Abstract
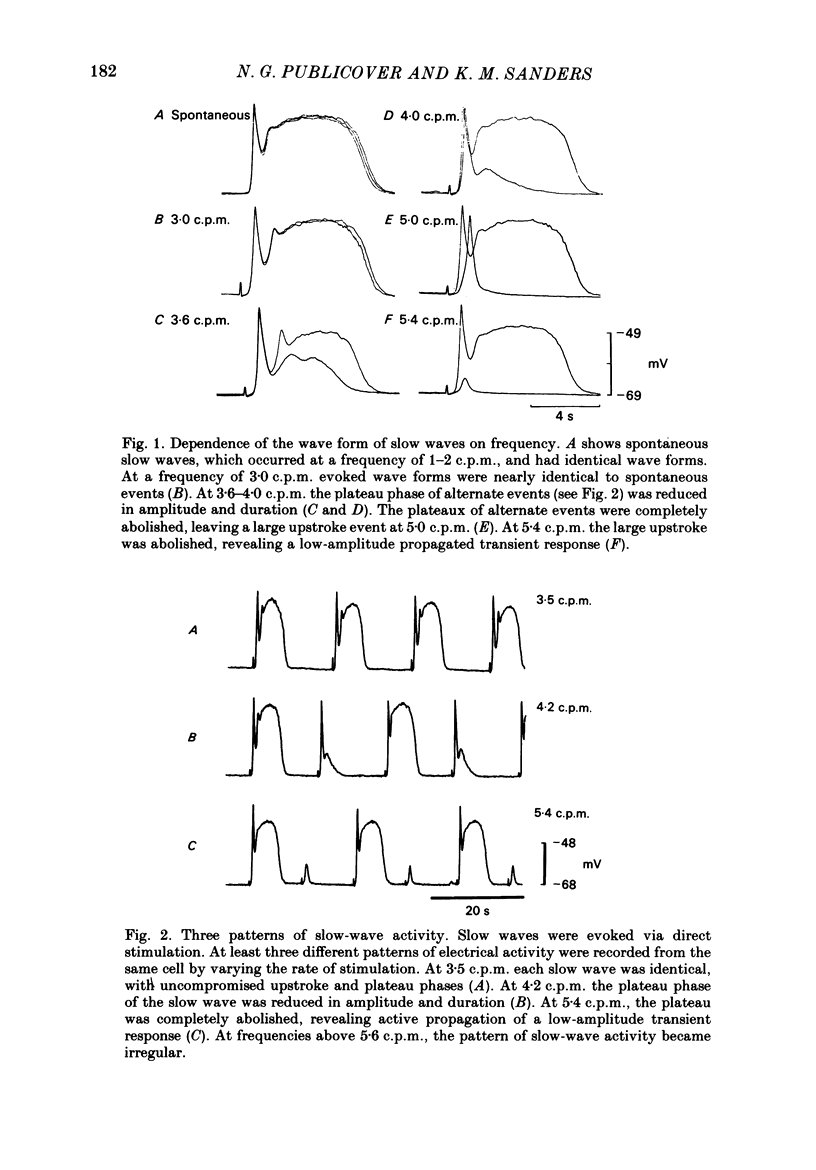
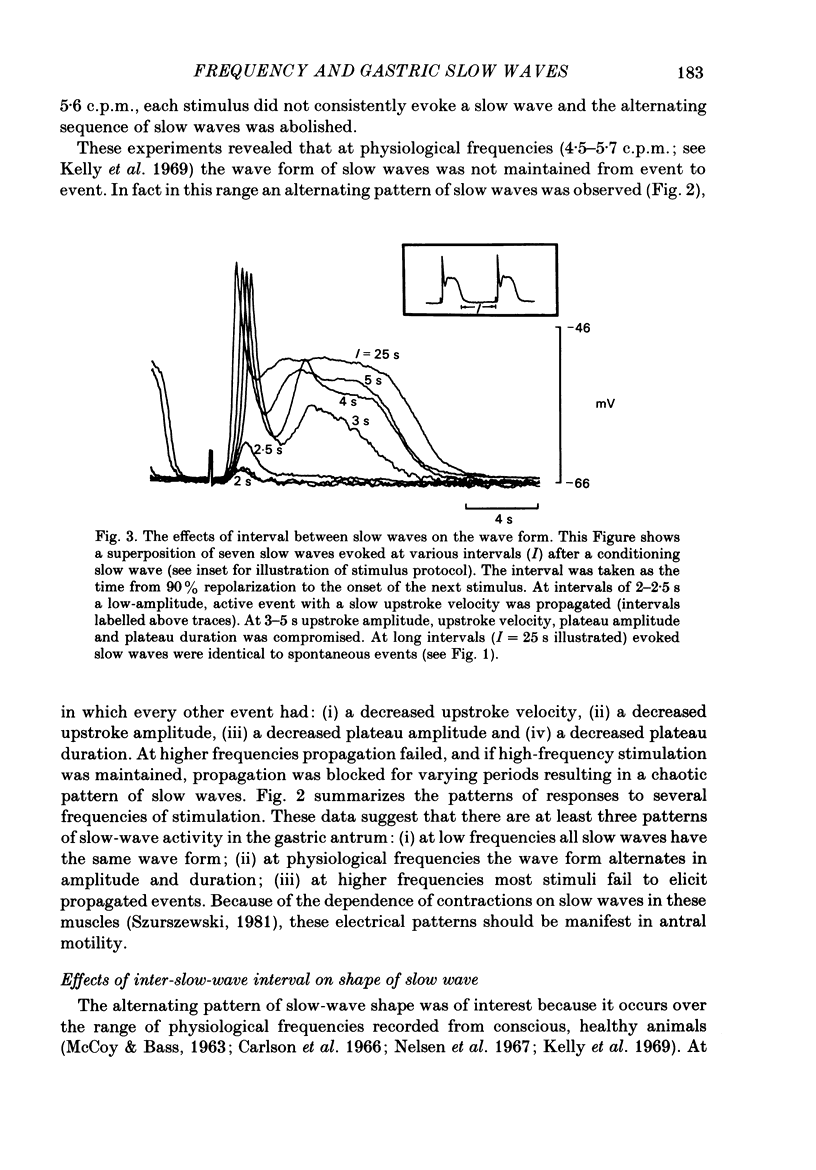
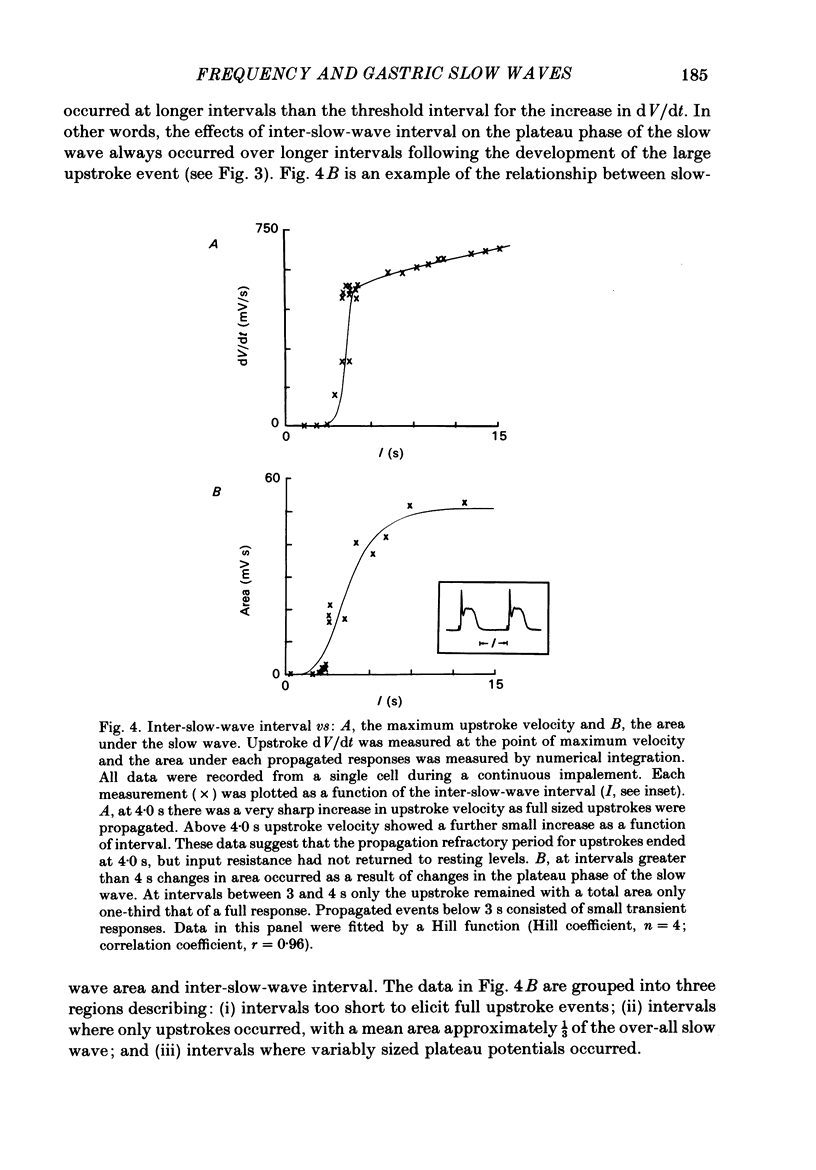
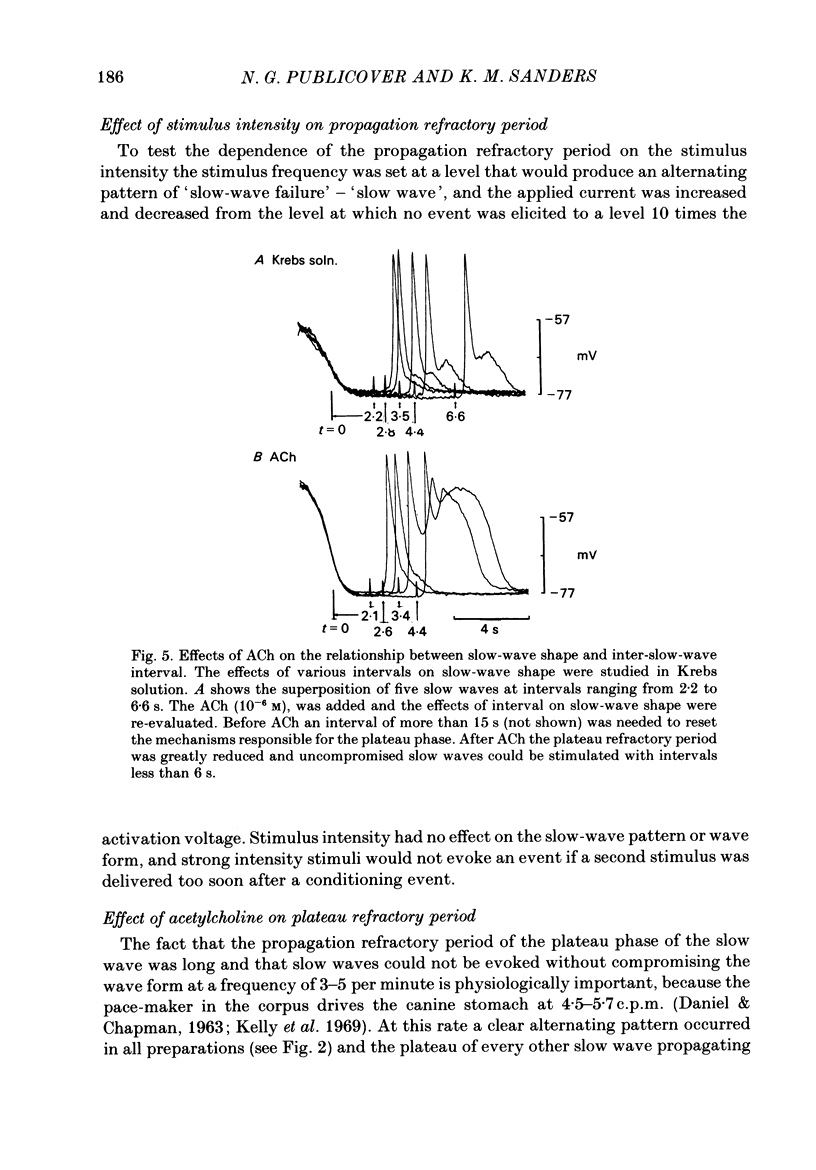
Experiments were performed to test the effects of frequency on the wave form of electrical slow waves in canine antral circular muscle. At frequencies between 3.0 and 5.6 cycles per minute antral slow waves revealed an alternating wave form pattern. At physiological frequencies antral muscle was incapable of consistently propagating mechanically productive slow waves. Two components of the slow wave were identified on the basis of propagation refractory period. At inter-slow-wave intervals of 3-14 s, the amplitude and duration of the plateau phase wave decreased, but the upstroke phase of the slow wave was minimally affected. Intervals of 2.5-4 s resulted in a normal upstroke event but abolished the plateau. At shorter intervals the upstroke phase of the slow wave was greatly reduced or abolished. The absolute propagation refractory period averaged 2.8 +/- 0.9 s (n = 7) following repolarization of a 'conditioning' slow wave. Slow waves failed to propagate within the absolute propagation refractory period. Acetylcholine decreased the interval required for the plateau phase of the slow wave to recover and permitted conduction of mechanically productive slow waves at or above physiological frequencies. The data presented suggest that gastric motility is modulated by extrinsic and intrinsic factors which regulate slow-wave frequency.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carlson H. C., Code C. F., Nelson R. A. Motor action of the canine gastroduodenal junction: a cineradiographic, pressure, and electric study. Am J Dig Dis. 1966 Feb;11(2):155–172. doi: 10.1007/BF02239239. [DOI] [PubMed] [Google Scholar]
- Cooke A. R. Control of gastric emptying and motility. Gastroenterology. 1975 Apr;68(4 Pt 1):804–816. [PubMed] [Google Scholar]
- DANIEL E. E., CHAPMAN K. M. Electrical activity of the gastrointestinal tract as an indication of mechanical activity. Am J Dig Dis. 1963 Jan;8:54–102. doi: 10.1007/BF02233560. [DOI] [PubMed] [Google Scholar]
- Kelly K. A., Code C. F., Elveback L. R. Patterns of canine gastric electrical activity. Am J Physiol. 1969 Aug;217(2):461–470. doi: 10.1152/ajplegacy.1969.217.2.461. [DOI] [PubMed] [Google Scholar]
- MCCOY E. J., BASS P. CHRONIC ELECTRICAL ACTIVITY OF GASTRODUODENAL AREA: EFFECTS OF FOOD AND CERTAIN CATECHOLAMINES. Am J Physiol. 1963 Sep;205:439–445. doi: 10.1152/ajplegacy.1963.205.3.439. [DOI] [PubMed] [Google Scholar]
- Morgan K. G., Szurszewski J. H. Mechanisms of phasic and tonic actions of pentagastrin on canine gastric smooth muscle. J Physiol. 1980 Apr;301:229–242. doi: 10.1113/jphysiol.1980.sp013201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelsen T. S., Eigenbrodt E. H., Keoshian L. A., Bunker C., Johnson L. Alterations in muscular and electrical activity of the stomach following vagotomy. Arch Surg. 1967 Jun;94(6):821–835. doi: 10.1001/archsurg.1967.01330120075015. [DOI] [PubMed] [Google Scholar]
- Papasova M. P., Nagai T., Prosser C. L. Two-component slow waves in smooth muscle of cat stomach. Am J Physiol. 1968 Apr;214(4):695–702. doi: 10.1152/ajplegacy.1968.214.4.695. [DOI] [PubMed] [Google Scholar]
- Sarna S. K., Daniel E. E. Electrical stimulation of gastric electrical control activity. Am J Physiol. 1973 Jul;225(1):125–131. doi: 10.1152/ajplegacy.1973.225.1.125. [DOI] [PubMed] [Google Scholar]
- Szurszewski J. H. Mechanism of action of pentagastrin and acetylcholine on the longitudinal muscle of the canine antrum. J Physiol. 1975 Nov;252(2):335–361. doi: 10.1113/jphysiol.1975.sp011147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Sharkawy T. Y., Morgan K. G., Szurszewski J. H. Intracellular electrical activity of canine and human gastric smooth muscle. J Physiol. 1978 Jun;279:291–307. doi: 10.1113/jphysiol.1978.sp012345. [DOI] [PMC free article] [PubMed] [Google Scholar]


