Abstract
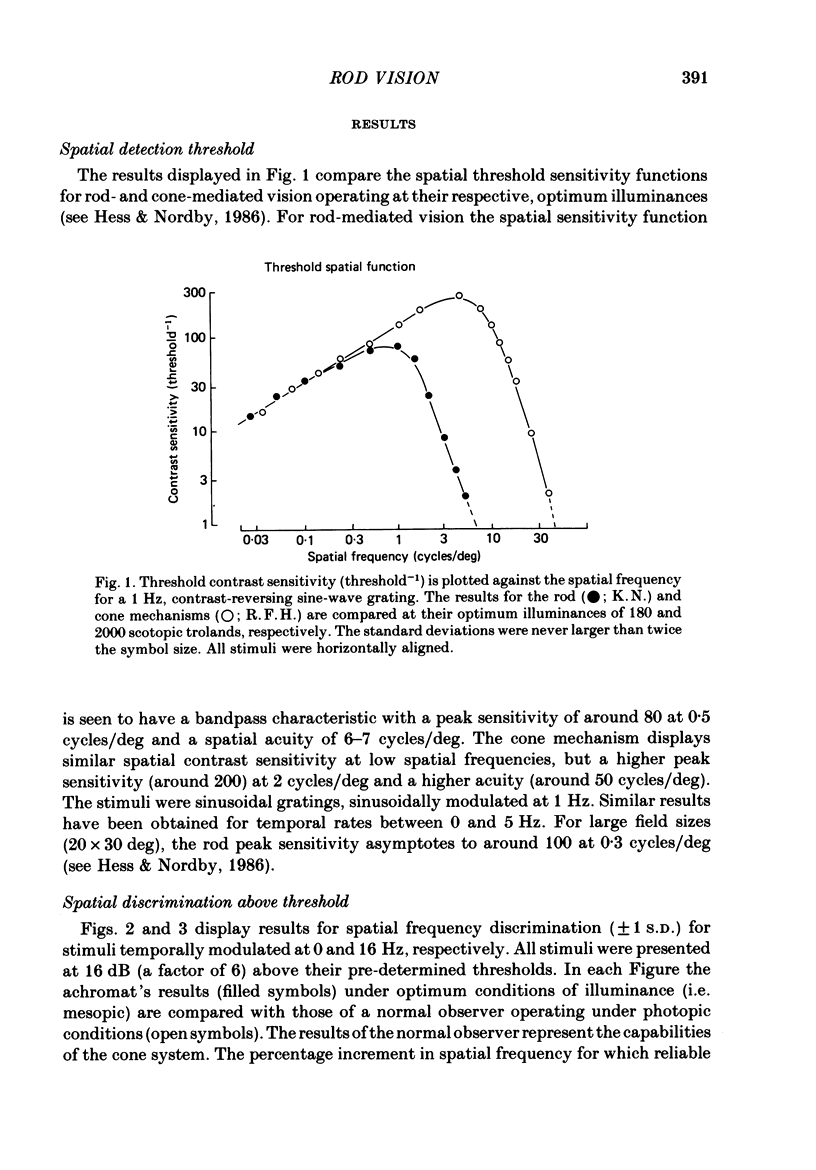
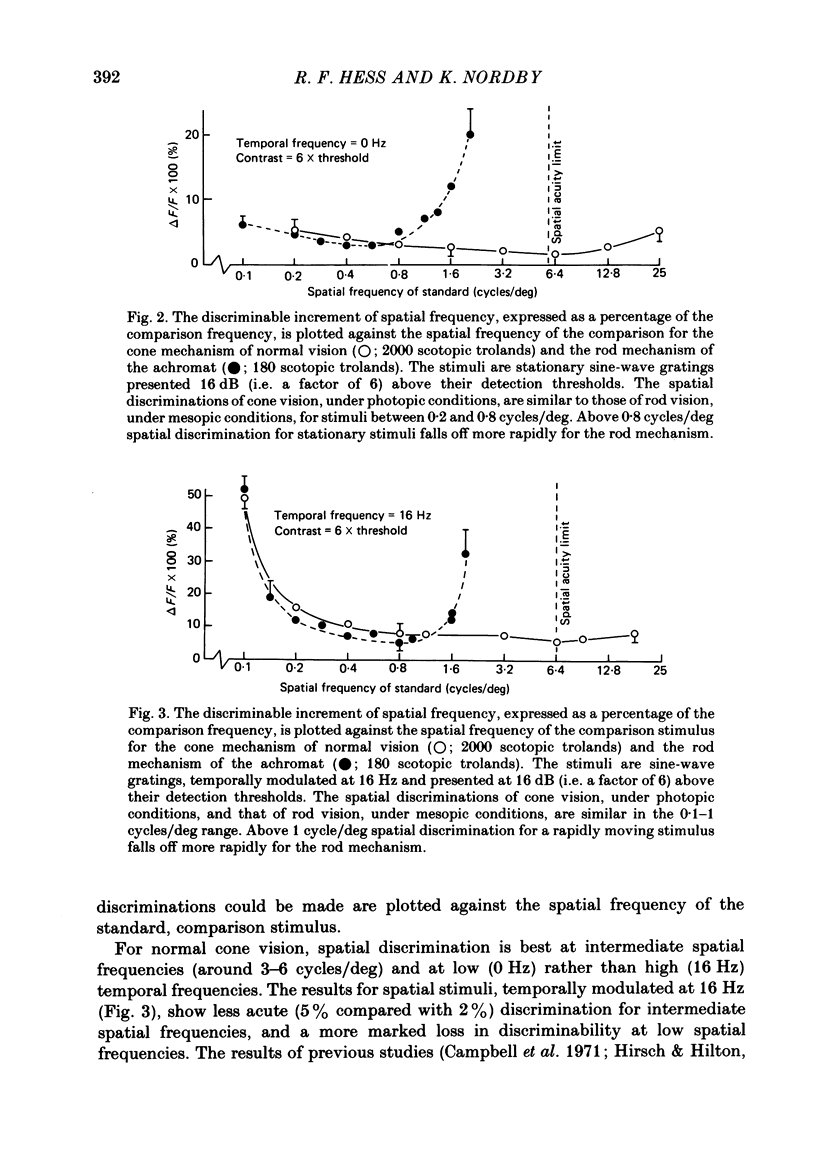
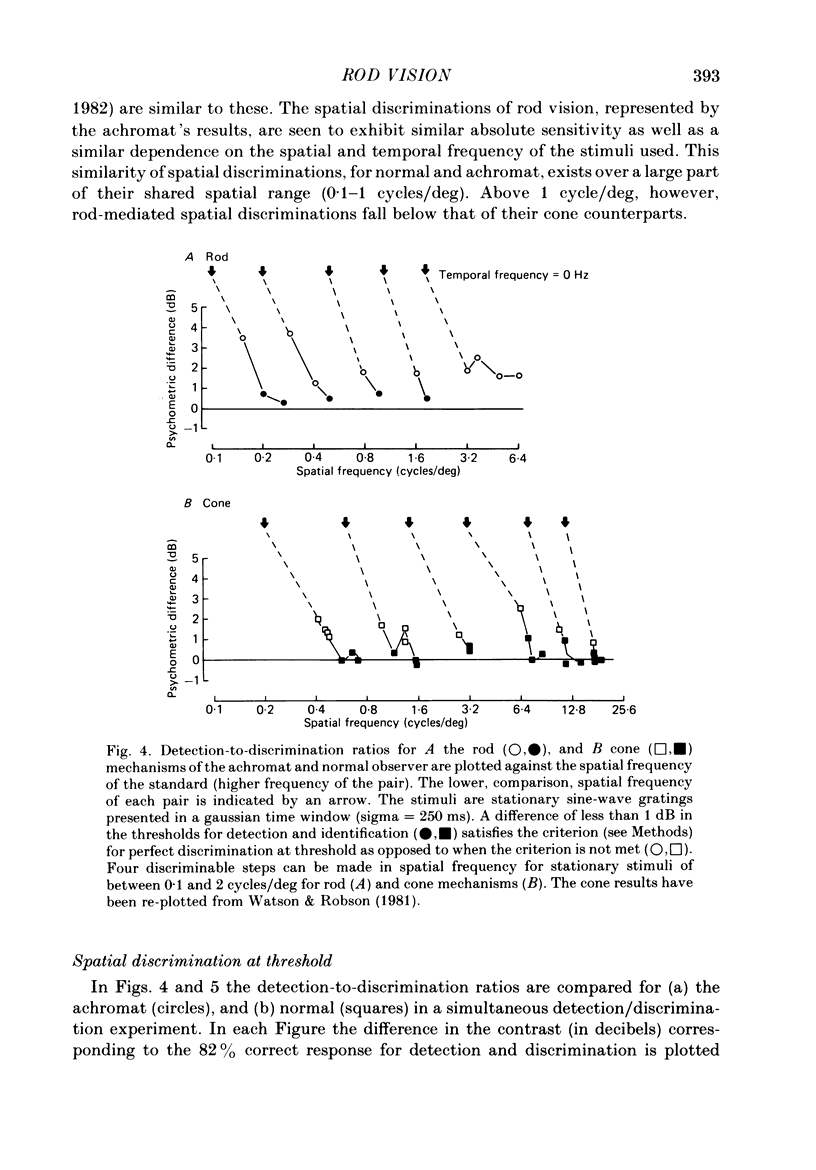
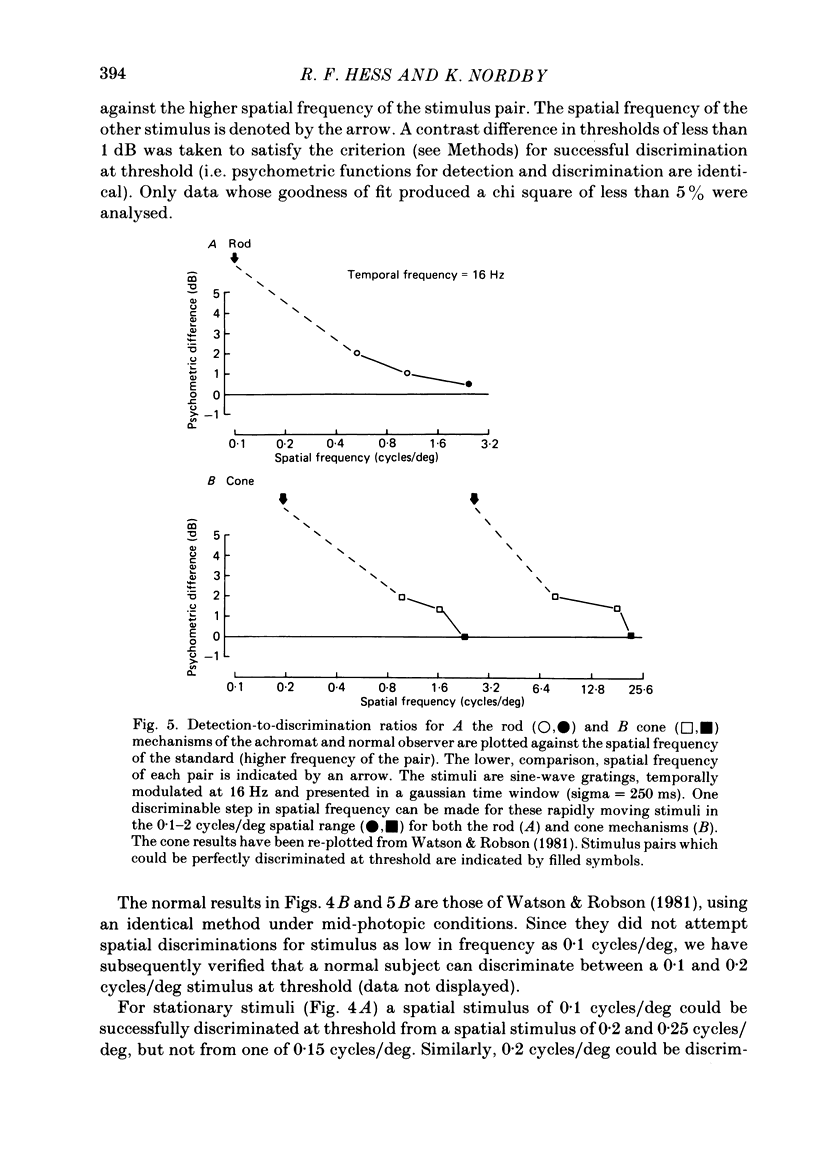
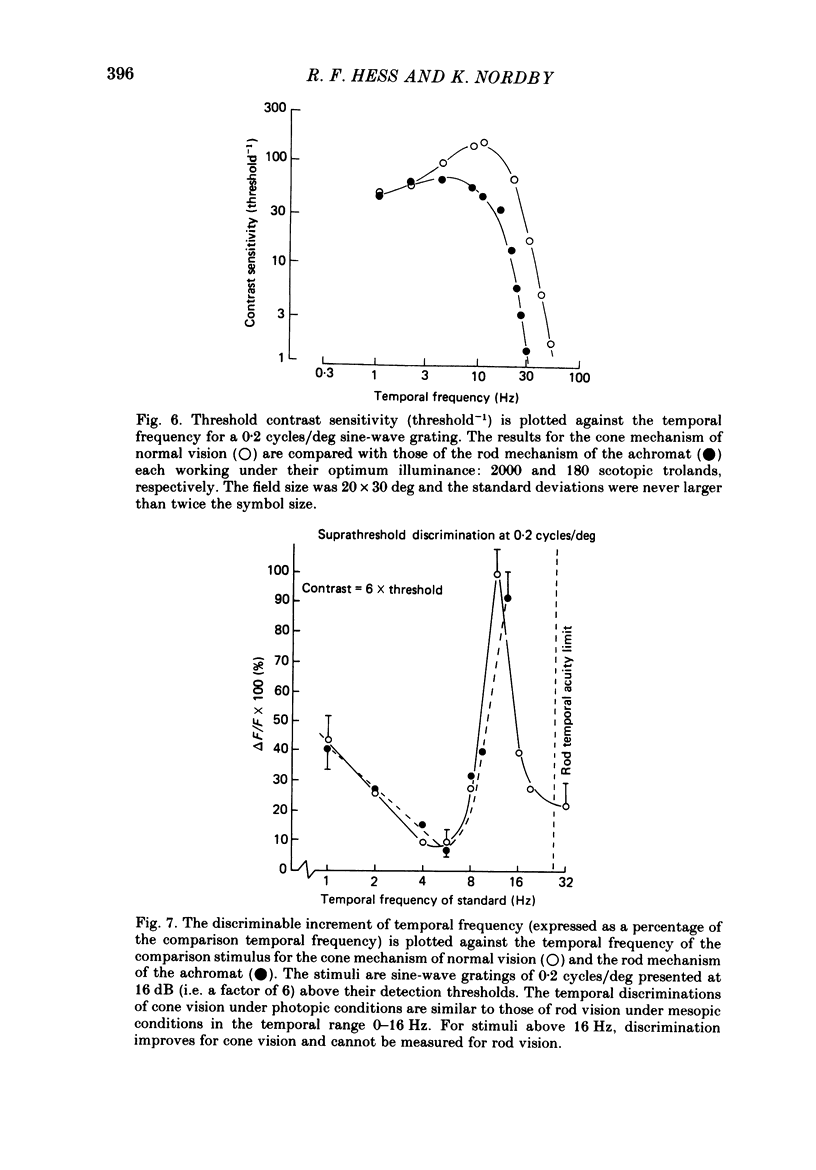
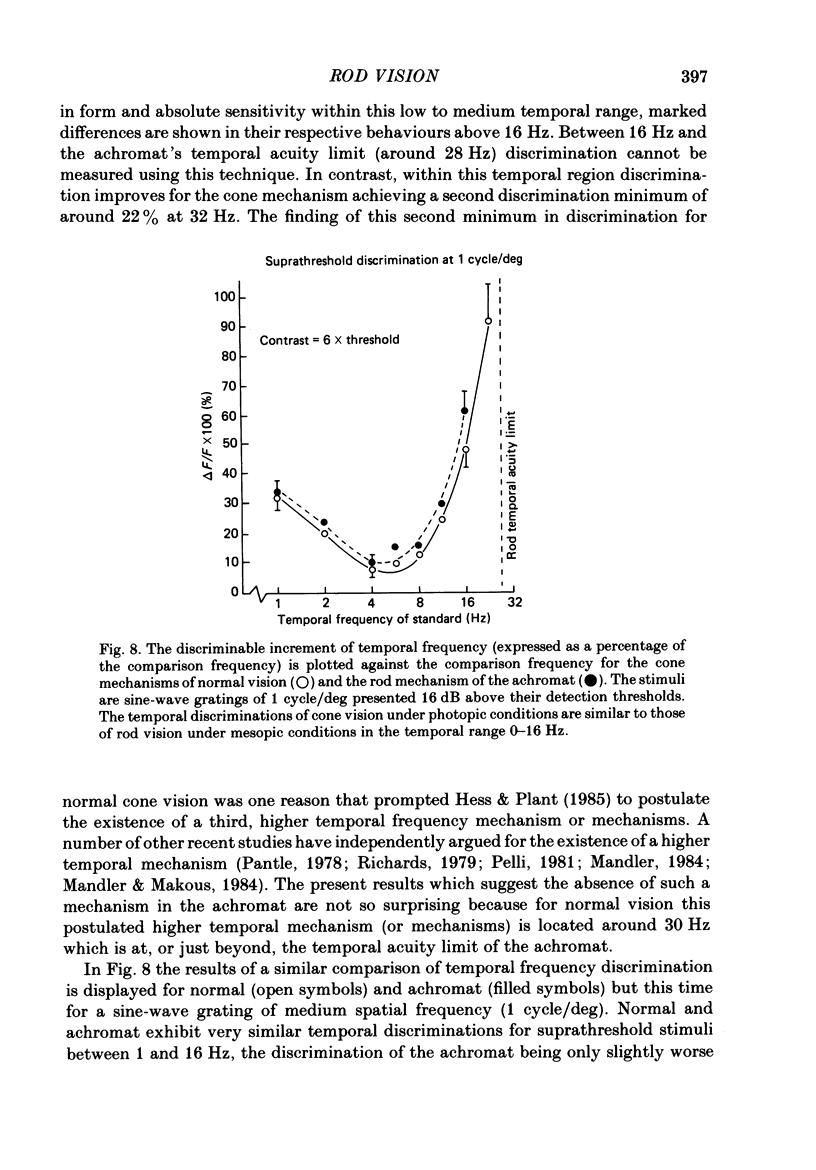
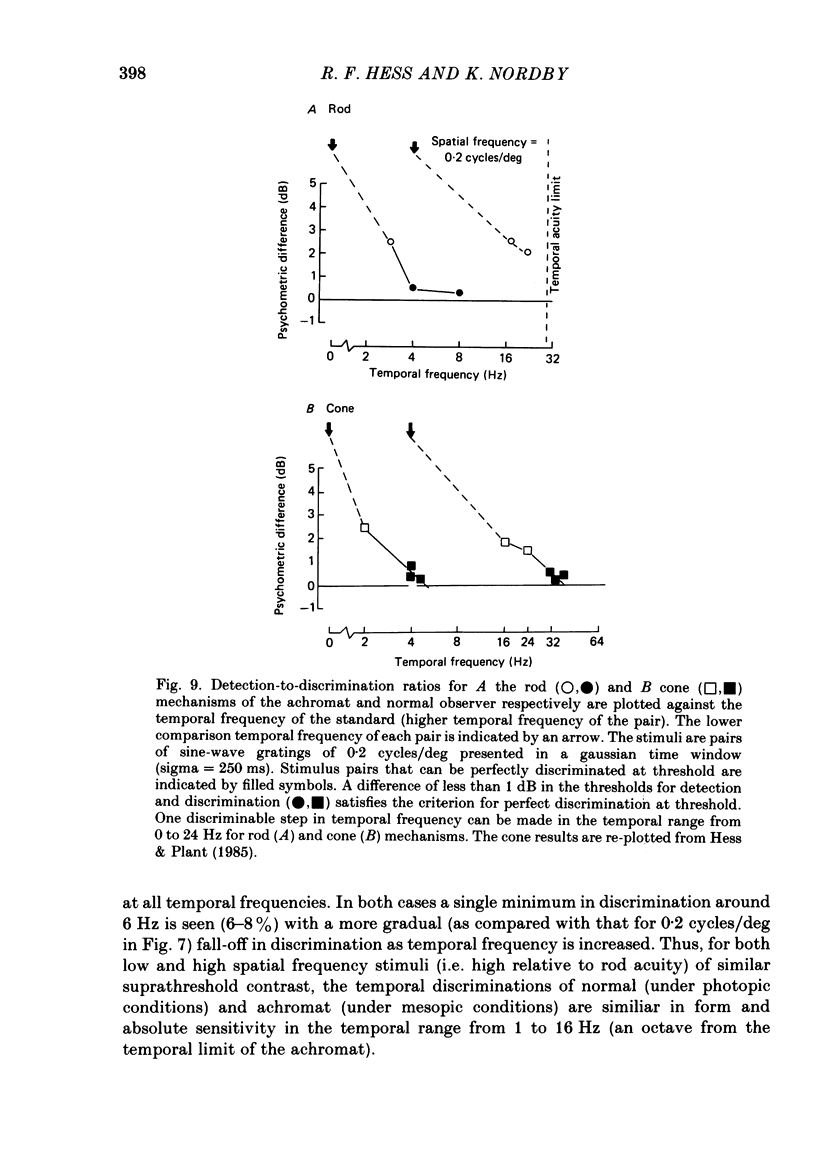
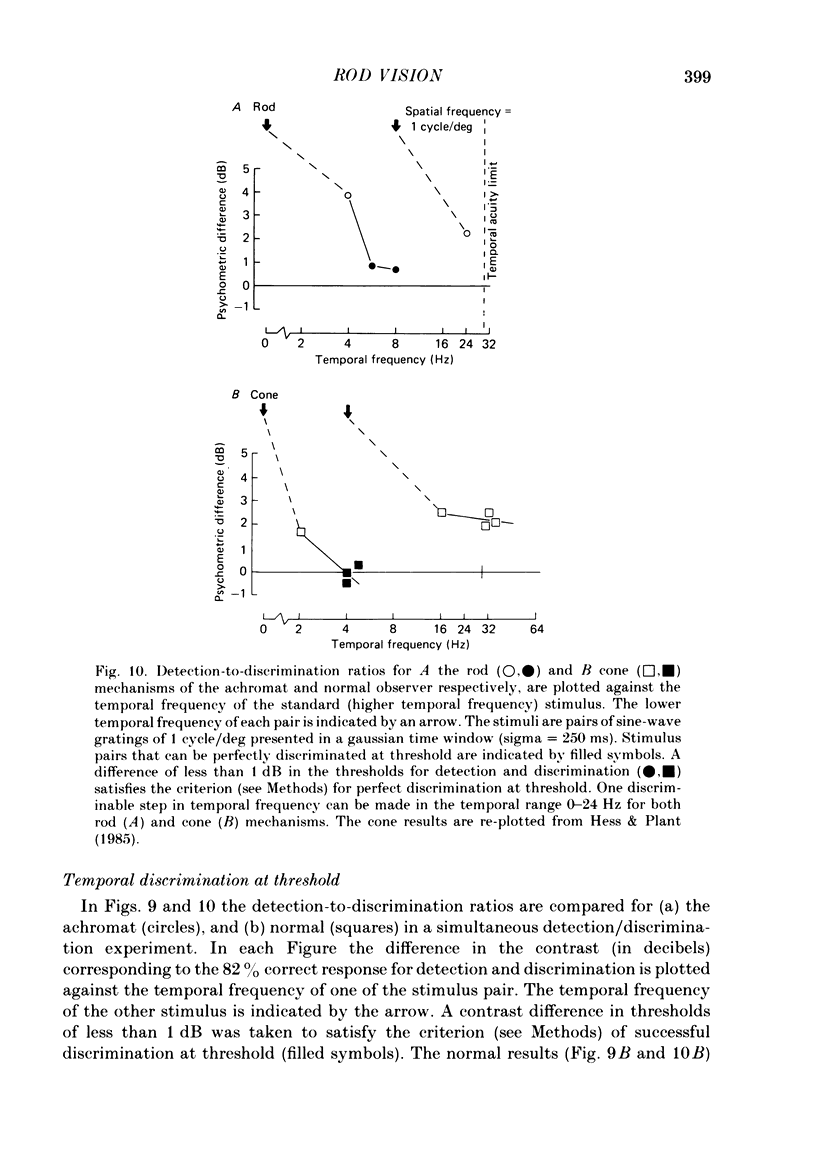
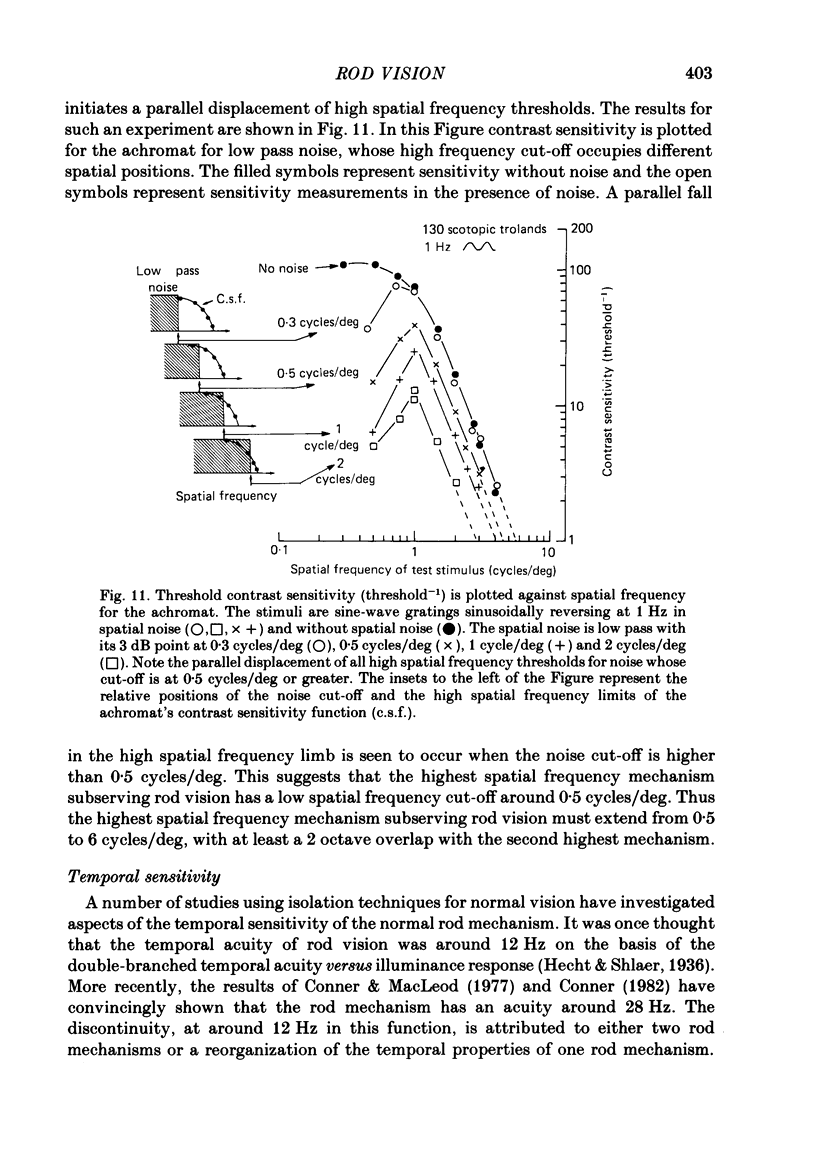
The spatial and temporal properties of rod vision were measured for stimuli at and above the detection threshold in an achromat whose spectral sensitivity, dark adaptation, spatial and temporal thresholds and Stiles-Crawford effect suggest the presence of only a normally functioning rod system. The properties of rod and cone vision were compared at illuminances where their respective sensitivities were optimum. The threshold spatial sensitivity of the rod mechanism under optimum illumination (180 scotopic trolands) exhibits bandpass properties with a peak sensitivity of around 80 at 0.5 cycles/deg and a spatial acuity of 6-7 cycles/deg. The threshold temporal sensitivity also exhibits bandpass properties under these conditions with a peak sensitivity of around 80 at 5 Hz and a temporal acuity of 30 Hz. For stimuli of low spatial frequency (less than 0.3 cycles/deg) and low temporal frequency, the threshold sensitivities of rod- and cone-mediated vision are identical. Rod- and cone-mediated vision display comparable spatial and temporal discrimination for targets of equal suprathreshold contrast over the low to mid spatial and temporal range that they share. Rod-mediated discriminations fall below those of cone vision above 1 cycle/deg for spatial judgements and above 15 Hz for temporal judgements. The number of discriminable steps in spatial frequency and temporal frequency at threshold is similar for rod and cone vision over the spatio-temporal frequency range that they share. Over this range rod- and cone-mediated vision can discriminate four steps in spatial frequency and one step in temporal frequency. These results suggest that rod vision shows comparable spatio-temporal discrimination performance to cone vision and that it is subserved by at least five spatial and two temporal labelled detectors. The response of the highest spatial frequency filter subserving rod vision extends from 0.5 to 6 cycles/deg.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell F. W., Green D. G. Optical and retinal factors affecting visual resolution. J Physiol. 1965 Dec;181(3):576–593. doi: 10.1113/jphysiol.1965.sp007784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F. W., Nachmias J., Jukes J. Spatial-frequency discrimination in human vision. J Opt Soc Am. 1970 Apr;60(4):555–559. doi: 10.1364/josa.60.000555. [DOI] [PubMed] [Google Scholar]
- Campbell F. W., Robson J. G. Application of Fourier analysis to the visibility of gratings. J Physiol. 1968 Aug;197(3):551–566. doi: 10.1113/jphysiol.1968.sp008574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner J. D., MacLeod D. I. Rod photoreceptors detect rapid flicker. Science. 1977 Feb 18;195(4279):698–699. doi: 10.1126/science.841308. [DOI] [PubMed] [Google Scholar]
- Conner J. D. The temporal properties of rod vision. J Physiol. 1982 Nov;332:139–155. doi: 10.1113/jphysiol.1982.sp014406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daitch J. M., Green D. G. Contrast sensitivity of the human peripheral retina. Vision Res. 1969 Aug;9(8):947–952. doi: 10.1016/0042-6989(69)90100-x. [DOI] [PubMed] [Google Scholar]
- Green D. G. Visual acuity in the blue cone monochromat. J Physiol. 1972 Apr;222(2):419–426. doi: 10.1113/jphysiol.1972.sp009806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess R. F., Nordby K. Spatial and temporal limits of vision in the achromat. J Physiol. 1986 Feb;371:365–385. doi: 10.1113/jphysiol.1986.sp015981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch J., Hylton R. Limits of spatial-frequency discrimination as evidence of neural interpolation. J Opt Soc Am. 1982 Oct;72(10):1367–1374. doi: 10.1364/josa.72.001367. [DOI] [PubMed] [Google Scholar]
- Howell E. R., Hess R. F. The functional area for summation to threshold for sinusoidal gratings. Vision Res. 1978;18(4):369–374. doi: 10.1016/0042-6989(78)90045-7. [DOI] [PubMed] [Google Scholar]
- Mandler M. B., Makous W. A three channel model of temporal frequency perception. Vision Res. 1984;24(12):1881–1887. doi: 10.1016/0042-6989(84)90021-x. [DOI] [PubMed] [Google Scholar]
- Mandler M. B. Temporal frequency discrimination above threshold. Vision Res. 1984;24(12):1873–1880. doi: 10.1016/0042-6989(84)90020-8. [DOI] [PubMed] [Google Scholar]
- Nordby K., Stabell B., Stabell U. Dark-adaptation of the human rod system. Vision Res. 1984;24(8):841–849. doi: 10.1016/0042-6989(84)90156-1. [DOI] [PubMed] [Google Scholar]
- Pantle A. J. Temporal frequency response characteristic of motion channels measured with three different psychophysical techniques. Percept Psychophys. 1978 Sep;24(3):285–294. doi: 10.3758/bf03206100. [DOI] [PubMed] [Google Scholar]
- Richards W. Quantifying sensory channels: generalizing colorimetry to orientation and texture, touch, and tones. Sens Processes. 1979 Sep;3(3):207–229. [PubMed] [Google Scholar]
- Smith R. A., Jr Luminance-dependent changes in mesopic visual contrast sensitivity. J Physiol. 1973 Apr;230(1):115–135. doi: 10.1113/jphysiol.1973.sp010178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson P. Discrimination of moving gratings at and above detection threshold. Vision Res. 1983;23(12):1533–1538. doi: 10.1016/0042-6989(83)90166-9. [DOI] [PubMed] [Google Scholar]
- Watson A. B. Probability summation over time. Vision Res. 1979;19(5):515–522. doi: 10.1016/0042-6989(79)90136-6. [DOI] [PubMed] [Google Scholar]
- Watson A. B., Robson J. G. Discrimination at threshold: labelled detectors in human vision. Vision Res. 1981;21(7):1115–1122. doi: 10.1016/0042-6989(81)90014-6. [DOI] [PubMed] [Google Scholar]


