Abstract
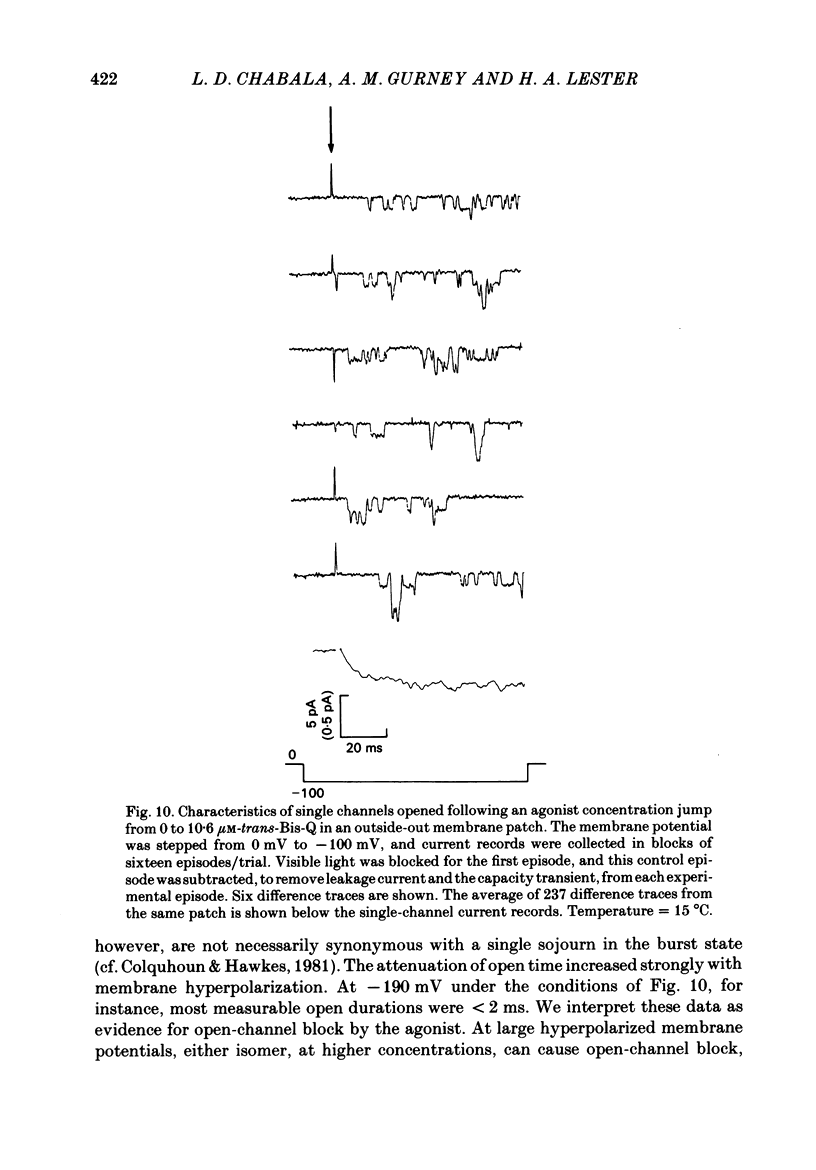
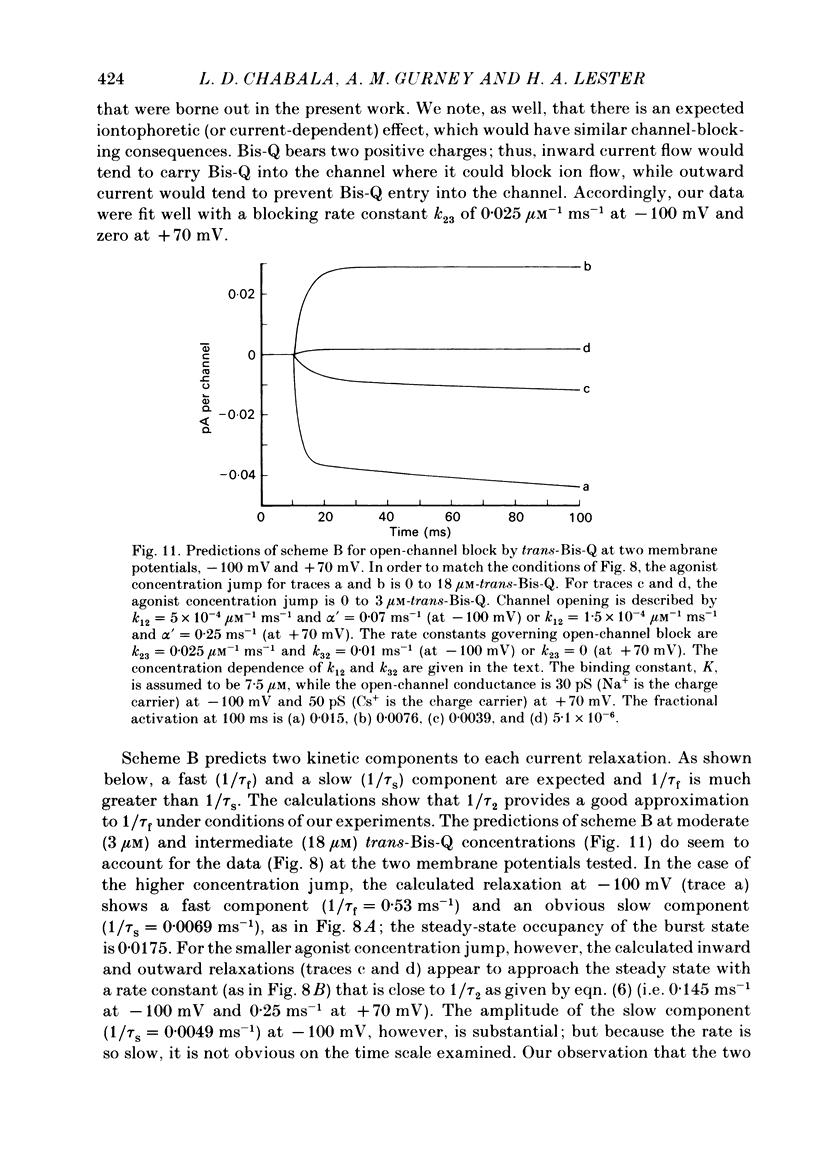
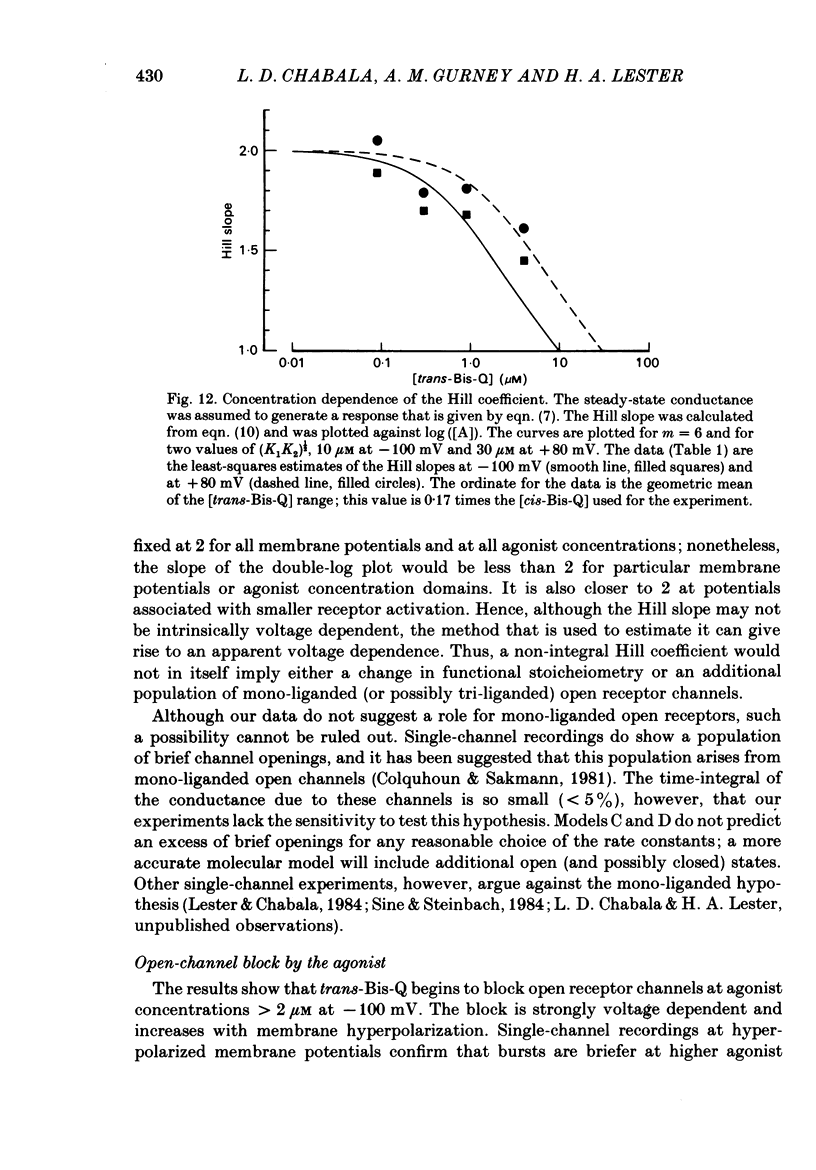
Whole-cell or single-channel currents through acetylcholine (ACh) receptor channels were studied in voltage-clamped rat myoballs or in excised membrane patches from myoballs. The recording pipette contained CsCl to suppress outward currents, and tetrodotoxin was used to help suppress Na+ currents. To minimize problems associated with bath applied agonists, myoballs were bathed in a solution containing the inactive (cis) isomer of the photo-isomerizable azobenzene derivative, Bis-Q. Calibrated light flashes of varying intensity were presented to produce concentration jumps of agonist, trans-Bis-Q. The resulting whole-cell current relaxations through ACh channels approach a steady state along an exponential time course, then decline as the newly created agonist diffuses away over the next few seconds. The dose-response relationship was inferred from Hill (double-log) plots for myoballs bathed in 500 nM-cis-Bis-Q at three membrane potentials. At low agonist concentrations (less than 300 nM-trans-Bis-Q), the slope of the Hill plot averaged 1.62 at -150 mV, 1.89 at -100 mV, and 2.05 at +80 mV. These results are consistent with an apparent agonist affinity constant that decreases with membrane depolarization and shifts the responses further down on the dose-response curve. When the myoballs were bathed in higher concentrations of cis-Bis-Q (1.5-20 microM), the slope of the Hill plot was reduced at all membrane potentials, although it was still closer to two at positive potentials. This is expected from the known sigmoid shape of the dose-response relation. The shallow dependence of the Hill slope on agonist concentration suggests the presence of negative cooperativity in the over-all binding of agonist molecules. Following treatment of the membrane with dithiothreitol to reduce disulphide groups, the Hill slope for the reversibly bound agonist, trans-Bis-Q, remained near two. The kinetics of currents at hyperpolarized membrane potentials became complicated at higher agonist concentrations in a manner that was consistent with open-channel block by trans-Bis-Q; the currents showed a slow secondary increase in conductance. Averaged single-channel recordings at higher agonist concentrations resemble macroscopic relaxations under comparable conditions. Furthermore, those recordings also suggested that open channels are blocked by trans-Bis-Q at concentrations greater than 2 microM; the block depends strongly on membrane potential and increases with hyperpolarization. Currents at positive membrane potentials showed no evidence of open-channel block.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF















Selected References
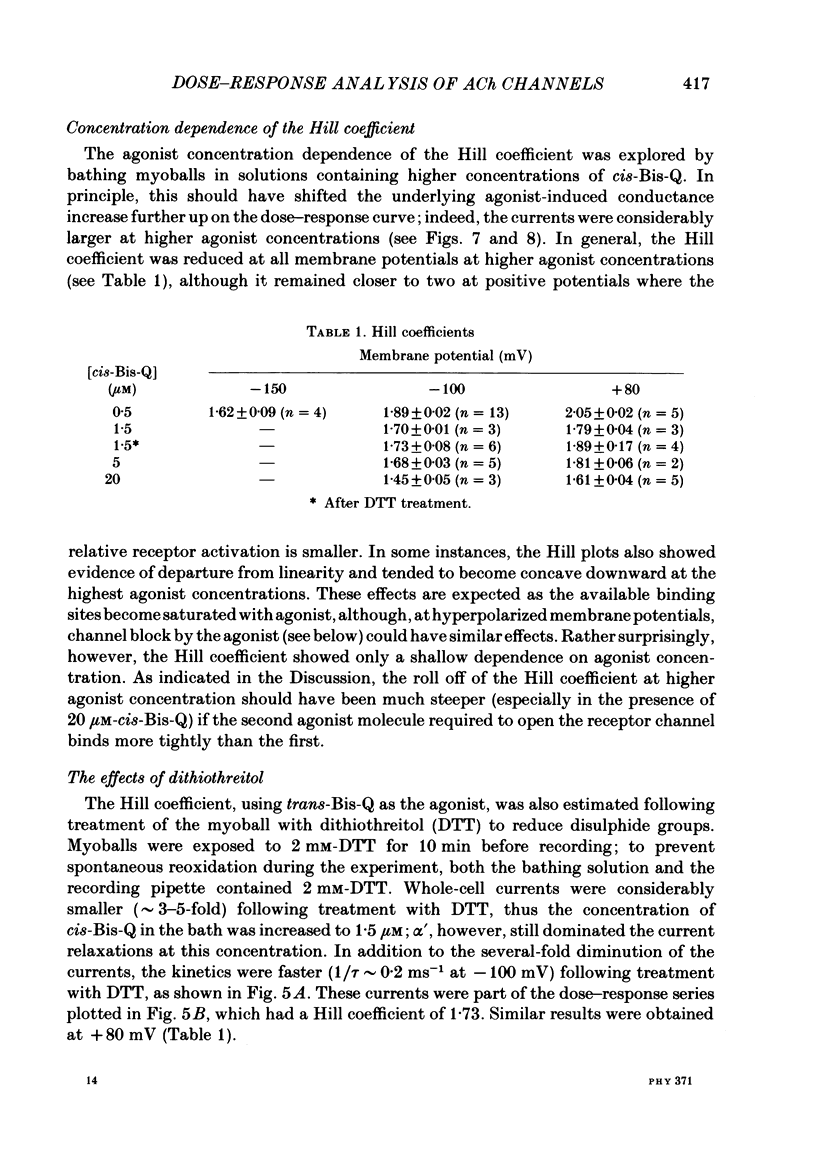
These references are in PubMed. This may not be the complete list of references from this article.
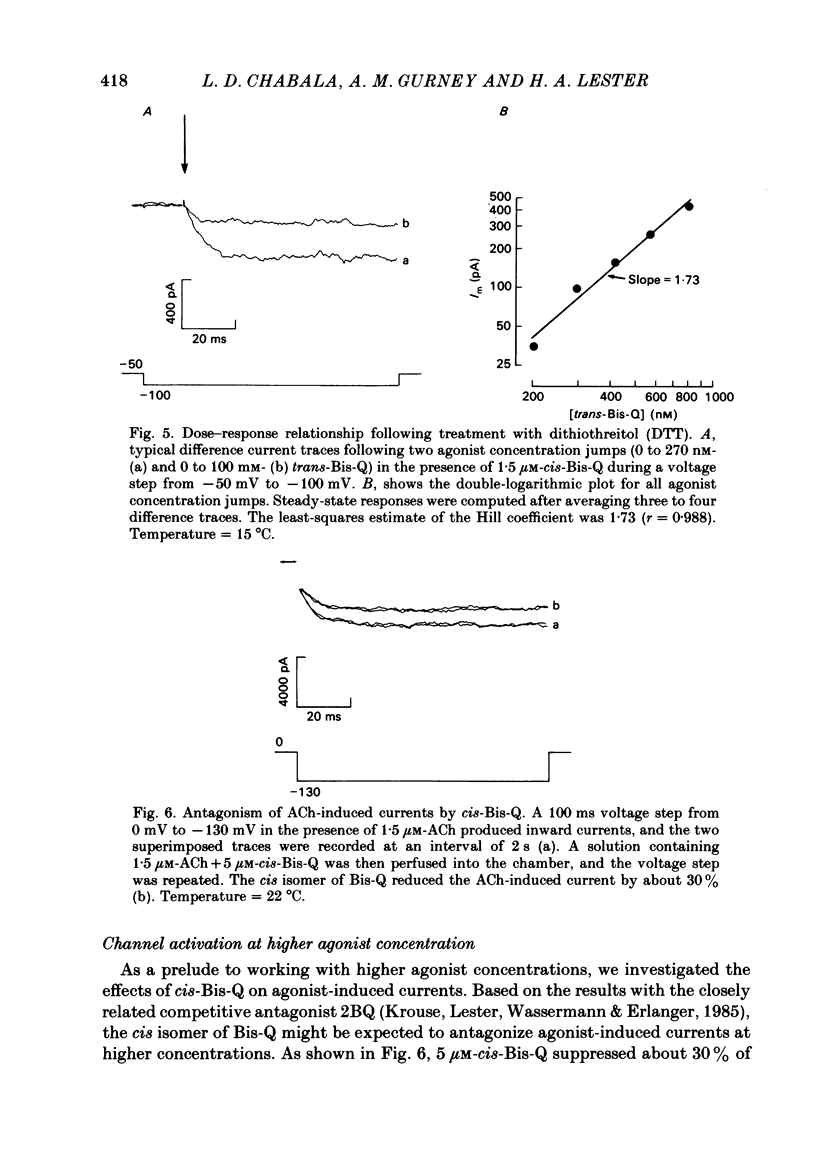
- Adams P. R. An analysis of the dose-response curve at voltage-clamped frog-endplates. Pflugers Arch. 1975 Oct 28;360(2):145–153. doi: 10.1007/BF00580537. [DOI] [PubMed] [Google Scholar]
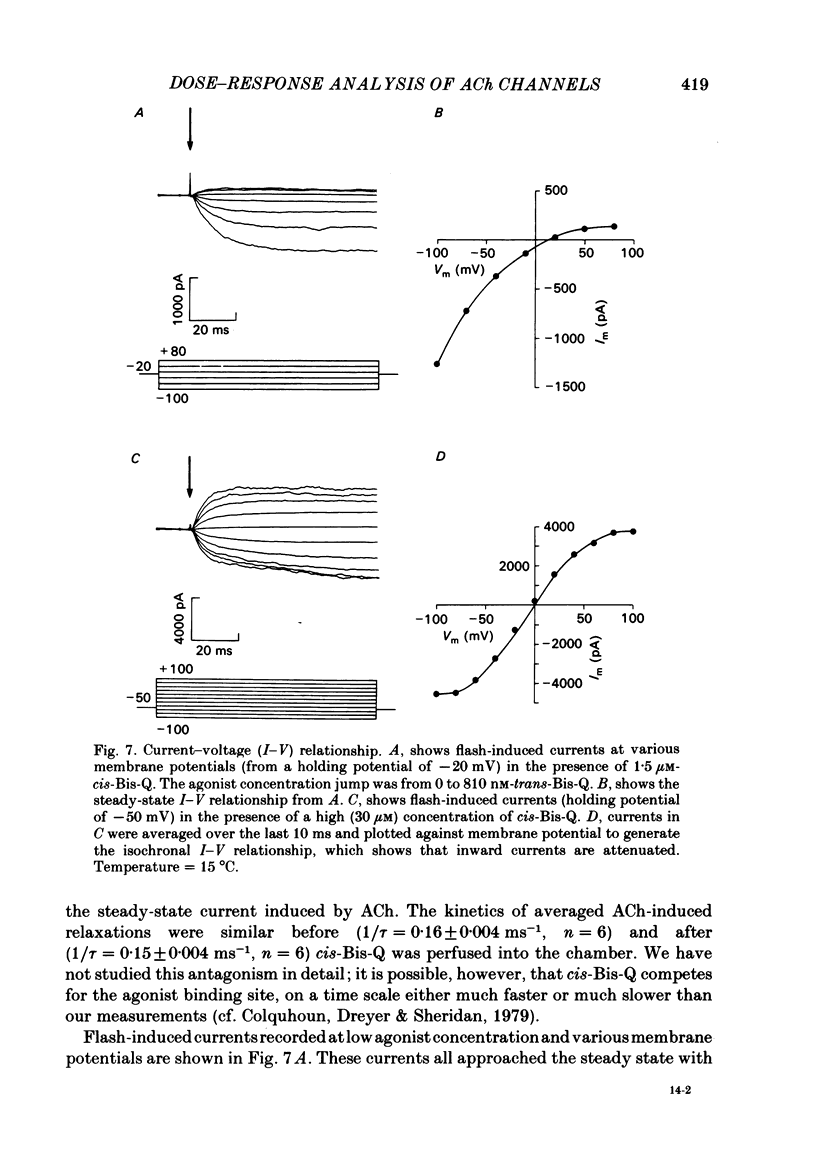
- Adams P. R. Drug blockade of open end-plate channels. J Physiol. 1976 Sep;260(3):531–552. doi: 10.1113/jphysiol.1976.sp011530. [DOI] [PMC free article] [PubMed] [Google Scholar]
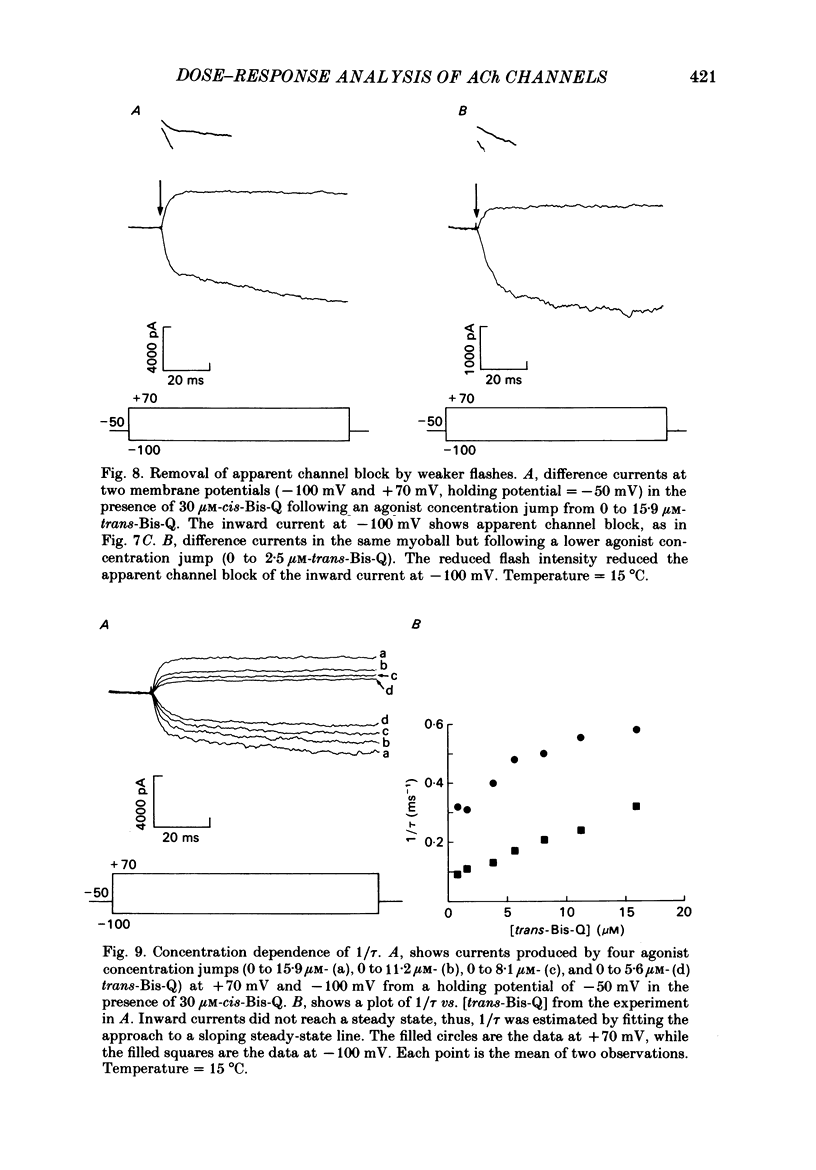
- Adams P. R. Kinetics of agonist conductance changes during hyperolarization at frog endplates. Br J Pharmacol. 1975 Feb;53(2):308–310. doi: 10.1111/j.1476-5381.1975.tb07364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Sakmann B. Decamethonium both opens and blocks endplate channels. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2994–2998. doi: 10.1073/pnas.75.6.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Haim D., Dreyer F., Peper K. Acetylcholine receptor: modification of synaptic gating mechanism after treatment with a disulfide bond reducing agent. Pflugers Arch. 1975 Mar 22;355(1):19–26. doi: 10.1007/BF00584796. [DOI] [PubMed] [Google Scholar]
- Chabala L. D., Gurney A. M., Lester H. A. Photoactivation and dissociation of agonist molecules at the nicotinic acetylcholine receptor in voltage-clamped rat myoballs. Biophys J. 1985 Aug;48(2):241–246. doi: 10.1016/S0006-3495(85)83777-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabala L. D., Sheridan R. E., Hodge D. C., Power J. N., Walsh M. P. A microscope stage temperature controller for the study of whole-cell or single-channel currents. Pflugers Arch. 1985 Aug;404(4):374–377. doi: 10.1007/BF00585351. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Dreyer F., Sheridan R. E. The actions of tubocurarine at the frog neuromuscular junction. J Physiol. 1979 Aug;293:247–284. doi: 10.1113/jphysiol.1979.sp012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of single ion channels. Proc R Soc Lond B Biol Sci. 1981 Mar 6;211(1183):205–235. doi: 10.1098/rspb.1981.0003. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fluctuations in the microsecond time range of the current through single acetylcholine receptor ion channels. Nature. 1981 Dec 3;294(5840):464–466. doi: 10.1038/294464a0. [DOI] [PubMed] [Google Scholar]
- Dionne V. E., Steinbach J. H., Stevens C. F. An analysis of the dose-response relationship at voltage-clamped frog neuromuscular junctions. J Physiol. 1978 Aug;281:421–444. doi: 10.1113/jphysiol.1978.sp012431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer F., Peper K., Sterz R. Determination of dose-response curves by quantitative ionophoresis at the frog neuromuscular junction. J Physiol. 1978 Aug;281:395–419. doi: 10.1113/jphysiol.1978.sp012430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Horn R., Brodwick M. S. Acetylcholine-induced current in perfused rat myoballs. J Gen Physiol. 1980 Mar;75(3):297–321. doi: 10.1085/jgp.75.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin A., Bartels E. Effects of blocking sulfhydryl groups and of reducing disulfide bonds on the acetylcholine-activated permeability system of the electroplax. Biochim Biophys Acta. 1966 Nov 8;126(3):525–535. doi: 10.1016/0926-6585(66)90010-0. [DOI] [PubMed] [Google Scholar]
- Karlin A. On the application of "a plausible model" of allosteric proteins to the receptor for acetylcholine. J Theor Biol. 1967 Aug;16(2):306–320. doi: 10.1016/0022-5193(67)90011-2. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegel D. R., Wolf B. D., Sheridan R. E., Lester H. A. Software for electrophysiological experiments with a personal computer. J Neurosci Methods. 1985 Feb;12(4):317–330. doi: 10.1016/0165-0270(85)90016-0. [DOI] [PubMed] [Google Scholar]
- Krouse M. E., Lester H. A., Wassermann N. H., Erlanger B. F. Rates and equilibria for a photoisomerizable antagonist at the acetylcholine receptor of Electrophorus electroplaques. J Gen Physiol. 1985 Aug;86(2):235–256. doi: 10.1085/jgp.86.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land B. R., Salpeter E. E., Salpeter M. M. Kinetic parameters for acetylcholine interaction in intact neuromuscular junction. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7200–7204. doi: 10.1073/pnas.78.11.7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester H. A., Changeux J. P., Sheridan R. E. Conductance increases produced by bath application of cholinergic agonists to Electrophorus electroplaques. J Gen Physiol. 1975 Jun;65(6):797–816. doi: 10.1085/jgp.65.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester H. A., Koblin D. D., Sheridan R. E. Role of voltage-sensitive receptors in nicotinic transmission. Biophys J. 1978 Mar;21(3):181–194. doi: 10.1016/S0006-3495(78)85518-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester H. A., Krouse M. E., Nass M. M., Wassermann N. H., Erlanger B. F. A covalently bound photoisomerizable agonist: comparison with reversibly bound agonists at Electrophorus electroplaques. J Gen Physiol. 1980 Feb;75(2):207–232. doi: 10.1085/jgp.75.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester H. A., Nerbonne J. M. Physiological and pharmacological manipulations with light flashes. Annu Rev Biophys Bioeng. 1982;11:151–175. doi: 10.1146/annurev.bb.11.060182.001055. [DOI] [PubMed] [Google Scholar]
- Lewis C. A., Stevens C. F. Acetylcholine receptor channel ionic selectivity: ions experience an aqueous environment. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6110–6113. doi: 10.1073/pnas.80.19.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976 Apr 29;260(5554):799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Voltage-dependence of drug-induced conductance in frog neuromuscular junction. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2140–2144. doi: 10.1073/pnas.72.6.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerbonne J. M., Sheridan R. E., Chabala L. D., Lester H. A. cis-3,3'-Bis-[alpha-(trimethylammonium)methyl]azobenzene (cis-Bis-Q). Purification and properties at acetylcholine receptors of Electrophorus electroplaques. Mol Pharmacol. 1983 Mar;23(2):344–349. [PubMed] [Google Scholar]
- Peper K., Bradley R. J., Dreyer F. The acetylcholine receptor at the neuromuscular junction. Physiol Rev. 1982 Oct;62(4 Pt 1):1271–1340. doi: 10.1152/physrev.1982.62.4.1271. [DOI] [PubMed] [Google Scholar]
- Rang H. P., Ritter J. M. The effect of disulfide bond reduction on the properties of cholinergic receptors in chick muscle. Mol Pharmacol. 1971 Nov;7(6):620–631. [PubMed] [Google Scholar]
- STEPHENSON R. P. A modification of receptor theory. Br J Pharmacol Chemother. 1956 Dec;11(4):379–393. doi: 10.1111/j.1476-5381.1956.tb00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan R. E., Lester H. A. Functional stoichiometry at the nicotinic receptor. The photon cross section for phase 1 corresponds to two bis-Q molecules per channel. J Gen Physiol. 1982 Oct;80(4):499–515. doi: 10.1085/jgp.80.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan R. E., Lester H. A. Rates and equilibria at the acetylcholine receptor of Electrophorus electroplaques: a study of neurally evoked postsynaptic currents and of voltage-jump relaxations. J Gen Physiol. 1977 Aug;70(2):187–219. [PMC free article] [PubMed] [Google Scholar]
- Sheridan R. E., Lester H. A. Relaxation measurements on the acetylcholine receptor. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3496–3500. doi: 10.1073/pnas.72.9.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Activation of a nicotinic acetylcholine receptor. Biophys J. 1984 Jan;45(1):175–185. doi: 10.1016/S0006-3495(84)84146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrar D. A. Effects of dithiothreitol on end-plate currents. J Physiol. 1978 Mar;276:403–417. doi: 10.1113/jphysiol.1978.sp012243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. W., Richardson C. A., McNamee M. G. Effects of thio-group modifications of Torpedo californica acetylcholine receptor on ion flux activation and inactivation kinetics. Biochemistry. 1984 May 22;23(11):2329–2338. doi: 10.1021/bi00306a002. [DOI] [PubMed] [Google Scholar]


