Abstract
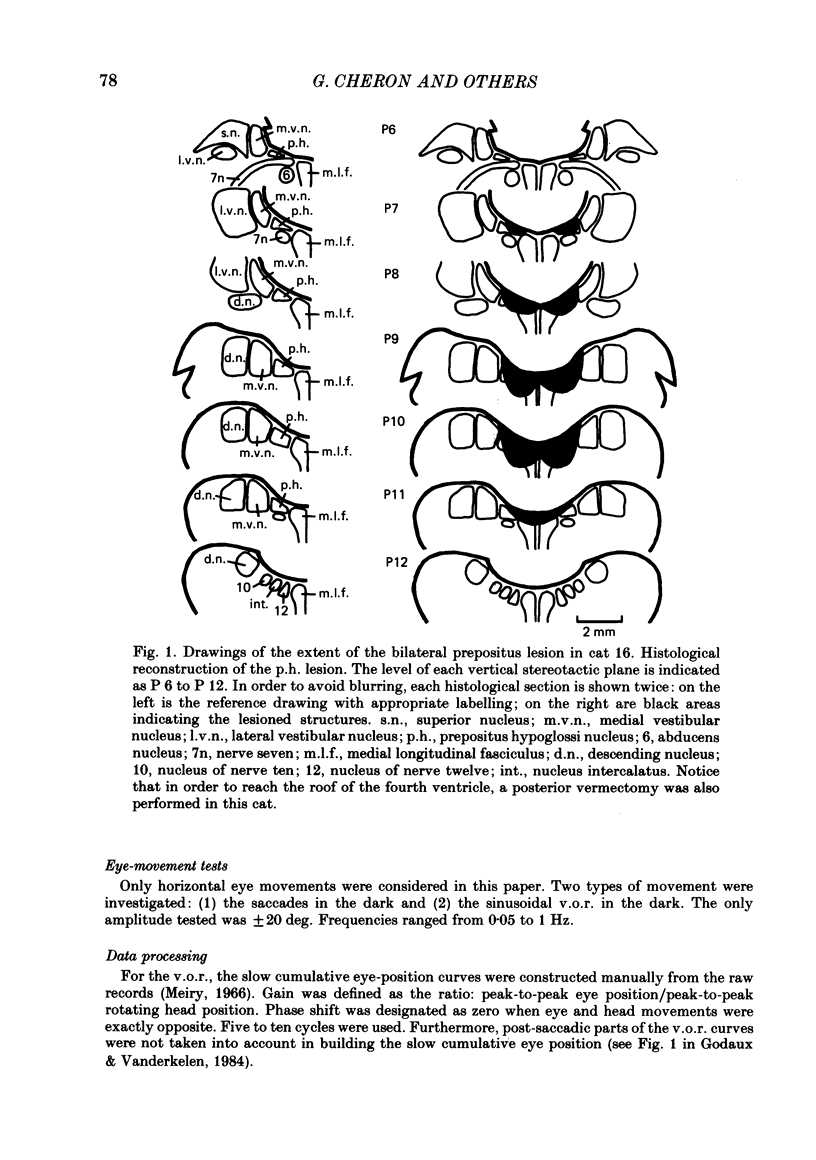
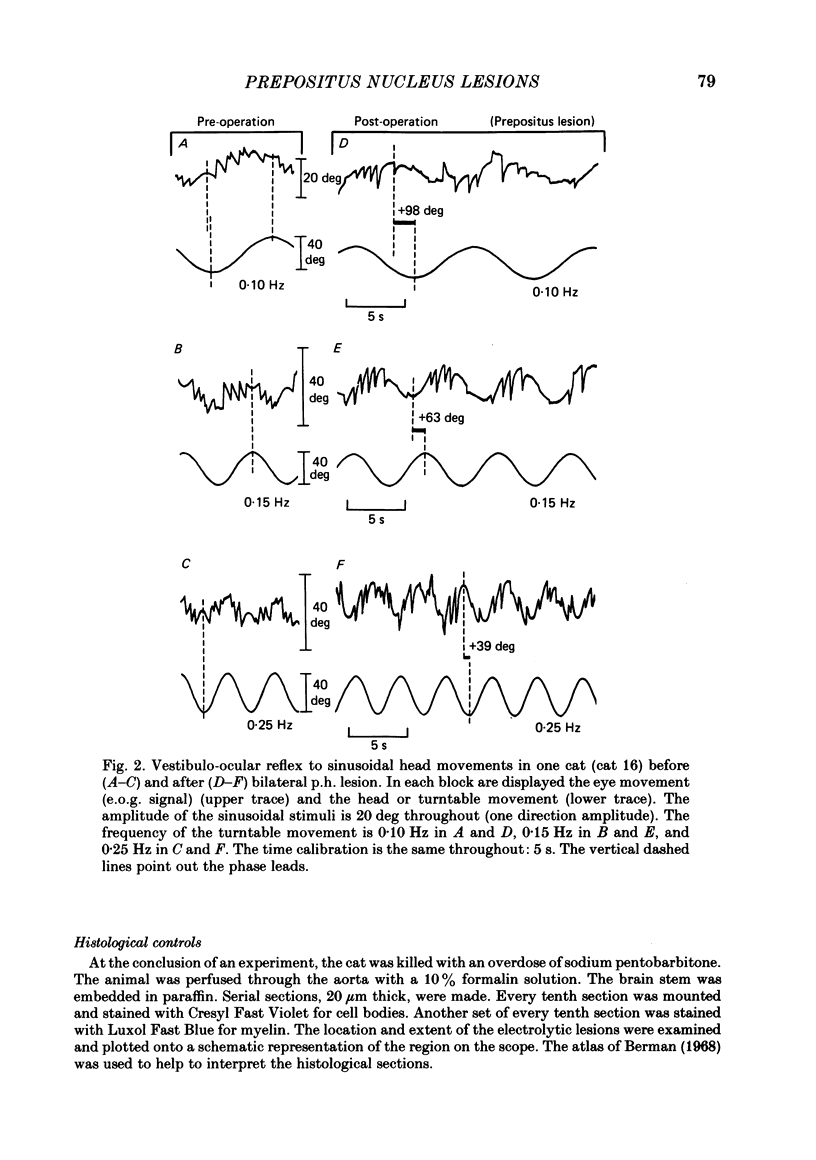
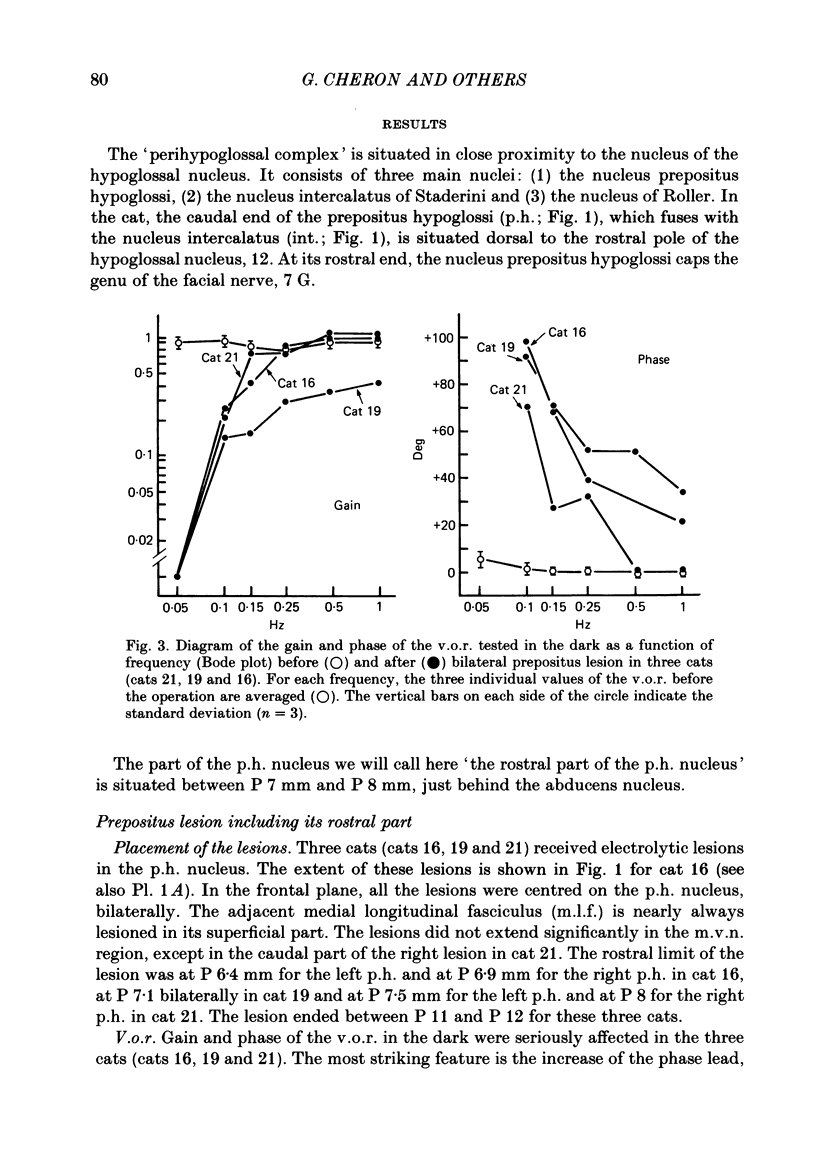
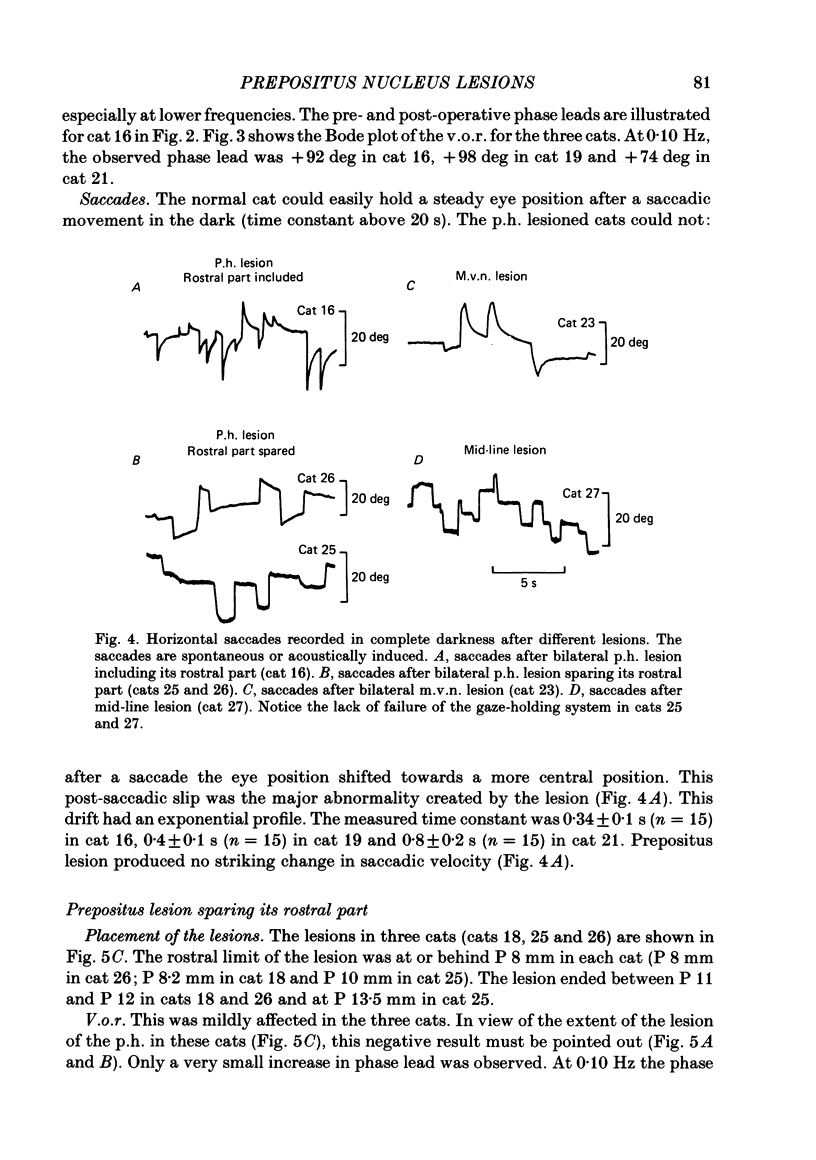
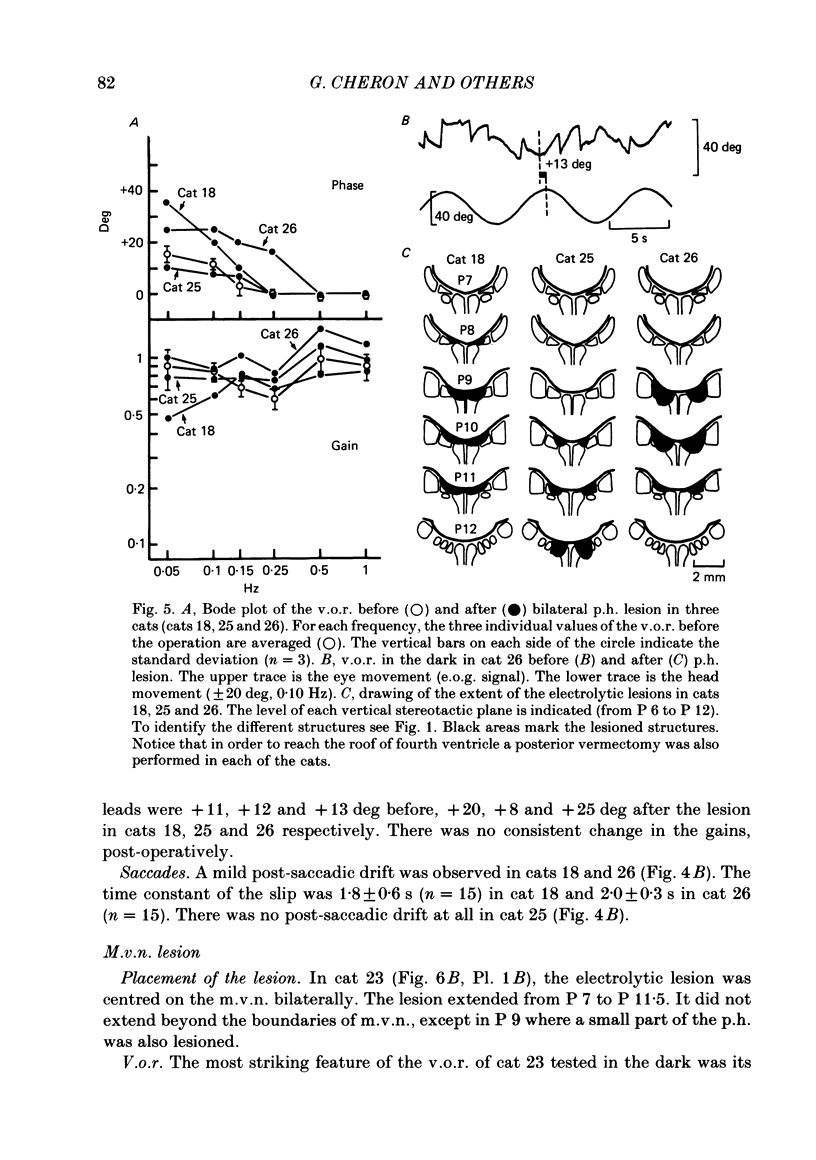
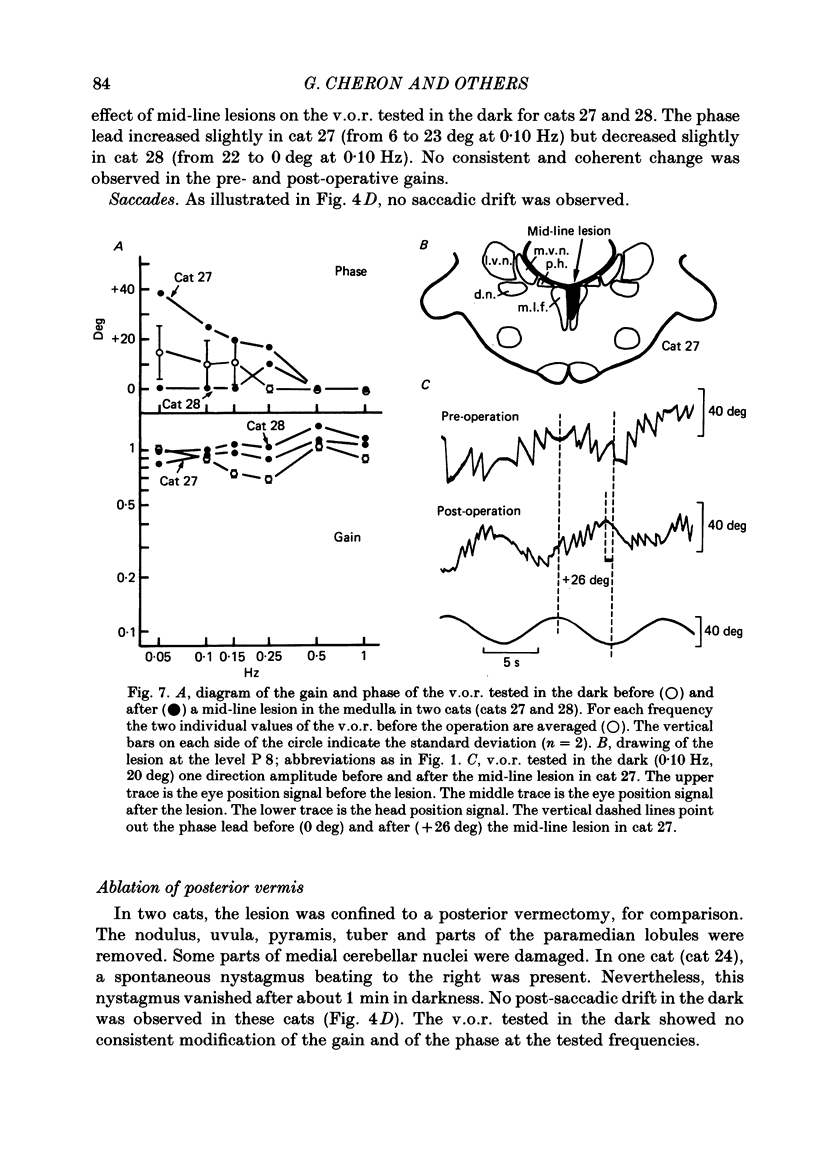
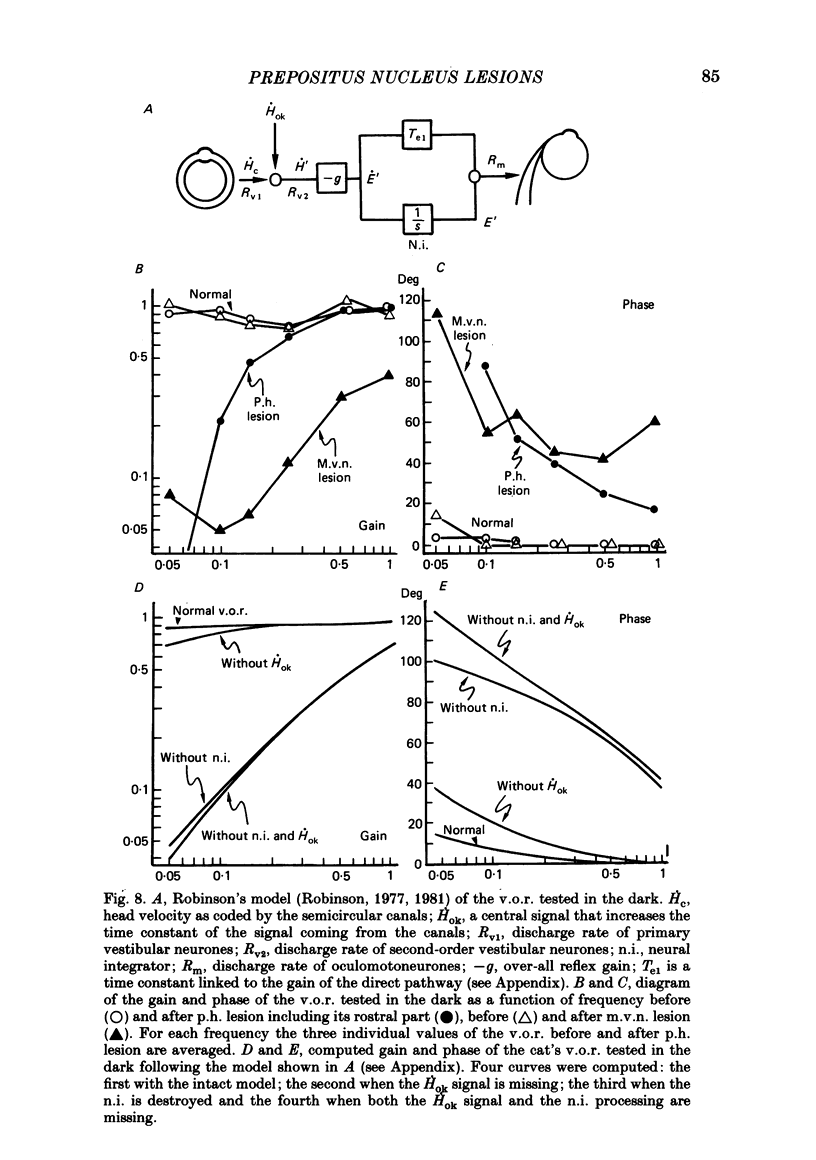
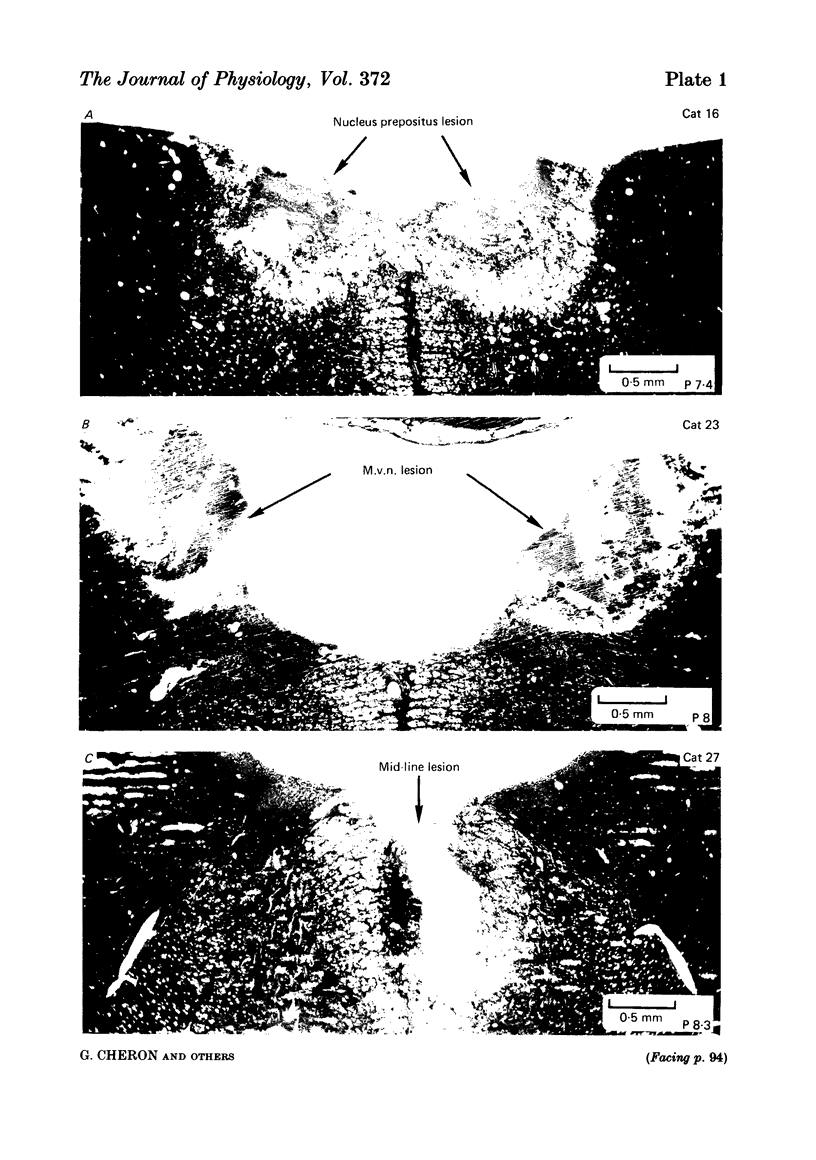
The effects of bilateral electrolytic lesions within and around the prepositus hypoglossi (p.h.) nucleus on horizontal saccades in the dark and on the horizontal sinusoidal vestibulo-ocular reflex (v.o.r.) in the dark were studied. After p.h. lesion, including its rostral part between P 7 and P 8, the v.o.r. showed a phase lead as much as about 90 deg at 0.10 Hz. A significant gain reduction paralleled that phase lead at lower frequencies. A large post-saccadic drift was also observed, the time constant of which ranged from 0.3 to 0.6 s. After p.h. lesion extending from P 8 to P 11 (but sparing the rostral part of the p.h.), no significant gain or phase lead change was observed. Post-saccadic drift was either missing or weak. A bilateral medial vestibular nucleus (m.v.n.) lesion from P 7 to P 11 produced a marked gain decrease, paralleled by a marked phase advance. A post-saccadic drift was observed (tau = 0.6 s). A surgical mid-line lesion from P 7 to P 11 (depth: about 2 mm) was followed by no remarkable change in the gain and in the phase of the v.o.r. No post-saccadic drift was observed after such lesion. It was concluded that (i) both the horizontal v.o.r. integration processing, and the horizontal saccadic integration processing were destroyed when an electrolytic lesion was made 'in the region of' the rostral part of the p.h. nucleus, and that (ii) the posterior four-fifths of the p.h. was the location of neither the horizontal v.o.r. integrator nor the horizontal saccadic integrator.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker R., Berthoz A. Is the prepositus hypoglossi nucleus the source of another vestibulo-ocular pathway? Brain Res. 1975 Mar 14;86(1):121–127. doi: 10.1016/0006-8993(75)90643-5. [DOI] [PubMed] [Google Scholar]
- Baker R., Gresty M., Berthoz A. Neuronal activity in the prepositus hypoglossi nucleus correlated with vertical and horizontal eye movement in the cat. Brain Res. 1976 Jan 16;101(2):366–371. doi: 10.1016/0006-8993(76)90278-x. [DOI] [PubMed] [Google Scholar]
- Balaban C. D. A projection from nucleus reticularis tegmenti pontis of Bechterew to the medial vestibular nucleus in rabbits. Exp Brain Res. 1983;51(2):304–309. doi: 10.1007/BF00237207. [DOI] [PubMed] [Google Scholar]
- Blanks R. H., Estes M. S., Markham C. H. Physiologic characteristics of vestibular first-order canal neurons in the cat. II. Response to constant angular acceleration. J Neurophysiol. 1975 Sep;38(5):1250–1268. doi: 10.1152/jn.1975.38.5.1250. [DOI] [PubMed] [Google Scholar]
- Blanks R. H., Volkind R., Precht W., Baker R. Responses of cat prepositus hypoglossi neurons to horizontal angular acceleration. Neuroscience. 1977;2(3):391–403. doi: 10.1016/0306-4522(77)90005-7. [DOI] [PubMed] [Google Scholar]
- Carleton S. C., Carpenter M. B. Afferent and efferent connections of the medial, inferior and lateral vestibular nuclei in the cat and monkey. Brain Res. 1983 Nov 14;278(1-2):29–51. doi: 10.1016/0006-8993(83)90223-8. [DOI] [PubMed] [Google Scholar]
- Cheron G., Gillis P., Godaux E. Lesions in the cat prepositus complex: effects on the optokinetic system. J Physiol. 1986 Mar;372:95–111. doi: 10.1113/jphysiol.1986.sp015999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B., Komatsuzaki A. Eye movements induced by stimulation of the pontine reticular formation: evidence for integration in oculomotor pathways. Exp Neurol. 1972 Jul;36(1):101–117. doi: 10.1016/0014-4886(72)90139-2. [DOI] [PubMed] [Google Scholar]
- Cohen B., Matsuo V., Raphan T. Quantitative analysis of the velocity characteristics of optokinetic nystagmus and optokinetic after-nystagmus. J Physiol. 1977 Sep;270(2):321–344. doi: 10.1113/jphysiol.1977.sp011955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demer J. L., Robinson D. A. Different time constants for optokinetic and vestibular nystagmus with a single velocity-storage element. Brain Res. 1983 Oct 3;276(1):173–177. doi: 10.1016/0006-8993(83)90560-7. [DOI] [PubMed] [Google Scholar]
- Fernandez C., Goldberg J. M. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. II. Response to sinusoidal stimulation and dynamics of peripheral vestibular system. J Neurophysiol. 1971 Jul;34(4):661–675. doi: 10.1152/jn.1971.34.4.661. [DOI] [PubMed] [Google Scholar]
- Fuchs A. F., Kimm J. Unit activity in vestibular nucleus of the alert monkey during horizontal angular acceleration and eye movement. J Neurophysiol. 1975 Sep;38(5):1140–1161. doi: 10.1152/jn.1975.38.5.1140. [DOI] [PubMed] [Google Scholar]
- Galiana H. L., Outerbridge J. S. A bilateral model for central neural pathways in vestibuloocular reflex. J Neurophysiol. 1984 Feb;51(2):210–241. doi: 10.1152/jn.1984.51.2.210. [DOI] [PubMed] [Google Scholar]
- Godaux E., Gobert C., Halleux J. Vestibuloocular reflex, optokinetic response, and their interactions in the alert cat. Exp Neurol. 1983 Apr;80(1):42–54. doi: 10.1016/0014-4886(83)90005-5. [DOI] [PubMed] [Google Scholar]
- Godaux E., Laune J. M. The saccadic system and the vestibulo-ocular reflex in the cat do not share the same integrator. Neurosci Lett. 1983 Aug 8;38(3):263–268. doi: 10.1016/0304-3940(83)90379-8. [DOI] [PubMed] [Google Scholar]
- Godaux E., Vanderkelen B. Vestibulo-ocular reflex, optokinetic response and their interactions in the cerebellectomized cat. J Physiol. 1984 Jan;346:155–170. doi: 10.1113/jphysiol.1984.sp015013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel A. M. Direct and indirect preoculomotor pathways of the brainstem: an autoradiographic study of the pontine reticular formation in the cat. J Comp Neurol. 1977 Sep 1;175(1):37–78. doi: 10.1002/cne.901750105. [DOI] [PubMed] [Google Scholar]
- Graybiel A. M., Hartwieg E. A. Some afferent connections of the oculomotor complex in the cat: an experimental study with tracer techniques. Brain Res. 1974 Dec 13;81(3):543–551. doi: 10.1016/0006-8993(74)90850-6. [DOI] [PubMed] [Google Scholar]
- Gresty M., Baker R. Neurons with visual receptive field, eye movement and neck displacement sensitivity within and around the nucleus prepositus hypoglossi in the alert cat. Exp Brain Res. 1976 Feb 26;24(4):429–433. doi: 10.1007/BF00235008. [DOI] [PubMed] [Google Scholar]
- Henn V., Lang W., Hepp K., Reisine H. Experimental gaze palsies in monkeys and their relation to human pathology. Brain. 1984 Jun;107(Pt 2):619–636. doi: 10.1093/brain/107.2.619. [DOI] [PubMed] [Google Scholar]
- Hikosaka O., Igusa Y., Imai H. Firing pattern of prepositus hypoglossi and adjacent reticular neurons related to vestibular nystagmus in the cat. Brain Res. 1978 Apr 14;144(2):395–403. doi: 10.1016/0006-8993(78)90167-1. [DOI] [PubMed] [Google Scholar]
- Keller E. L., Daniels P. D. Oculomotor related interaction of vestibular and visual stimulation in vestibular nucleus cells in alert monkey. Exp Neurol. 1975 Jan;46(1):187–198. doi: 10.1016/0014-4886(75)90041-2. [DOI] [PubMed] [Google Scholar]
- Keller E. L. Participation of medial pontine reticular formation in eye movement generation in monkey. J Neurophysiol. 1974 Mar;37(2):316–332. doi: 10.1152/jn.1974.37.2.316. [DOI] [PubMed] [Google Scholar]
- Keller E. L., Precht W. Visual-vestibular responses in vestibular nuclear neurons in the intact and cerebellectomized, alert cat. Neuroscience. 1979;4(11):1599–1613. doi: 10.1016/0306-4522(79)90023-x. [DOI] [PubMed] [Google Scholar]
- Lopez-Barneo J., Darlot C., Berthoz A., Baker R. Neuronal activity in prepositus nucleus correlated with eye movement in the alert cat. J Neurophysiol. 1982 Feb;47(2):329–352. doi: 10.1152/jn.1982.47.2.329. [DOI] [PubMed] [Google Scholar]
- Lopez-Barneo J., Darlot C., Berthoz A. Functional role of the prepositus hypoglossi nucleus in the control of gaze. Prog Brain Res. 1979;50:667–679. doi: 10.1016/S0079-6123(08)60864-X. [DOI] [PubMed] [Google Scholar]
- Lopez-Barneo J., Ribas J., Delgado-Garcia J. M. Identification of prepositus neurons projecting to the oculomotor nucleus in the alert cat. Brain Res. 1981 Jun 9;214(1):174–179. doi: 10.1016/0006-8993(81)90450-9. [DOI] [PubMed] [Google Scholar]
- McCrea R. A., Baker R., Delgado-Garcia J. Afferent and efferent organization of the prepositus hypoglossi nucleus. Prog Brain Res. 1979;50:653–665. doi: 10.1016/S0079-6123(08)60863-8. [DOI] [PubMed] [Google Scholar]
- Pola J., Robinson D. A. Oculomotor signals in medial longitudinal fasciculus of the monkey. J Neurophysiol. 1978 Mar;41(2):245–259. doi: 10.1152/jn.1978.41.2.245. [DOI] [PubMed] [Google Scholar]
- ROBINSON D. A. THE MECHANICS OF HUMAN SACCADIC EYE MOVEMENT. J Physiol. 1964 Nov;174:245–264. doi: 10.1113/jphysiol.1964.sp007485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphan T., Matsuo V., Cohen B. Velocity storage in the vestibulo-ocular reflex arc (VOR). Exp Brain Res. 1979 Apr 2;35(2):229–248. doi: 10.1007/BF00236613. [DOI] [PubMed] [Google Scholar]
- Robinson D. A. Linear addition of optokinetic and vestibular signals in the vestibular nucleus. Exp Brain Res. 1977 Nov 24;30(2-3):447–450. doi: 10.1007/BF00237269. [DOI] [PubMed] [Google Scholar]
- Robinson D. A. The effect of cerebellectomy on the cat's bestibulo-ocular integrator. Brain Res. 1974 May 17;71(2-3):195–207. doi: 10.1016/0006-8993(74)90961-5. [DOI] [PubMed] [Google Scholar]
- Skavenski A. A., Robinson D. A. Role of abducens neurons in vestibuloocular reflex. J Neurophysiol. 1973 Jul;36(4):724–738. doi: 10.1152/jn.1973.36.4.724. [DOI] [PubMed] [Google Scholar]
- Westheimer G., Blair S. M. Function Organization of primate oculomotor system revealed by cerebellectomy. Exp Brain Res. 1974;21(5):463–472. doi: 10.1007/BF00237165. [DOI] [PubMed] [Google Scholar]
- Yingcharoen K., Rinvik E. Ultrastructural demonstration of a projection from the flocculus to the nucleus prepositus hypoglossi in the cat. Exp Brain Res. 1983;51(2):192–198. doi: 10.1007/BF00237194. [DOI] [PubMed] [Google Scholar]
- de Jong J. M., Cohen B., Matsuo V., Uemura T. Midsagittal pontomedullary brain stem section: effects on ocular adduction and nystagmus. Exp Neurol. 1980 Jun;68(3):420–442. doi: 10.1016/0014-4886(80)90098-9. [DOI] [PubMed] [Google Scholar]