Abstract
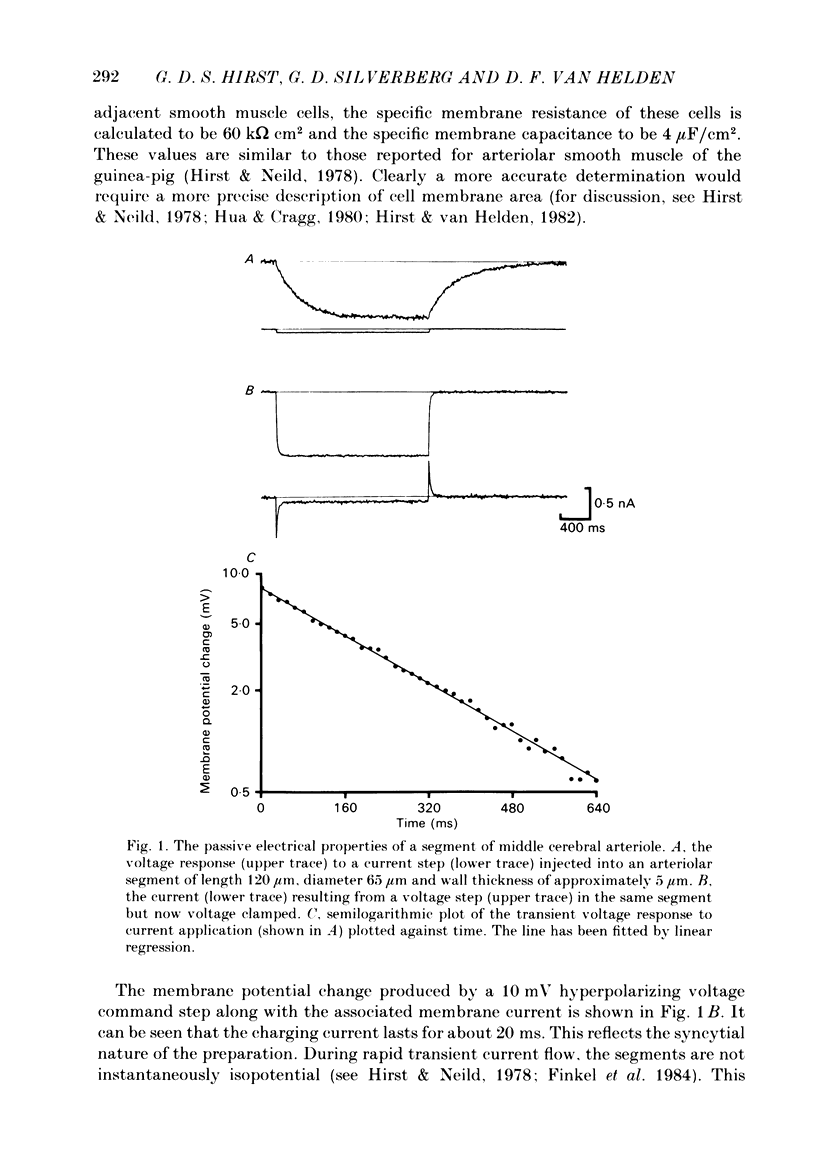
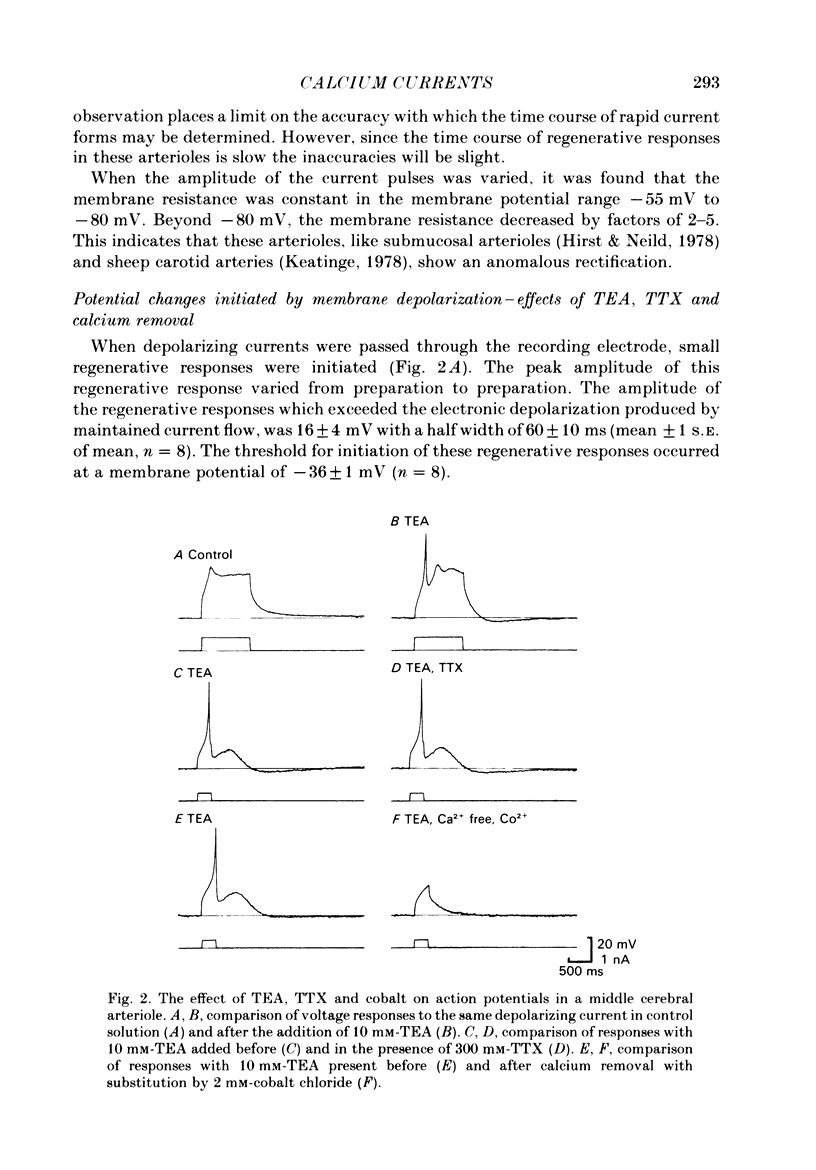
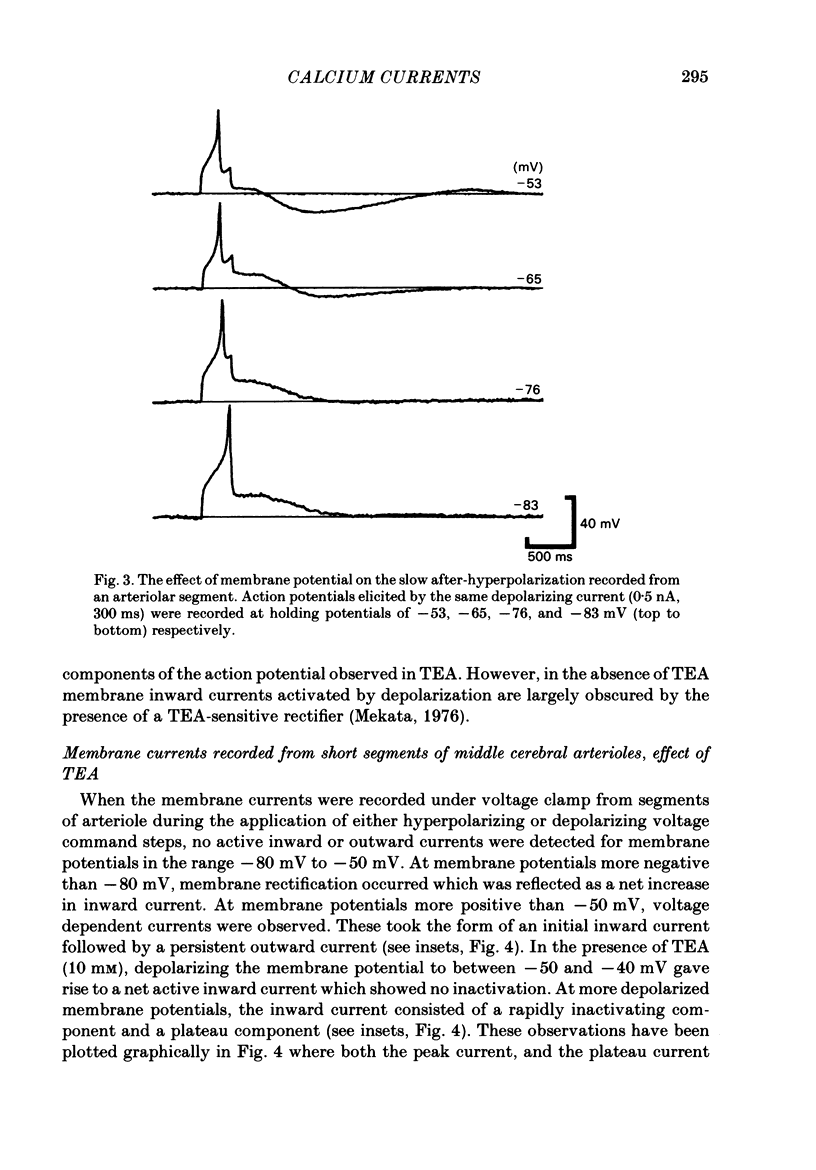
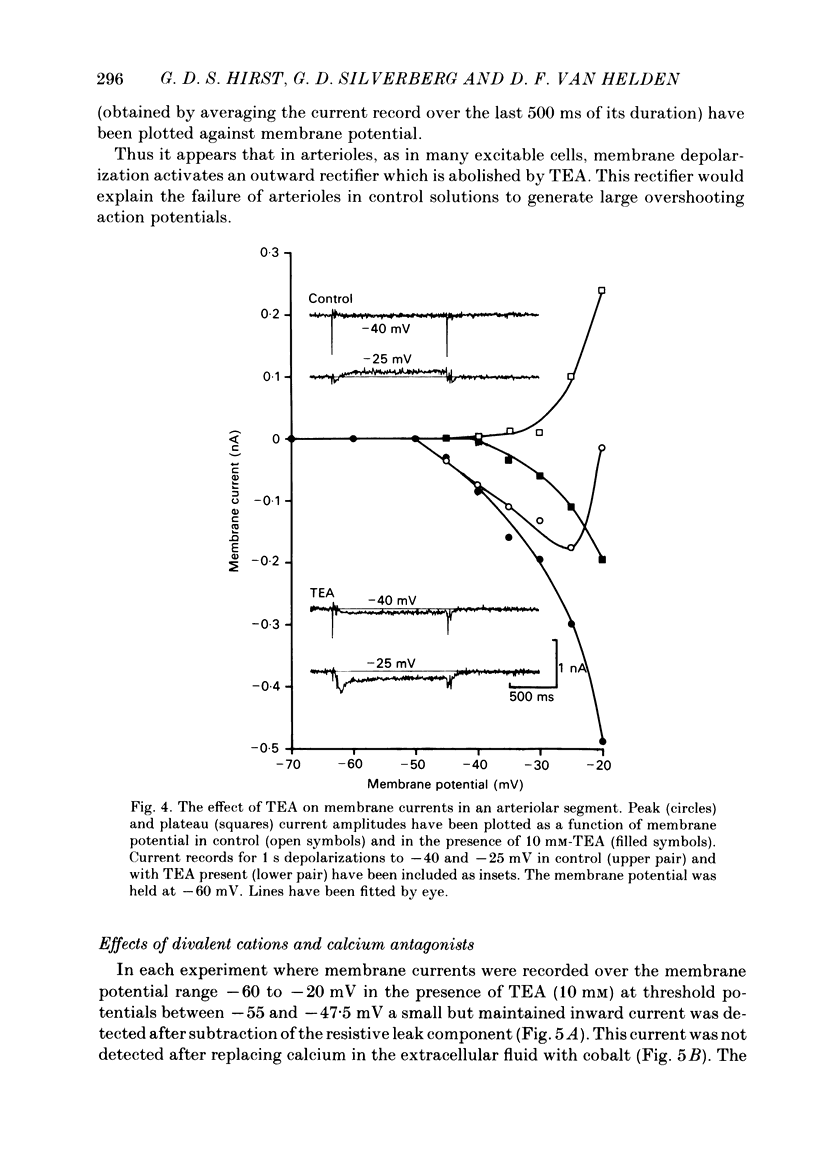
The active and passive electrical properties of isolated segments (length 120-220 microns, diameter 60-150 micron) of proximal rat middle cerebral arterioles (less than 1 mm from parent artery) were analysed using a single-electrode current or voltage clamp. The voltage response to a current step exhibited an exponential time course. The mean resistance and time constant was 102 M omega and 265 ms corresponding to approximate specific resistance and capacitance of 60 k omega/cm and 4 micro F/cm2. Membrane resistance was constant in the range -55 to -80 mV. At potentials more negative than -80 mV there was a decrease in membrane resistance resulting in activation of an inward rectifier. At membrane potentials less negative than -50 mV the membrane resistance decreased; larger depolarizations (greater than -40 mV) initiated small regenerative responses. External application of tetraethylammonium chloride caused membrane depolarization (10-15 mV), spontaneous discharge of action potentials and rhythmic arteriolar constriction. Action potentials studied with the membrane held at -60 mV had a large rapid depolarizing component, an after-depolarization and a small slower after-hyperpolarization. Tetrodotoxin (TTX) had no effect on the action potential. However, both the fast and slow components of the action potential were suppressed by extracellular removal of calcium ions and/or addition of cobalt ions, nifedipine or verapamil. Voltage-clamp studies demonstrated an inward rectifying current at membrane potentials more negative than -80 mV. At depolarized potentials at least four separate currents were activated; two separate calcium currents and two outward currents.
Full text
PDF



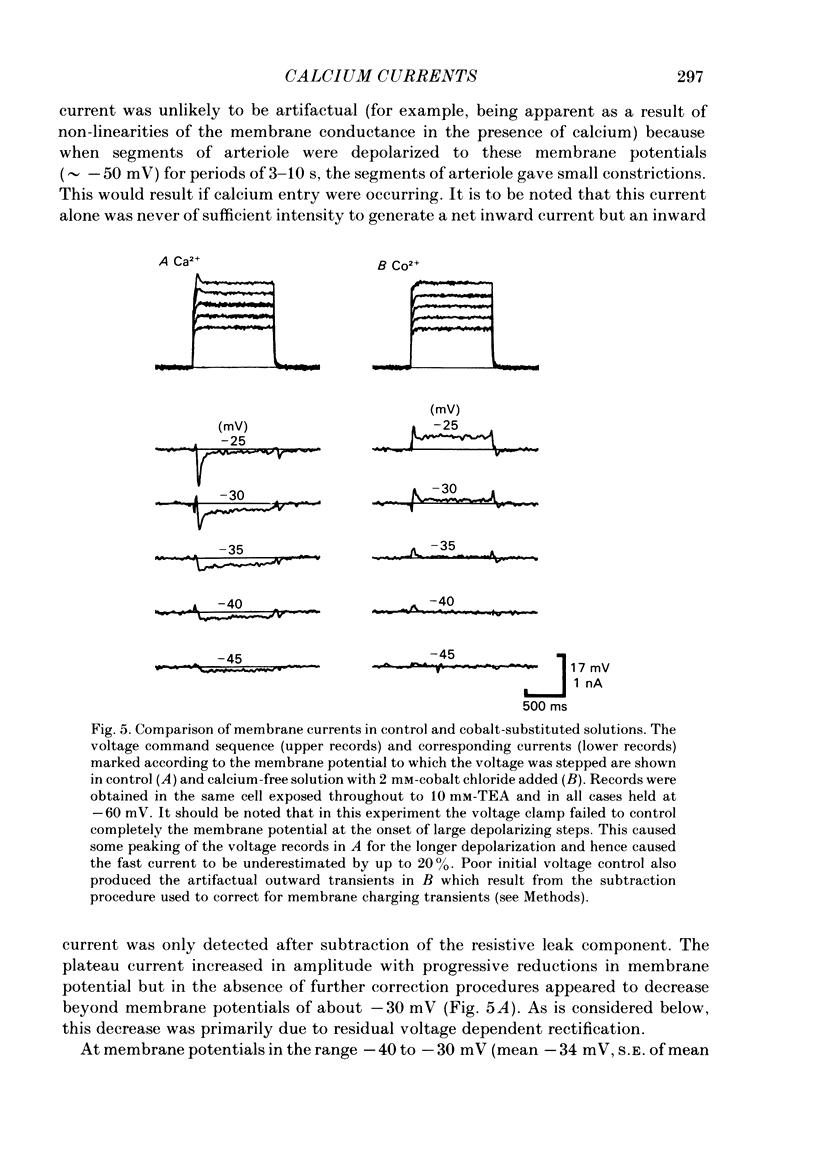
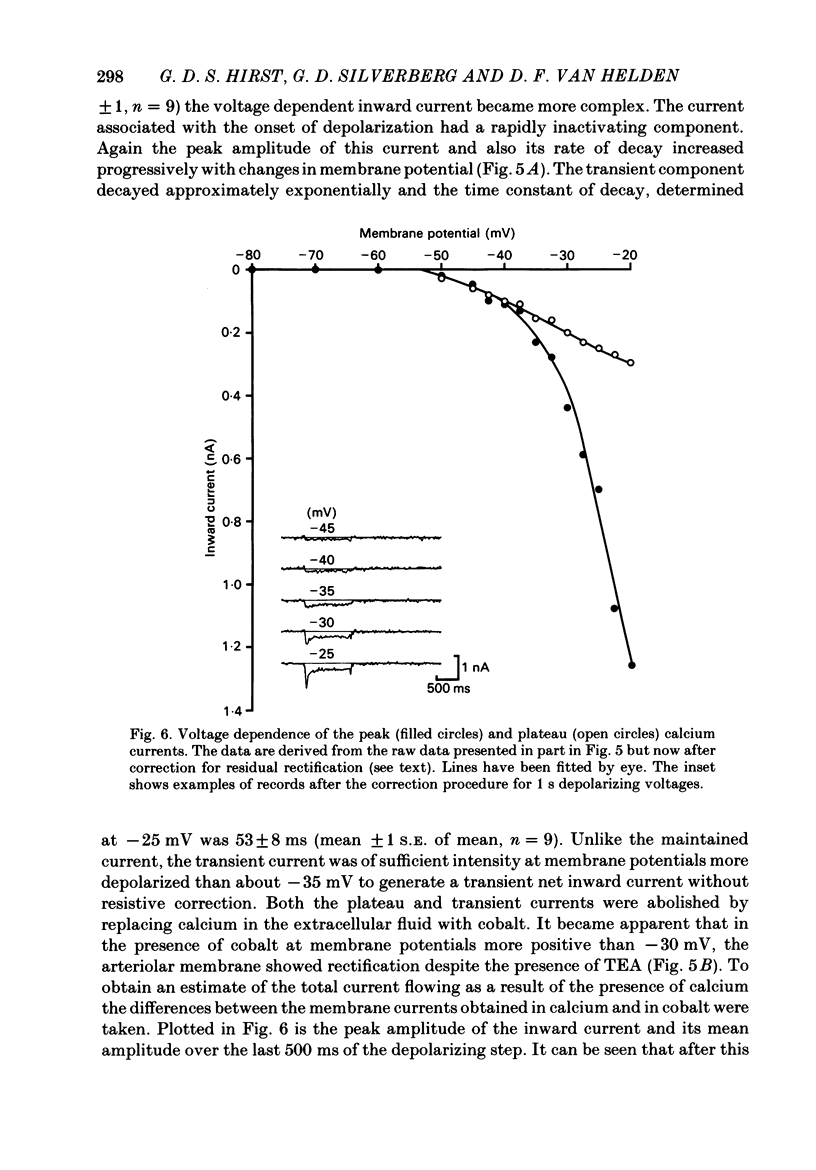
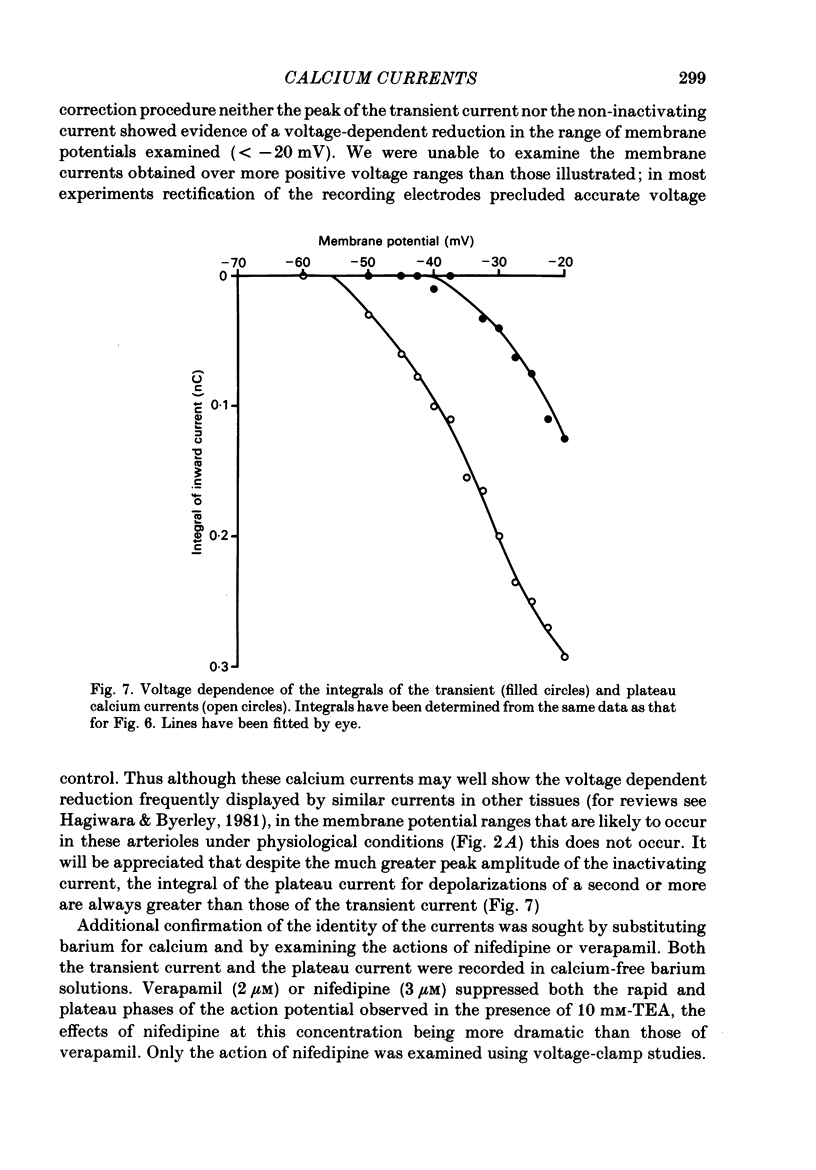
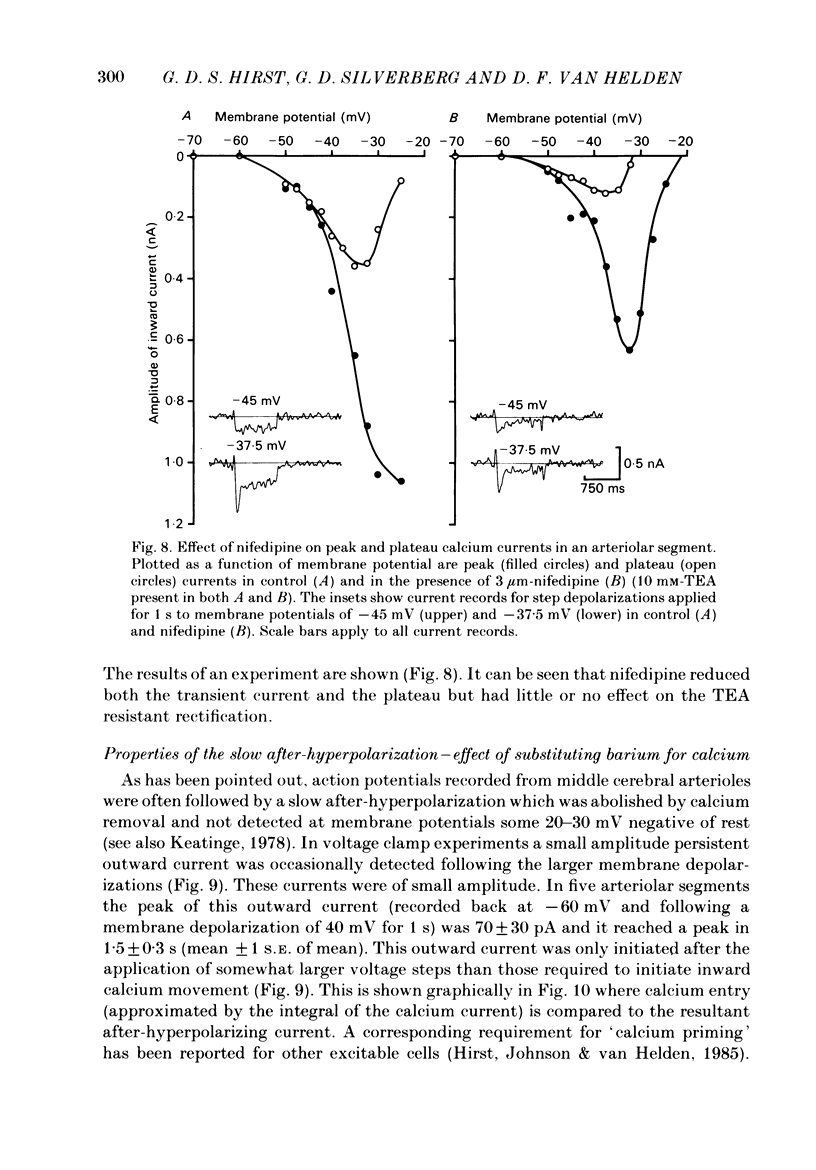
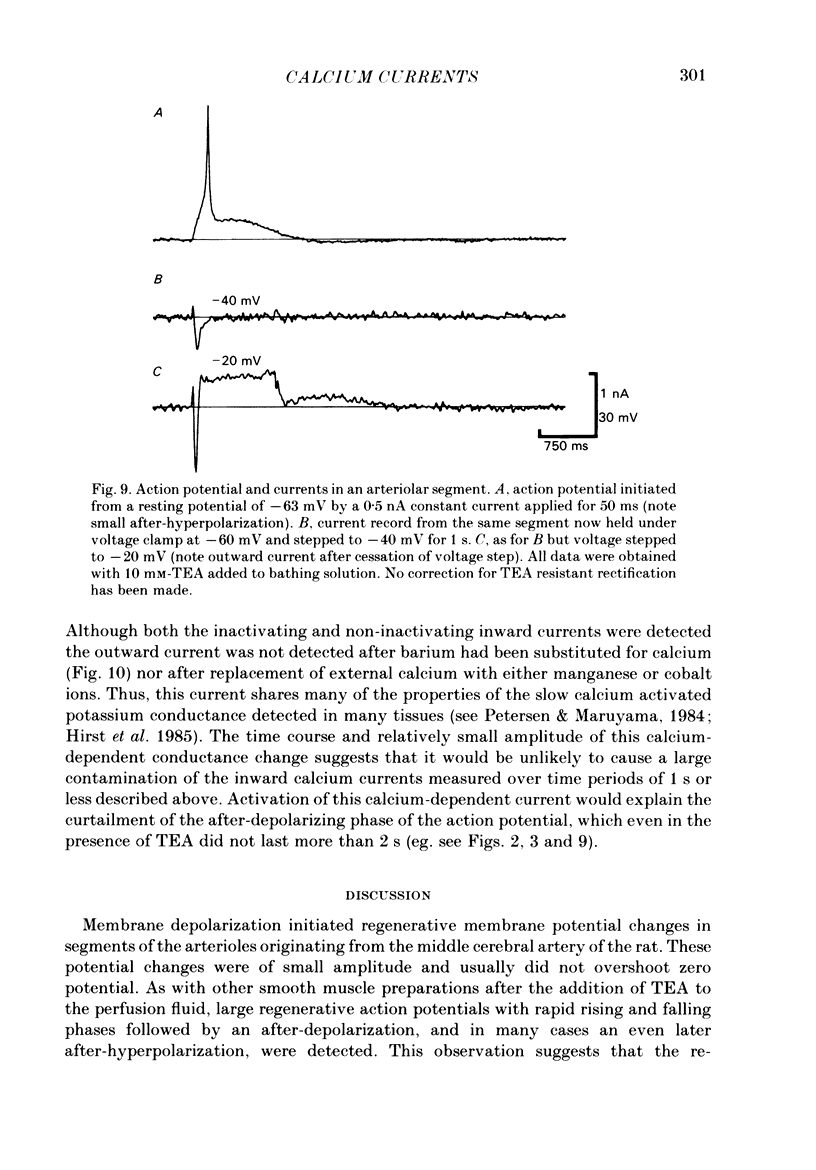
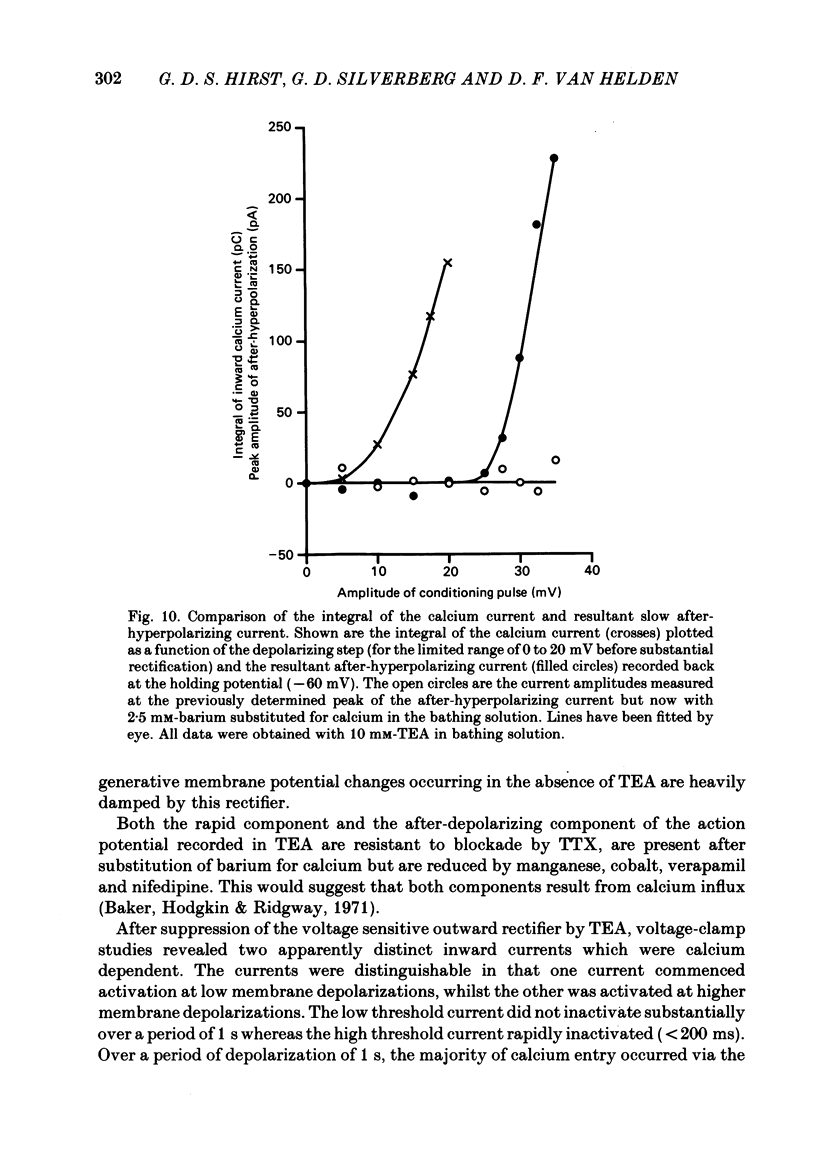


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C. Transmission from vasoconstrictor and vasodilator nerves to single smooth muscle cells of the guinea-pig uterine artery. J Physiol. 1969 Dec;205(3):695–708. doi: 10.1113/jphysiol.1969.sp008991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Kitamura K., Kuriyama H., Suzuki H. The membrane properties of the smooth muscle cells of the rabbit main pulmonary artery. J Physiol. 1977 Sep;271(1):41–61. doi: 10.1113/jphysiol.1977.sp011989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droogmans G., Raeymaekers L., Casteels R. Electro- and pharmacomechanical coupling in the smooth muscle cells of the rabbit ear artery. J Gen Physiol. 1977 Aug;70(2):129–148. doi: 10.1085/jgp.70.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel A. S., Hirst G. D., Van Helden D. F. Some properties of excitatory junction currents recorded from submucosal arterioles of guinea-pig ileum. J Physiol. 1984 Jun;351:87–98. doi: 10.1113/jphysiol.1984.sp015234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hill C. E., Hirst G. D., Silverberg G. D., van Helden D. F. Sympathetic innervation and excitability of arterioles originating from the rat middle cerebral artery. J Physiol. 1986 Feb;371:305–316. doi: 10.1113/jphysiol.1986.sp015976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C. E., Hirst G. D., van Helden D. F. Development of sympathetic innervation to proximal and distal arteries of the rat mesentery. J Physiol. 1983 May;338:129–147. doi: 10.1113/jphysiol.1983.sp014665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Holman M. E., Spence I. Two types of neurones in the myenteric plexus of duodenum in the guinea-pig. J Physiol. 1974 Jan;236(2):303–326. doi: 10.1113/jphysiol.1974.sp010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Johnson S. M., van Helden D. F. The calcium current in a myenteric neurone of the guinea-pig ileum. J Physiol. 1985 Apr;361:297–314. doi: 10.1113/jphysiol.1985.sp015647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. An analysis of excitatory junctional potentials recorded from arterioles. J Physiol. 1978 Jul;280:87–104. doi: 10.1113/jphysiol.1978.sp012374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O., Silverberg G. D. Noradrenaline receptors on the rat basilar artery. J Physiol. 1982 Jul;328:351–360. doi: 10.1113/jphysiol.1982.sp014268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D. Neuromuscular transmission in arterioles of guinea-pig submucosa. J Physiol. 1977 Dec;273(1):263–275. doi: 10.1113/jphysiol.1977.sp012093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., van Helden D. F. Ionic basis of the resting potential of submucosal arterioles in the ileum of the guinea-pig. J Physiol. 1982 Dec;333:53–67. doi: 10.1113/jphysiol.1982.sp014438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman M. E., Surprenant A. M. Some properties of the excitatory junction potentials recorded from saphenous arteries of rabbits. J Physiol. 1979 Feb;287:337–351. doi: 10.1113/jphysiol.1979.sp012663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua C., Cragg B. Measurements of smooth muscle cells in arterioles of guinea pig ileum. Acta Anat (Basel) 1980;107(2):224–230. doi: 10.1159/000145246. [DOI] [PubMed] [Google Scholar]
- Keatinge W. R. Ionic requirements for arterial action potential. J Physiol. 1968 Jan;194(1):169–182. doi: 10.1113/jphysiol.1968.sp008400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keatinge W. R. Mechanism of slow discharges of sheep carotid artery. J Physiol. 1978 Jun;279:275–289. doi: 10.1113/jphysiol.1978.sp012344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekata F. Rectification in the smooth muscle cell membrane of rabbit aorta. J Physiol. 1976 Jun;258(2):269–278. doi: 10.1113/jphysiol.1976.sp011419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H., Maruyama Y. Calcium-activated potassium channels and their role in secretion. Nature. 1984 Feb 23;307(5953):693–696. doi: 10.1038/307693a0. [DOI] [PubMed] [Google Scholar]
- Redfern P., Lundh H., Thesleff S. Tetrodotoxin resistant action potentials in denervated rat skeletal muscle. Eur J Pharmacol. 1970 Jul 15;11(2):263–265. doi: 10.1016/0014-2999(70)90056-7. [DOI] [PubMed] [Google Scholar]


