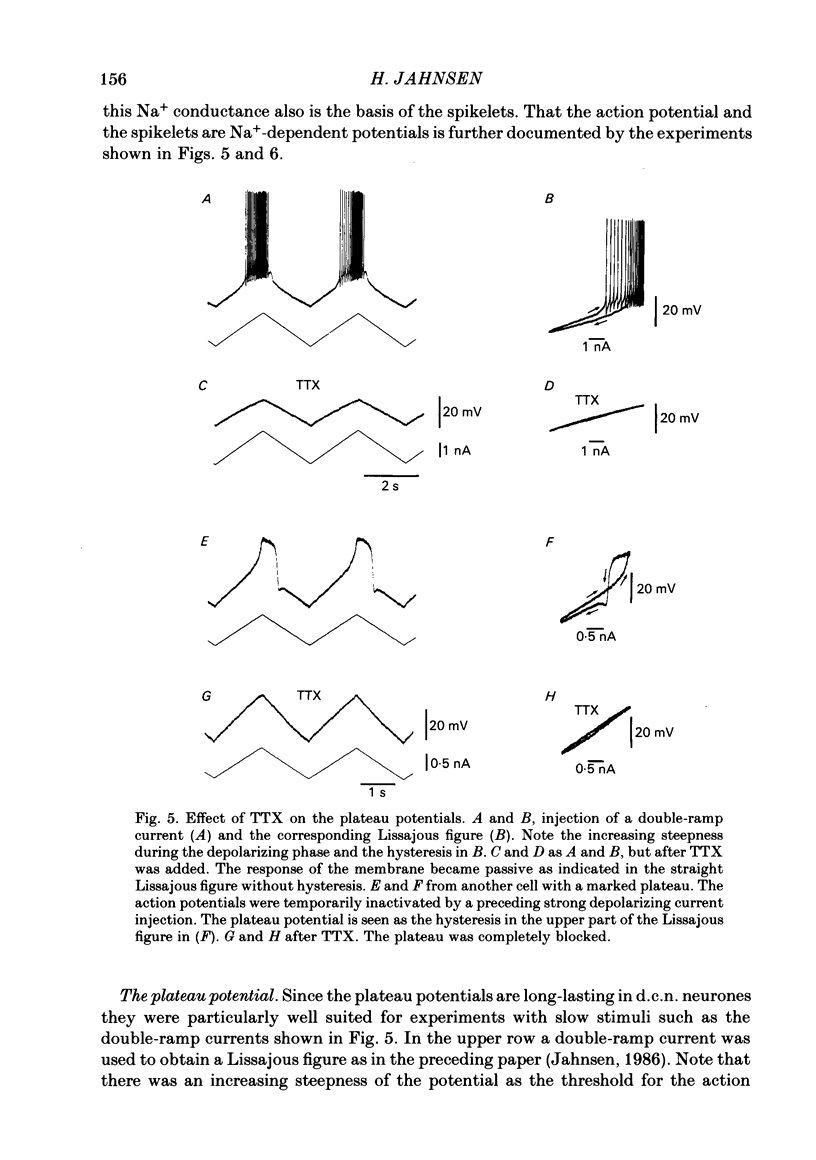
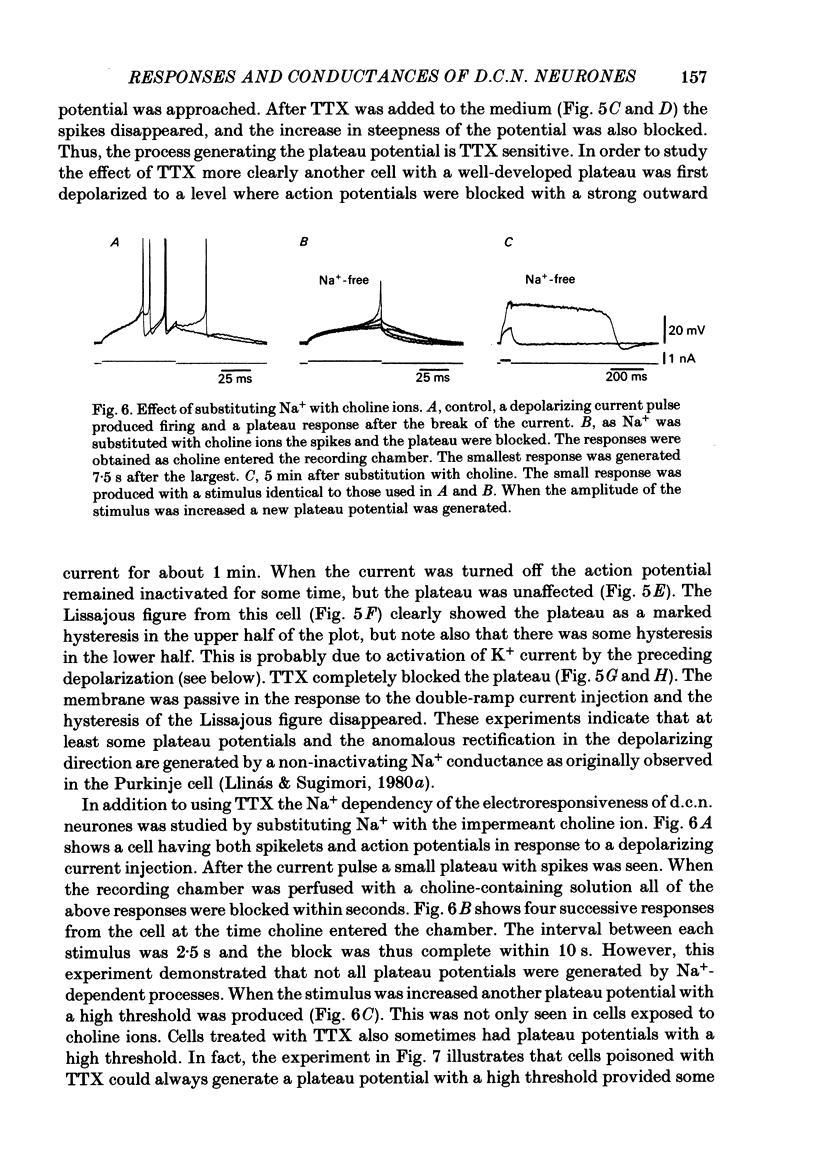
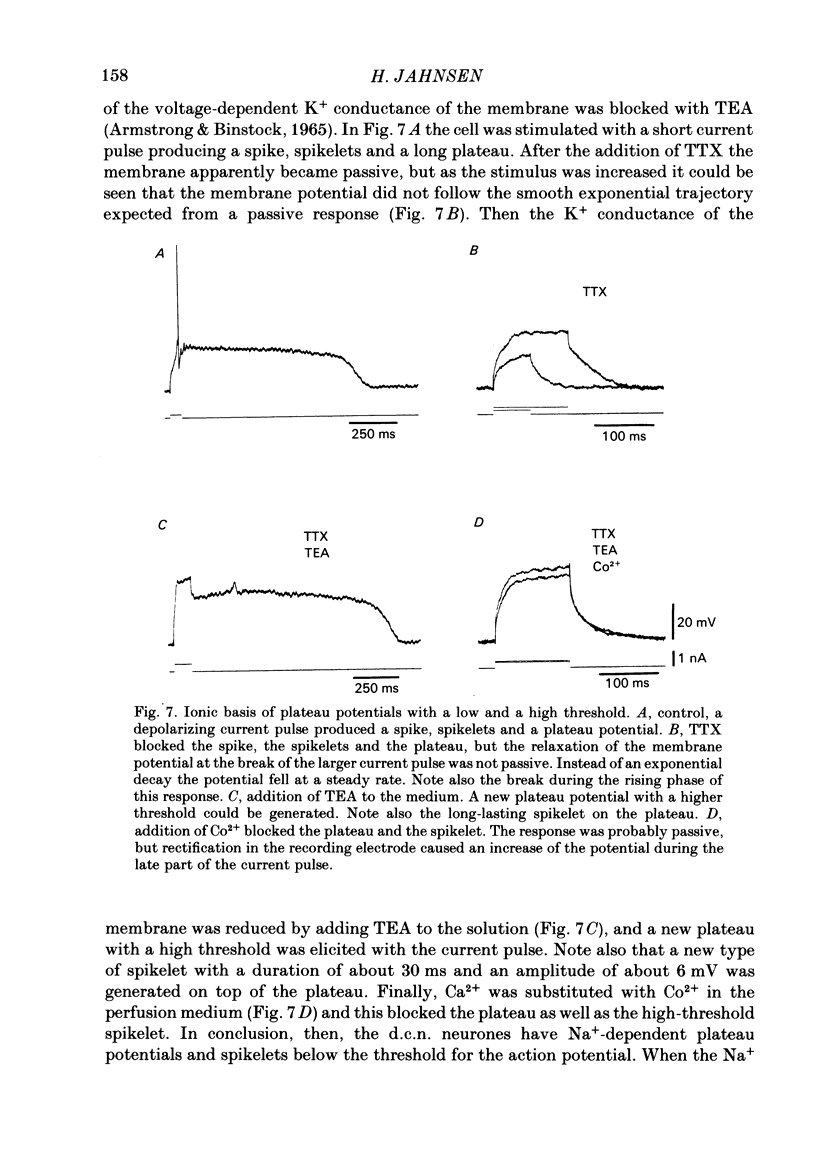
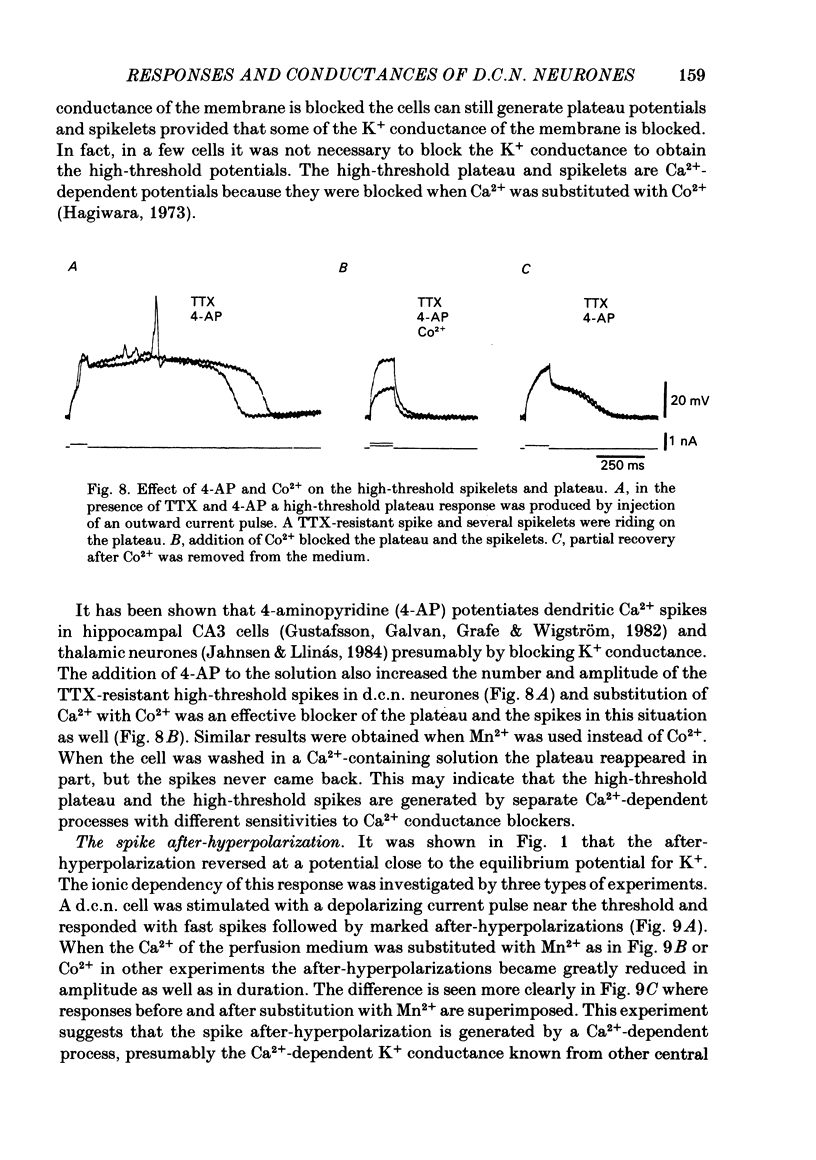
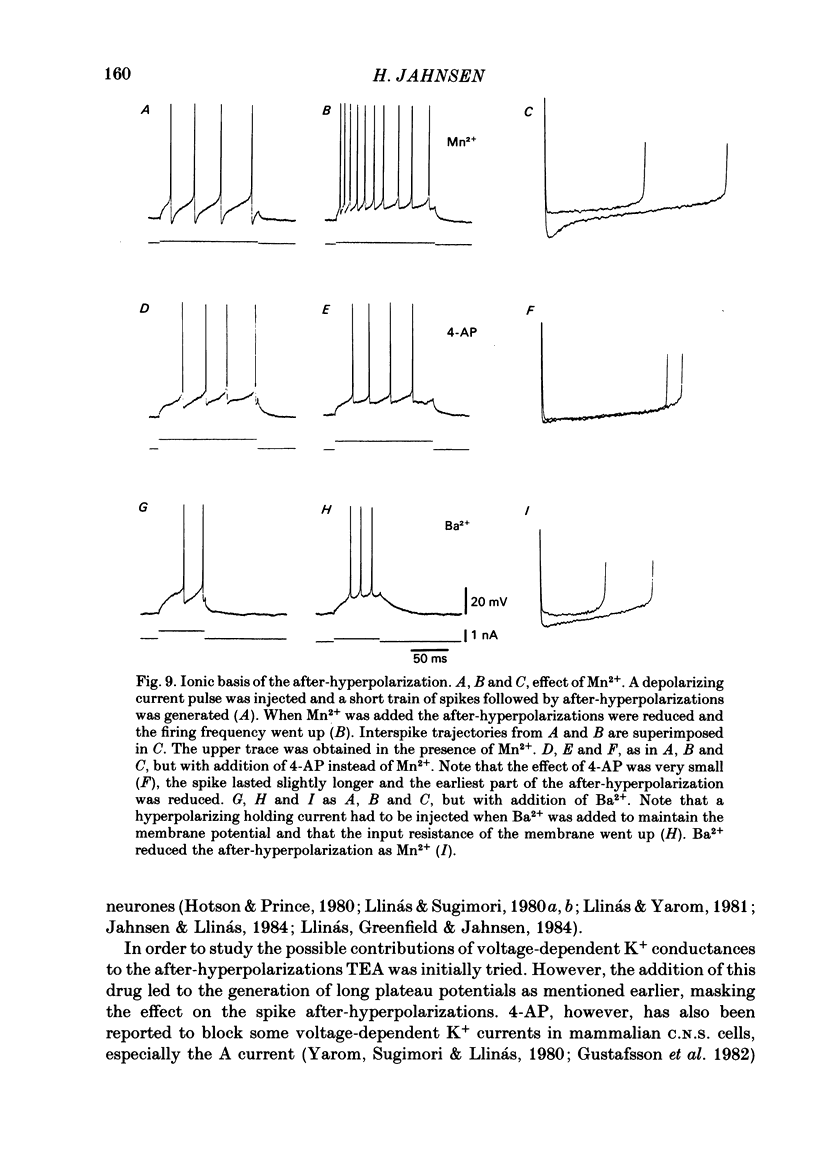
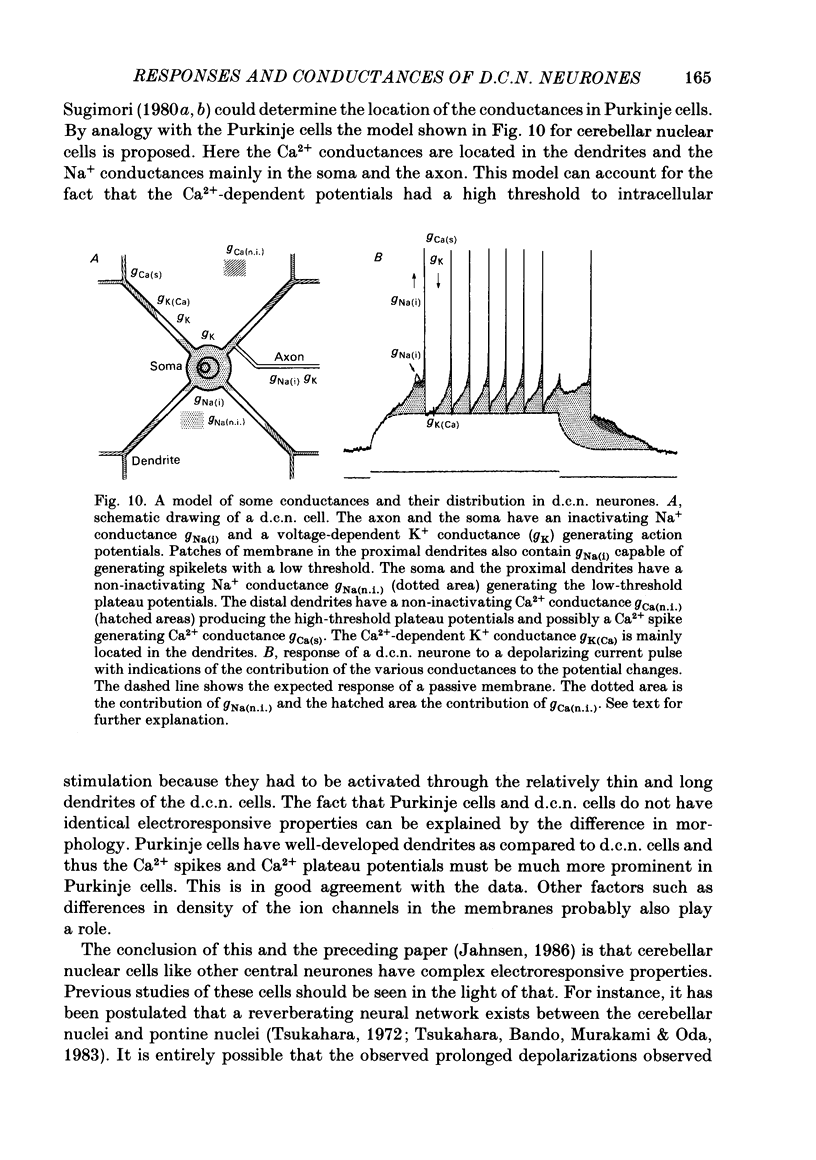
Abstract
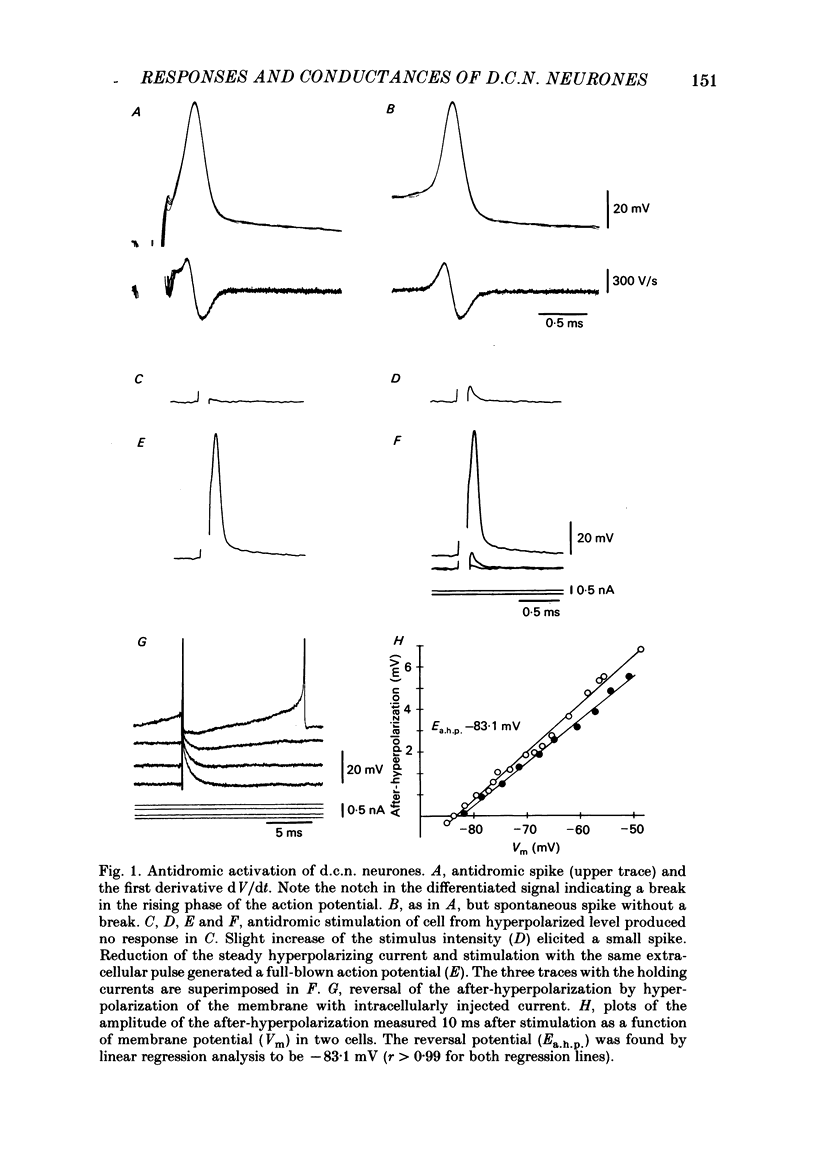
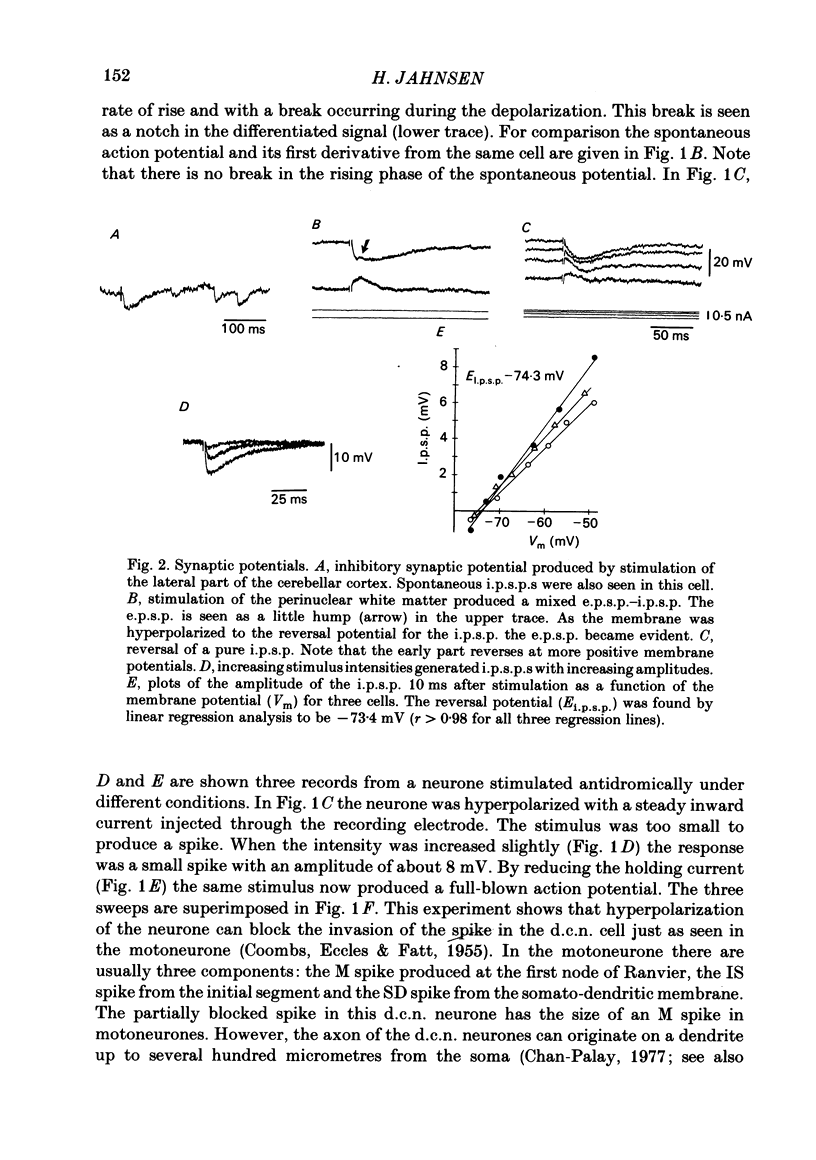
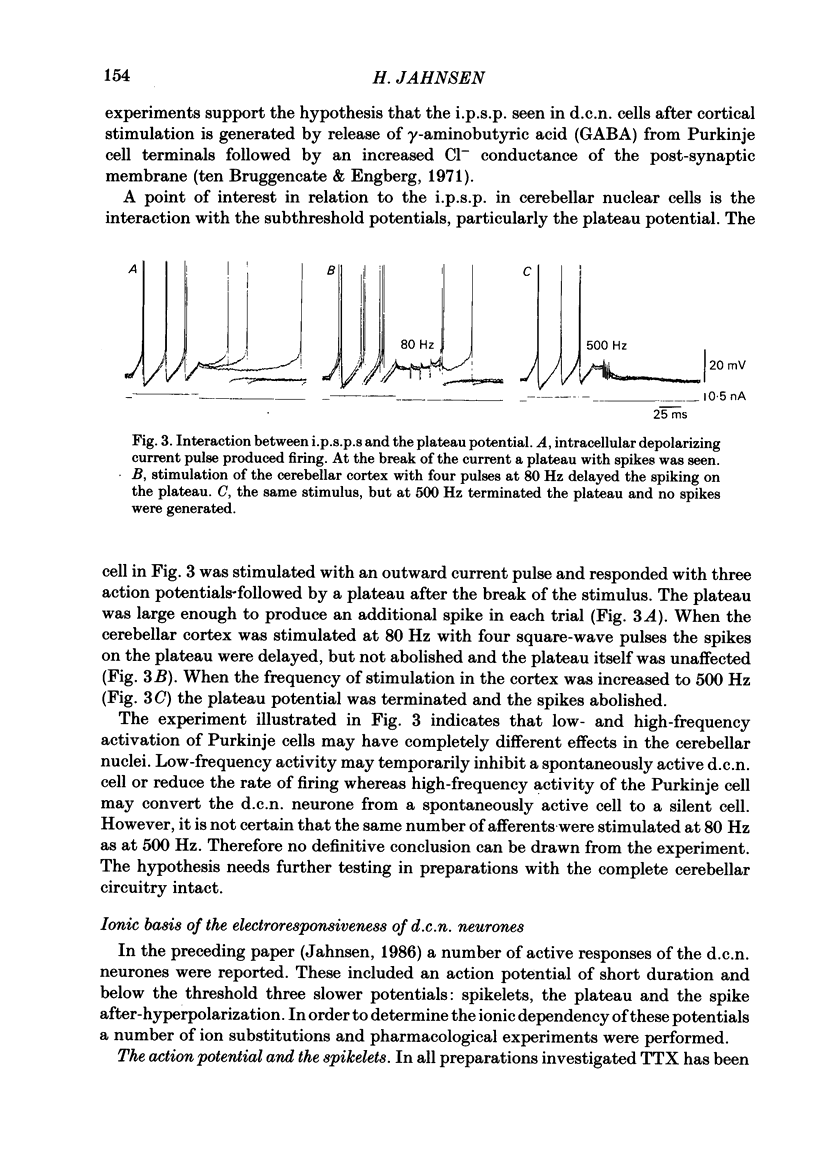
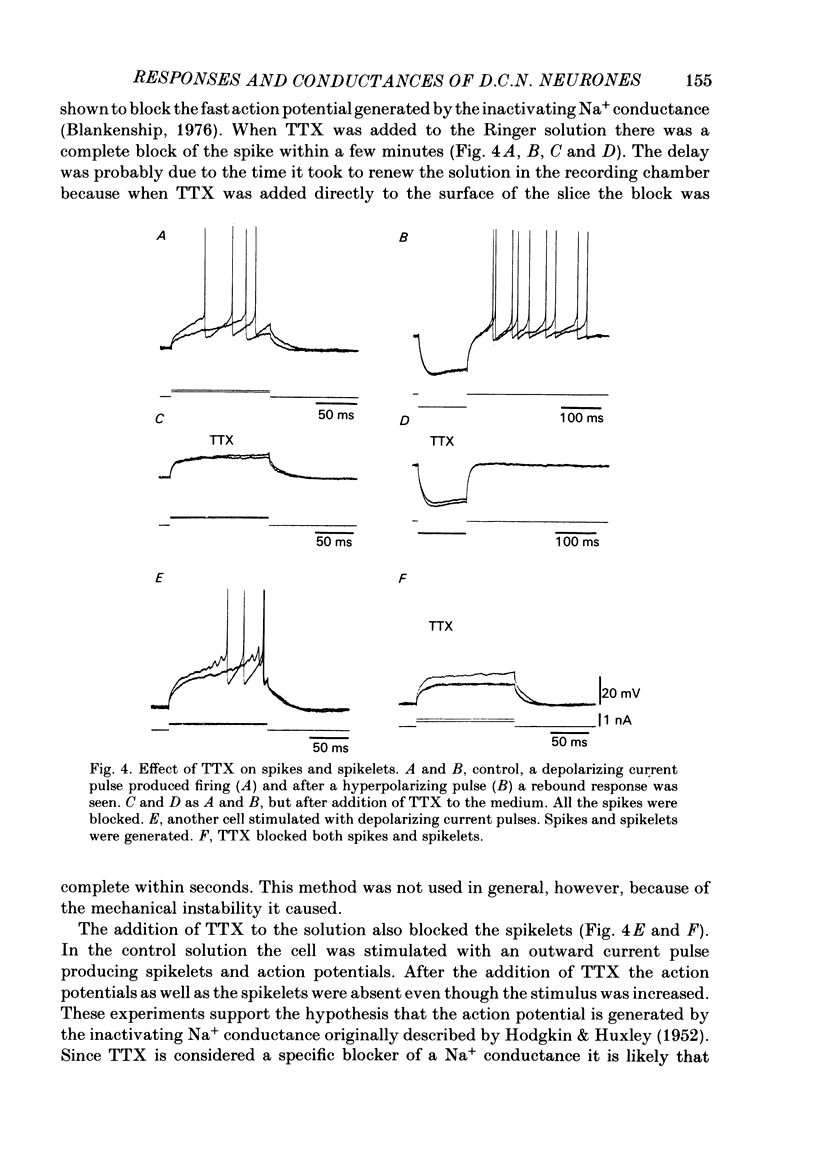
The responses of cerebellar nuclear cells to extracellular stimulation in a slice preparation were studied and the ionic basis of their electroresponsiveness was investigated with blockers of membrane conductances and with ion substitutions in the extracellular medium. The cells could be activated antidromically from the cerebellar cortex and the white matter surrounding the nuclei. The dominating response to orthodromic stimulation was an inhibitory synaptic potential presumably produced by activation of Purkinje cell fibres. The action potentials and the subthreshold spikelets were shown to be Na+ dependent and are presumably generated by a voltage-dependent inactivating Na+ conductance. Plateau potentials with a low threshold were also Na+ dependent, but these long-lasting potentials are probably produced by activation of a voltage-dependent non-inactivating Na+ conductance. Plateau potentials with a high threshold and high-threshold spikelets were Ca2+ dependent and seem to be generated by non-inactivating and possibly inactivating Ca2+ conductances. The spike after-hyperpolarizations had an early voltage-dependent K+ component and a late Ca2+-dependent K+ component. They are therefore produced by voltage-sensitive and Ca2+-dependent K+ conductances. By analogy with the distribution of conductances in Purkinje cells it is proposed that the Na+ conductances are mainly located in the somatic and axonal membrane and that the Ca2+ conductances are located in the dendrites. The functional implications of the complex electroresponsive properties of cerebellar nuclear cells are discussed.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG C. M., BINSTOCK L. ANOMALOUS RECTIFICATION IN THE SQUID GIANT AXON INJECTED WITH TETRAETHYLAMMONIUM CHLORIDE. J Gen Physiol. 1965 May;48:859–872. doi: 10.1085/jgp.48.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J., Bayer S. A. Embryonic development of the rat cerebellum. I. Delineation of the cerebellar primordium and early cell movements. J Comp Neurol. 1985 Jan 1;231(1):1–26. doi: 10.1002/cne.902310103. [DOI] [PubMed] [Google Scholar]
- Altman J., Bayer S. A. Prenatal development of the cerebellar system in the rat. I. Cytogenesis and histogenesis of the deep nuclei and the cortex of the cerebellum. J Comp Neurol. 1978 May 1;179(1):23–48. doi: 10.1002/cne.901790104. [DOI] [PubMed] [Google Scholar]
- Blankenship J. E. Tetrodotoxin: from poison to powerful tool. Perspect Biol Med. 1976 Summer;19(4):509–526. doi: 10.1353/pbm.1976.0071. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Griffith W. H. Persistent slow inward calcium current in voltage-clamped hippocampal neurones of the guinea-pig. J Physiol. 1983 Apr;337:303–320. doi: 10.1113/jphysiol.1983.sp014625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., ECCLES J. C., FATT P. The electrical properties of the motoneurone membrane. J Physiol. 1955 Nov 28;130(2):291–325. doi: 10.1113/jphysiol.1955.sp005411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell N. C., Ekerot C. F., Hesslow G. Interaction between responses in Purkinje cells evoked by climbing fibre impulses and parallel fibre volleys in the cat. J Physiol. 1983 Jul;340:225–238. doi: 10.1113/jphysiol.1983.sp014760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell N. C., Ekerot C. F., Hesslow G., Oscarsson O. Dendritic plateau potentials evoked in Purkinje cells by parallel fibre volleys in the cat. J Physiol. 1983 Jul;340:209–223. doi: 10.1113/jphysiol.1983.sp014759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. The action of antidromic impulses on the cerebellar Purkinje cells. J Physiol. 1966 Jan;182(2):316–345. doi: 10.1113/jphysiol.1966.sp007826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. The excitatory synaptic action of climbing fibres on the Purkinje cells of the cerebellum. J Physiol. 1966 Jan;182(2):268–296. doi: 10.1113/jphysiol.1966.sp007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Lux H. D. A voltage-sensitive persistent calcium conductance in neuronal somata of Helix. J Physiol. 1976 Jan;254(1):129–151. doi: 10.1113/jphysiol.1976.sp011225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan M., Grafe P., ten Bruggencate G. Convulsant actions of 4-aminopyridine on the guinea-pig olfactory cortex slice. Brain Res. 1982 Jun 3;241(1):75–86. doi: 10.1016/0006-8993(82)91230-6. [DOI] [PubMed] [Google Scholar]
- Gustafsson B., Galvan M., Grafe P., Wigström H. A transient outward current in a mammalian central neurone blocked by 4-aminopyridine. Nature. 1982 Sep 16;299(5880):252–254. doi: 10.1038/299252a0. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S. Ca spike. Adv Biophys. 1973;4:71–102. [PubMed] [Google Scholar]
- Halliwell J. V., Adams P. R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982 Oct 28;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Hess D. T. Cerebellar nucleo-cortical neurons projecting to the vermis of lobule VII in the rat. Brain Res. 1982 Sep 30;248(2):361–366. doi: 10.1016/0006-8993(82)90595-9. [DOI] [PubMed] [Google Scholar]
- Hotson J. R., Prince D. A. A calcium-activated hyperpolarization follows repetitive firing in hippocampal neurons. J Neurophysiol. 1980 Feb;43(2):409–419. doi: 10.1152/jn.1980.43.2.409. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J., Hultborn H., Jespersen B., Kiehn O. Intrinsic membrane properties causing a bistable behaviour of alpha-motoneurones. Exp Brain Res. 1984;55(2):391–394. doi: 10.1007/BF00237290. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J., Yarom Y. Intrinsic control of electroresponsive properties of transplanted mammalian brain neurons. Brain Res. 1985 Jun 3;335(2):372–376. doi: 10.1016/0006-8993(85)90497-4. [DOI] [PubMed] [Google Scholar]
- Ito M., Nisimaru N., Shibuki K. Destruction of inferior olive induces rapid depression in synaptic action of cerebellar Purkinje cells. Nature. 1979 Feb 15;277(5697):568–569. doi: 10.1038/277568a0. [DOI] [PubMed] [Google Scholar]
- Ito M., Orlov I., Shimoyama I. Reduction of the cerebellar stimulus effect on rat Deiters neurons after chemical destruction of the inferior olive. Exp Brain Res. 1978 Sep 15;33(1):143–145. doi: 10.1007/BF00238802. [DOI] [PubMed] [Google Scholar]
- Ito M., Yoshida M., Obata K., Kawai N., Udo M. Inhibitory control of intracerebellar nuclei by the purkinje cell axons. Exp Brain Res. 1970;10(1):64–80. doi: 10.1007/BF00340519. [DOI] [PubMed] [Google Scholar]
- Ito M., Yoshida M. The origin of cerebral-induced inhibition of Deiters neurones. I. Monosynaptic initiation of the inhibitory postsynaptic potentials. Exp Brain Res. 1966;2(4):330–349. doi: 10.1007/BF00234779. [DOI] [PubMed] [Google Scholar]
- Jahnsen H. Electrophysiological characteristics of neurones in the guinea-pig deep cerebellar nuclei in vitro. J Physiol. 1986 Mar;372:129–147. doi: 10.1113/jphysiol.1986.sp016001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnsen H., Llinás R. Ionic basis for the electro-responsiveness and oscillatory properties of guinea-pig thalamic neurones in vitro. J Physiol. 1984 Apr;349:227–247. doi: 10.1113/jphysiol.1984.sp015154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D., Hablitz J. J., Wilson W. A. Voltage clamp discloses slow inward current in hippocampal burst-firing neurones. Nature. 1980 Jul 24;286(5771):391–393. doi: 10.1038/286391a0. [DOI] [PubMed] [Google Scholar]
- Llinás R., Greenfield S. A., Jahnsen H. Electrophysiology of pars compacta cells in the in vitro substantia nigra--a possible mechanism for dendritic release. Brain Res. 1984 Feb 27;294(1):127–132. doi: 10.1016/0006-8993(84)91316-7. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol. 1980 Aug;305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980 Aug;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Yarom Y. Electrophysiology of mammalian inferior olivary neurones in vitro. Different types of voltage-dependent ionic conductances. J Physiol. 1981 Jun;315:549–567. doi: 10.1113/jphysiol.1981.sp013763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVicar B. A., Dudek F. E. Electrotonic coupling between pyramidal cells: a direct demonstration in rat hippocampal slices. Science. 1981 Aug 14;213(4509):782–785. doi: 10.1126/science.6266013. [DOI] [PubMed] [Google Scholar]
- Meech R. W. Calcium-dependent potassium activation in nervous tissues. Annu Rev Biophys Bioeng. 1978;7:1–18. doi: 10.1146/annurev.bb.07.060178.000245. [DOI] [PubMed] [Google Scholar]
- Montarolo P. G., Raschi F., Strata P. Are the climbing fibres essential for the Purkinje cell inhibitory action? Exp Brain Res. 1981;42(2):215–218. doi: 10.1007/BF00236909. [DOI] [PubMed] [Google Scholar]
- Murase K., Randić M. Electrophysiological properties of rat spinal dorsal horn neurones in vitro: calcium-dependent action potentials. J Physiol. 1983 Jan;334:141–153. doi: 10.1113/jphysiol.1983.sp014485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata K., Takeda K., Shinozaki H. Further study on pharmacological properties of the cerebellar-induced inhibition of deiters neurones. Exp Brain Res. 1970 Nov 26;11(4):327–342. doi: 10.1007/BF00237907. [DOI] [PubMed] [Google Scholar]
- Rogawski M. A., Barker J. L. Effects of 4-aminopyridine on calcium action potentials and calcium current under voltage clamp in spinal neurons. Brain Res. 1983 Nov 28;280(1):180–185. doi: 10.1016/0006-8993(83)91190-3. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Slawsky M. Probable calcium spikes in hippocampal neurons. Brain Res. 1977 Oct 21;135(1):157–161. doi: 10.1016/0006-8993(77)91060-5. [DOI] [PubMed] [Google Scholar]
- Schwindt P., Crill W. E. A persistent negative resistance in cat lumbar motoneurons. Brain Res. 1977 Jan 14;120(1):173–178. doi: 10.1016/0006-8993(77)90510-8. [DOI] [PubMed] [Google Scholar]
- Stafstrom C. E., Schwindt P. C., Crill W. E. Negative slope conductance due to a persistent subthreshold sodium current in cat neocortical neurons in vitro. Brain Res. 1982 Mar 18;236(1):221–226. doi: 10.1016/0006-8993(82)90050-6. [DOI] [PubMed] [Google Scholar]
- Tolbert D. L., Bantli H., Bloedel J. R. Anatomical and physiological evidence for a cerebellar nucleo-cortical projection in the cat. Neuroscience. 1976 Jun;1(3):205–217. doi: 10.1016/0306-4522(76)90078-6. [DOI] [PubMed] [Google Scholar]
- Tsukahara N., Bando T., Murakami F., Oda Y. Properties of cerebello-precerebellar reverberating circuits. Brain Res. 1983 Sep 12;274(2):249–259. doi: 10.1016/0006-8993(83)90702-3. [DOI] [PubMed] [Google Scholar]
- Tsukahara N. The properties of the cerebello-pontine reverberating circuit. Brain Res. 1972 May 12;40(1):67–71. doi: 10.1016/0006-8993(72)90108-4. [DOI] [PubMed] [Google Scholar]
- WERMAN R., GRUNDFEST H. Graded and all-or-none electrogenesis in arthropod muscle. II. The effects of alkali-earth and onium ions on lobster muscle fibers. J Gen Physiol. 1961 May;44:997–1027. doi: 10.1085/jgp.44.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. T., North R. A., Shefner S. A., Nishi S., Egan T. M. Membrane properties of rat locus coeruleus neurones. Neuroscience. 1984 Sep;13(1):137–156. doi: 10.1016/0306-4522(84)90265-3. [DOI] [PubMed] [Google Scholar]
- ten Bruggencate G., Engberg I. Iontophoretic studies in Deiters' nucleus of the inhibitory actions of GABA and related amino acids and the interactions of strychnine and picrotoxin. Brain Res. 1971 Feb 5;25(3):431–448. doi: 10.1016/0006-8993(71)90453-7. [DOI] [PubMed] [Google Scholar]


