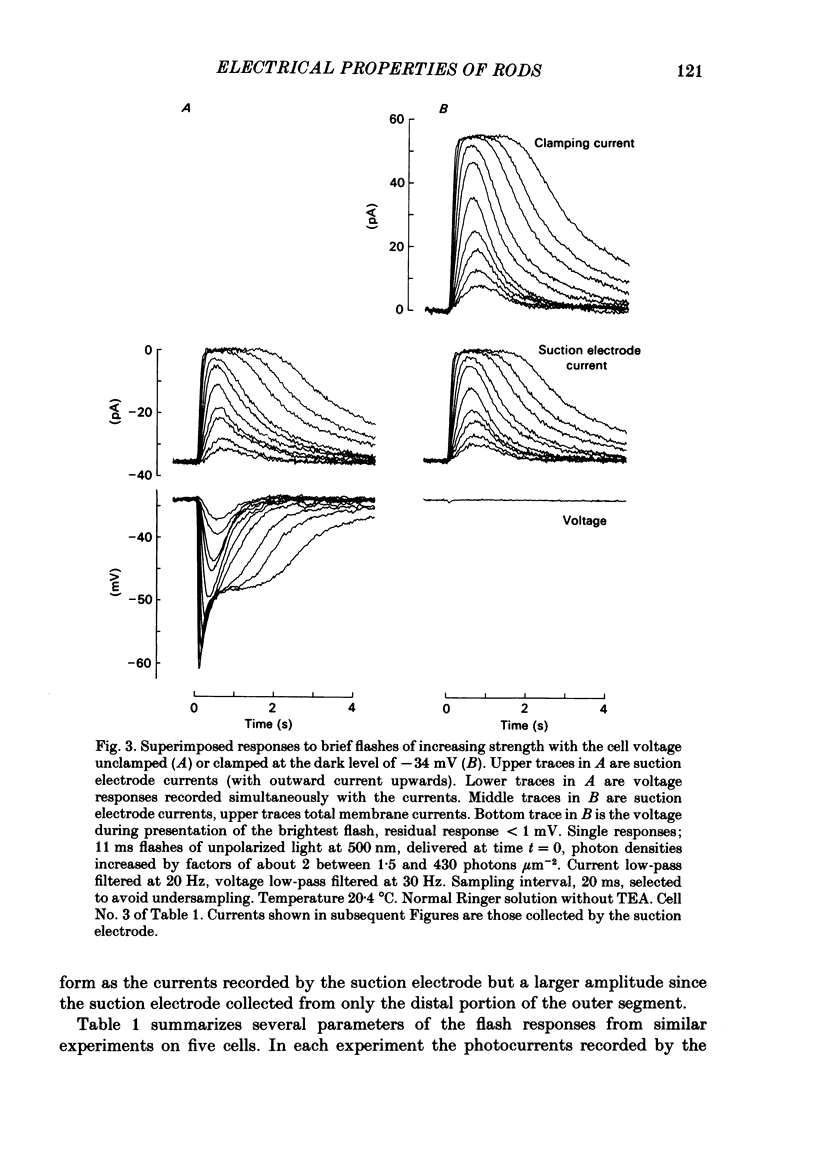
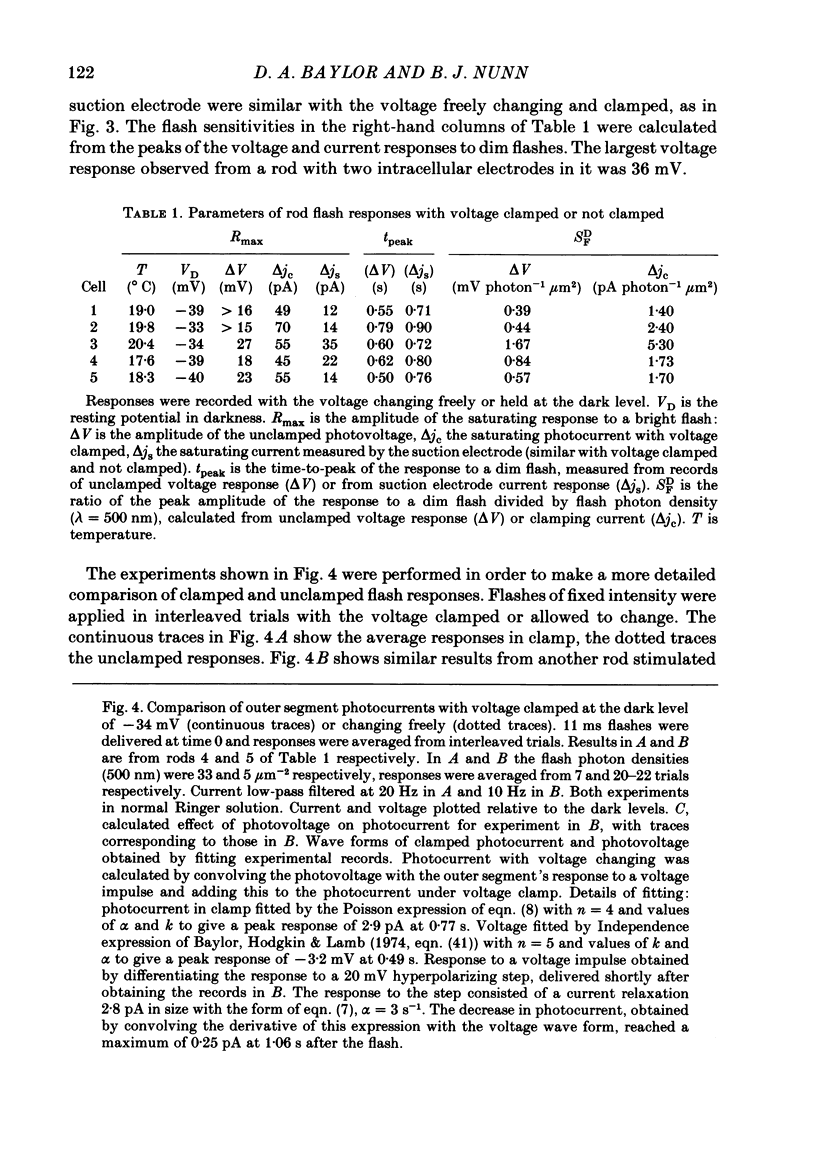
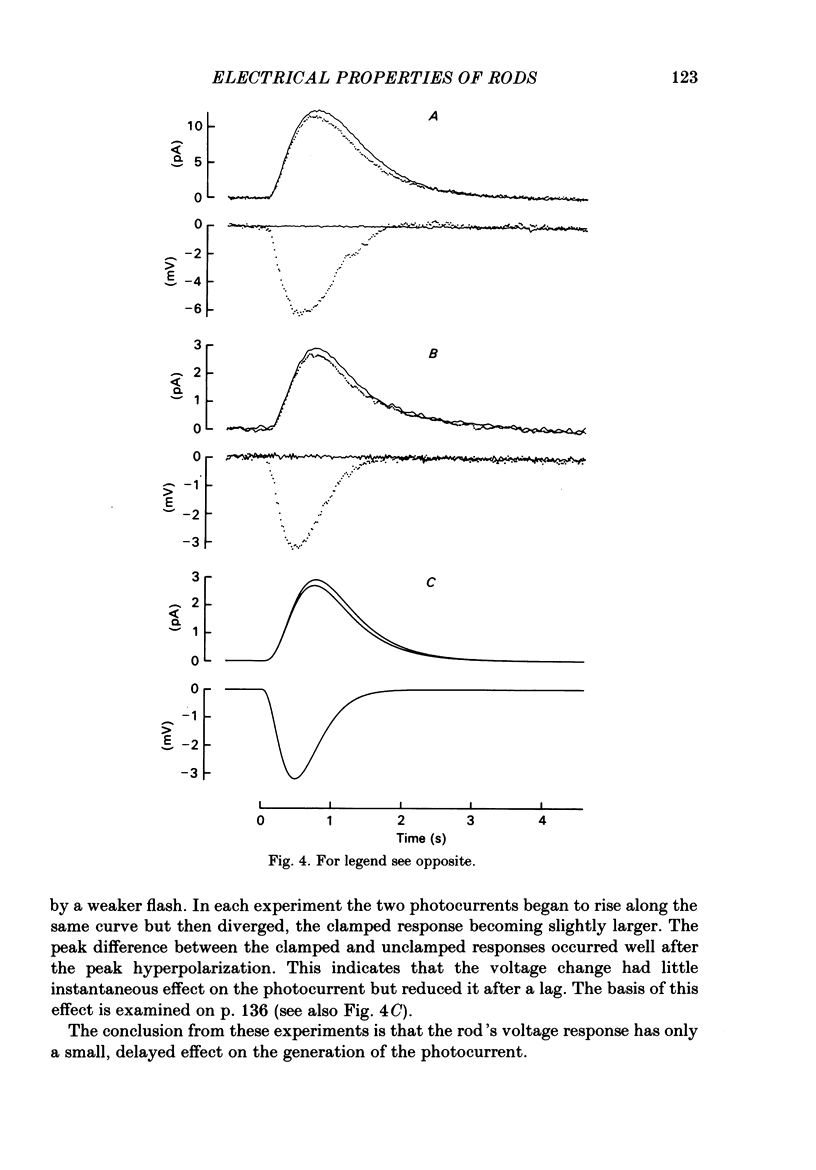
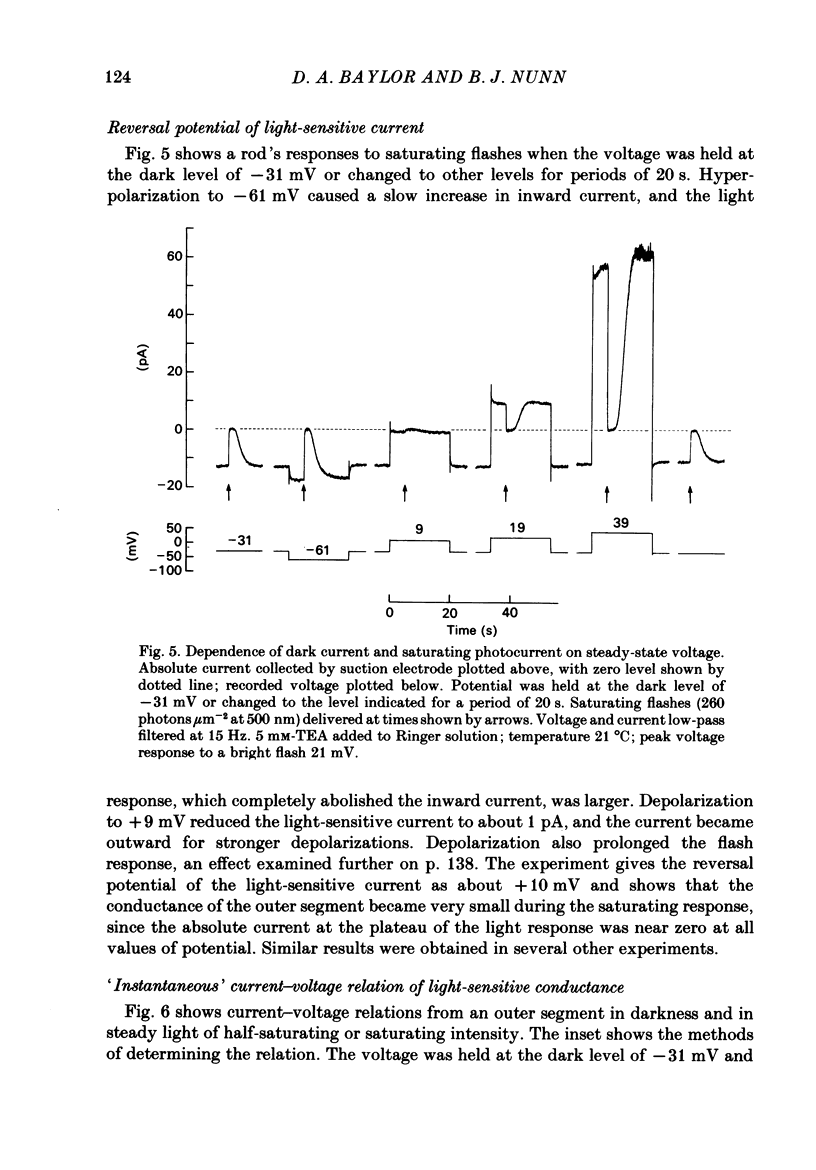
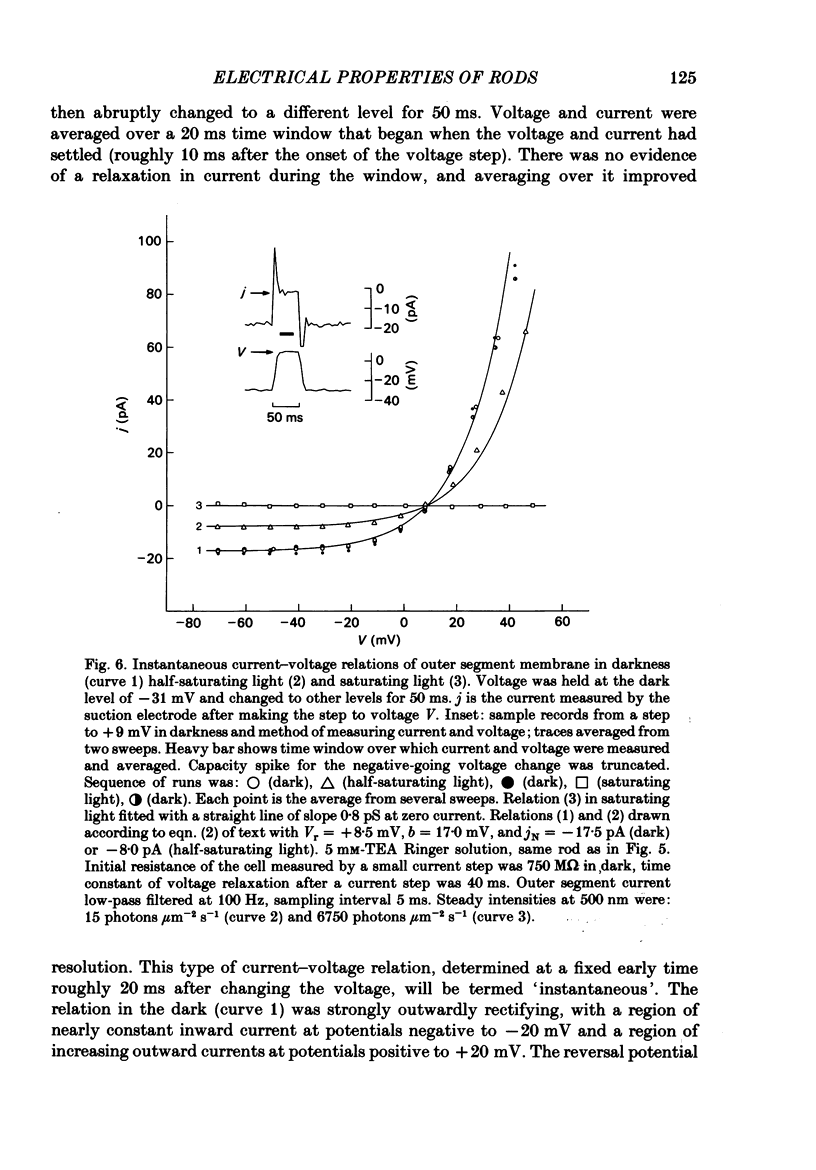

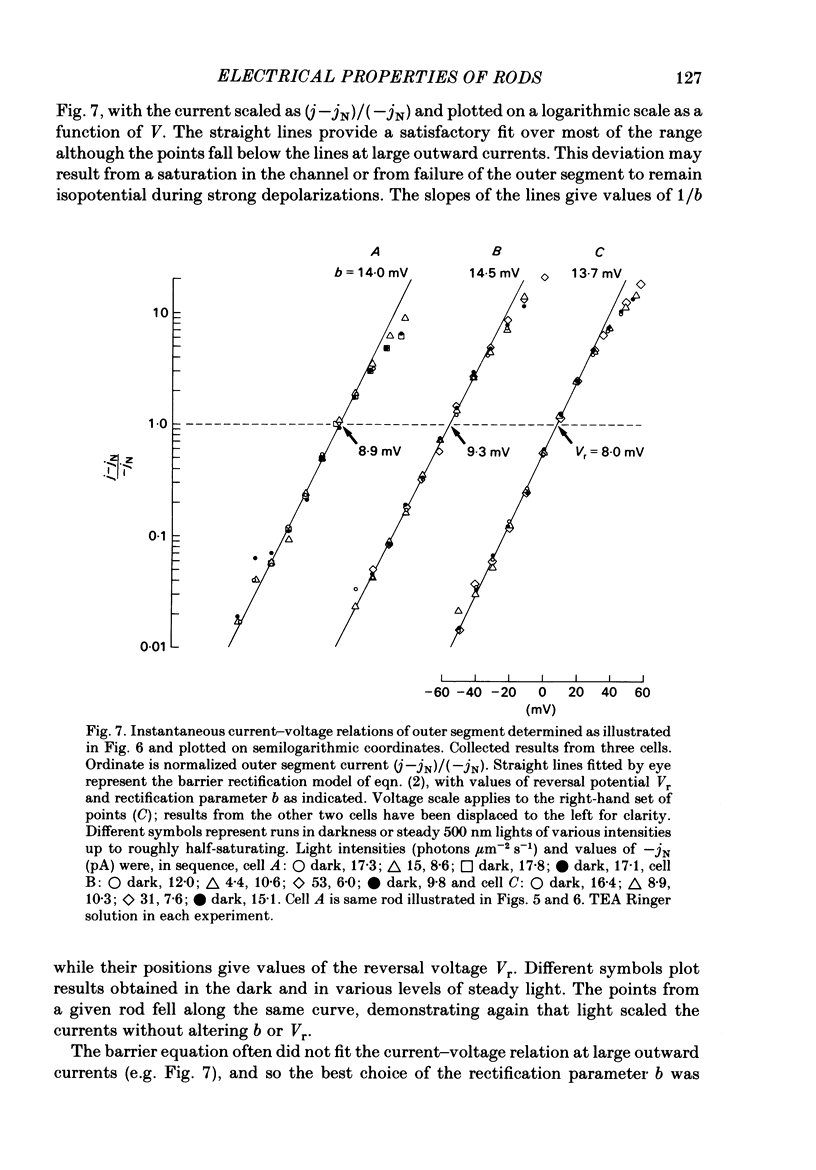







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attwell D., Wilson M. Behaviour of the rod network in the tiger salamander retina mediated by membrane properties of individual rods. J Physiol. 1980 Dec;309:287–315. doi: 10.1113/jphysiol.1980.sp013509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN P. K., GIBBONS I. R., WALD G. THE VISUAL CELLS AND VISUAL PIGMENT OF THE MUDPUPPY, NECTURUS. J Cell Biol. 1963 Oct;19:79–106. doi: 10.1083/jcb.19.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader C. R., Macleish P. R., Schwartz E. A. A voltage-clamp study of the light response in solitary rods of the tiger salamander. J Physiol. 1979 Nov;296:1–26. doi: 10.1113/jphysiol.1979.sp012988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian B. L., Fain G. L. The effects of low calcium and background light on the sensitivity of toad rods. J Physiol. 1982 Sep;330:307–329. doi: 10.1113/jphysiol.1982.sp014343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L., Lamb T. D. The electrical response of turtle cones to flashes and steps of light. J Physiol. 1974 Nov;242(3):685–727. doi: 10.1113/jphysiol.1974.sp010731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D. Local effects of bleaching in retinal rods of the toad. J Physiol. 1982 Jul;328:49–71. doi: 10.1113/jphysiol.1982.sp014252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. The membrane current of single rod outer segments. J Physiol. 1979 Mar;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Matthews G., Nunn B. J. Location and function of voltage-sensitive conductances in retinal rods of the salamander, Ambystoma tigrinum. J Physiol. 1984 Sep;354:203–223. doi: 10.1113/jphysiol.1984.sp015372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Matthews G., Yau K. W. Two components of electrical dark noise in toad retinal rod outer segments. J Physiol. 1980 Dec;309:591–621. doi: 10.1113/jphysiol.1980.sp013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. M., Hagiwara S., Koike H., Meech R. M. Membrane properties of a barnacle photoreceptor examined by the voltage clamp technique. J Physiol. 1970 Jun;208(2):385–413. doi: 10.1113/jphysiol.1970.sp009127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Coles J. A., Pinto L. H. Effects of injections of calcium and EGTA into the outer segments of retinal rods of Bufo marinus. J Physiol. 1977 Aug;269(3):707–722. doi: 10.1113/jphysiol.1977.sp011924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Pinto L. H. Ionic mechanism for the photoreceptor potential of the retina of Bufo marinus. J Physiol. 1974 Feb;236(3):575–591. doi: 10.1113/jphysiol.1974.sp010453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capovilla M., Caretta A., Cervetto L., Torre V. Ionic movements through light-sensitive channels of toad rods. J Physiol. 1983 Oct;343:295–310. doi: 10.1113/jphysiol.1983.sp014893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbs W. H., Pugh E. N., Jr Cyclic GMP can increase rod outer-segment light-sensitive current 10-fold without delay of excitation. Nature. 1985 Feb 14;313(6003):585–587. doi: 10.1038/313585a0. [DOI] [PubMed] [Google Scholar]
- Detwiler P. B., Hodgkin A. L., McNaughton P. A. A surprising property of electrical spread in the network of rods in the turtle's retina. Nature. 1978 Aug 10;274(5671):562–565. doi: 10.1038/274562a0. [DOI] [PubMed] [Google Scholar]
- Detwiler P. B., Hodgkin A. L., McNaughton P. A. Temporal and spatial characteristics of the voltage response of rods in the retina of the snapping turtle. J Physiol. 1980 Mar;300:213–250. doi: 10.1113/jphysiol.1980.sp013159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUORTES M. G., HODGKIN A. L. CHANGES IN TIME SCALE AND SENSITIVITY IN THE OMMATIDIA OF LIMULUS. J Physiol. 1964 Aug;172:239–263. doi: 10.1113/jphysiol.1964.sp007415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985 Jan 24;313(6000):310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- Gold G. H., Korenbrot J. I. Light-induced calcium release by intact retinal rods. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5557–5561. doi: 10.1073/pnas.77.9.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold G. H. Photoreceptor coupling in retina of the toad, Bufo marinus. II. Physiology. J Neurophysiol. 1979 Jan;42(1 Pt 1):311–328. doi: 10.1152/jn.1979.42.1.311. [DOI] [PubMed] [Google Scholar]
- Gray P., Attwell D. Kinetics of light-sensitive channels in vertebrate photoreceptors. Proc R Soc Lond B Biol Sci. 1985 Jan 22;223(1232):379–388. doi: 10.1098/rspb.1985.0007. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. The potassium permeability of a giant nerve fibre. J Physiol. 1955 Apr 28;128(1):61–88. doi: 10.1113/jphysiol.1955.sp005291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagins W. A., Penn R. D., Yoshikami S. Dark current and photocurrent in retinal rods. Biophys J. 1970 May;10(5):380–412. doi: 10.1016/S0006-3495(70)86308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J. The ionic selectivity and calcium dependence of the light-sensitive pathway in toad rods. J Physiol. 1985 Jan;358:447–468. doi: 10.1113/jphysiol.1985.sp015561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J., Yau K. W. Effect of ions on retinal rods from Bufo marinus. J Physiol. 1984 May;350:649–680. doi: 10.1113/jphysiol.1984.sp015223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., McNaughton P. A., Yau K. W. Spatial spread of activation and background desensitization in toad rod outer segments. J Physiol. 1981;319:463–496. doi: 10.1113/jphysiol.1981.sp013921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeish P. R., Schwartz E. A., Tachibana M. Control of the generator current in solitary rods of the Ambystoma tigrinum retina. J Physiol. 1984 Mar;348:645–664. doi: 10.1113/jphysiol.1984.sp015131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H. R., Torre V., Lamb T. D. Effects on the photoresponse of calcium buffers and cyclic GMP incorporated into the cytoplasm of retinal rods. Nature. 1985 Feb 14;313(6003):582–585. doi: 10.1038/313582a0. [DOI] [PubMed] [Google Scholar]
- Schwartz E. A. Electrical properties of the rod syncytium in the retina of the turtle. J Physiol. 1976 May;257(2):379–406. doi: 10.1113/jphysiol.1976.sp011374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Responses of single rods in the retina of the turtle. J Physiol. 1973 Aug;232(3):503–514. doi: 10.1113/jphysiol.1973.sp010283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau K. W., McNaughton P. A., Hodgkin A. L. Effect of ions on the light-sensitive current in retinal rods. Nature. 1981 Aug 6;292(5823):502–505. doi: 10.1038/292502a0. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Cation selectivity of light-sensitive conductance in retinal rods. Nature. 1984 May 24;309(5966):352–354. doi: 10.1038/309352a0. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Electrogenic Na-Ca exchange in retinal rod outer segment. Nature. 1984 Oct 18;311(5987):661–663. doi: 10.1038/311661a0. [DOI] [PubMed] [Google Scholar]
- Yoshikami S., George J. S., Hagins W. A. Light-induced calcium fluxes from outer segment layer of vertebrate retinas. Nature. 1980 Jul 24;286(5771):395–398. doi: 10.1038/286395a0. [DOI] [PubMed] [Google Scholar]


