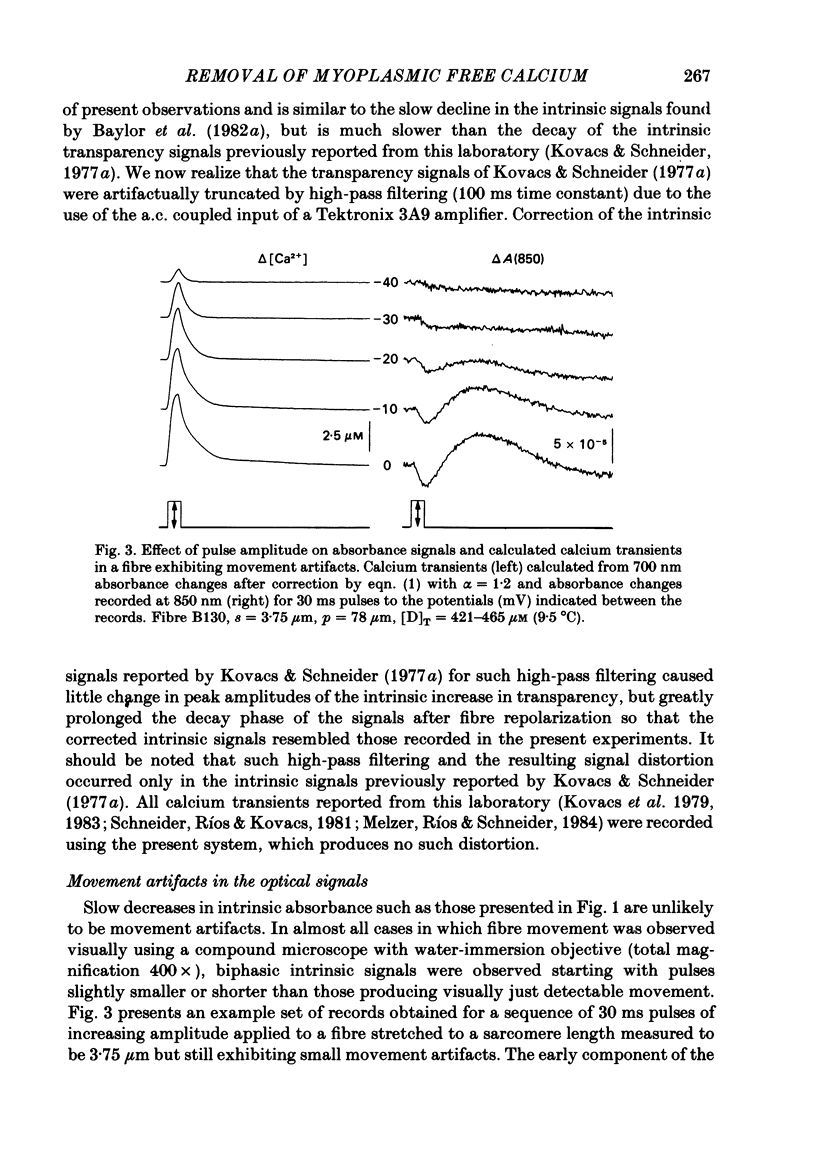
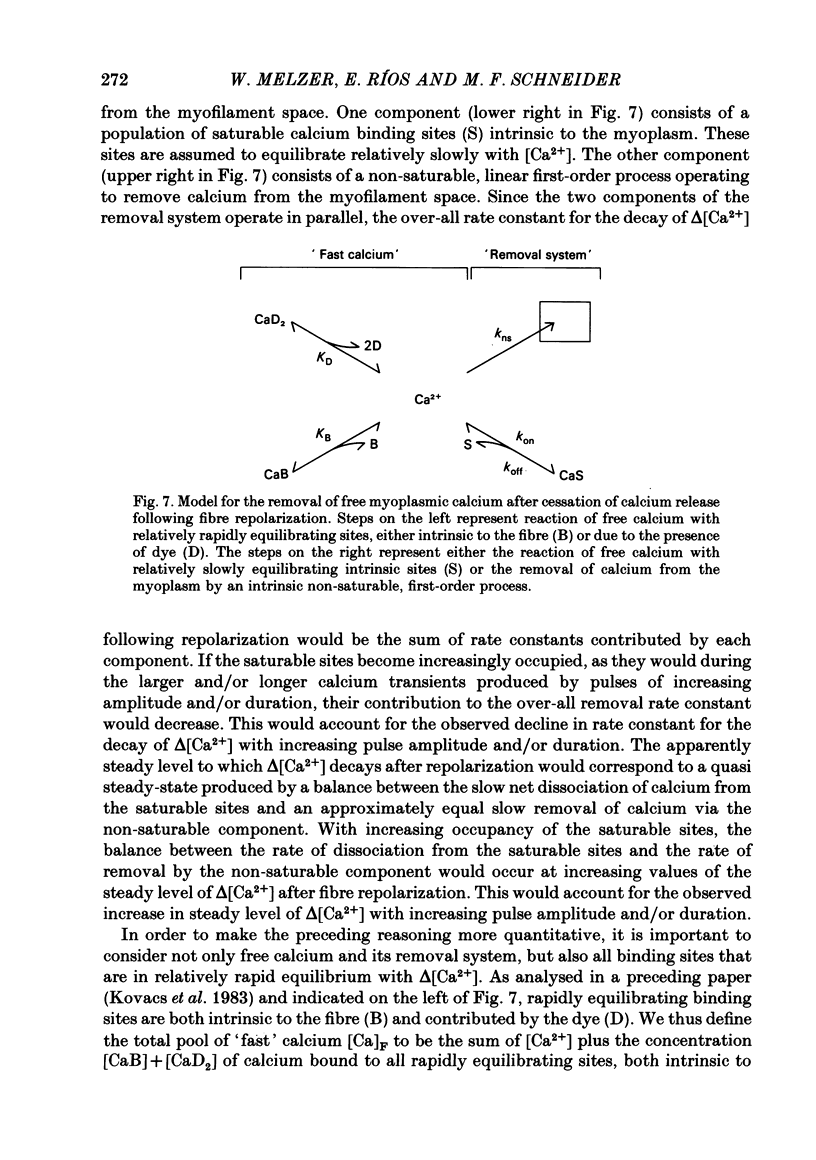
Abstract
Transient changes in intracellular free calcium concentration (delta [Ca2+]) in response to pulse depolarizations were monitored in isolated segments of single frog skeletal muscle fibres cut at both ends and voltage clamped at a holding potential of -90 mV in a double-Vaseline-gap chamber. Calcium transients were monitored optically using the metallochromic indicator dye Antipyrylazo III (APIII), which entered the fibre by diffusion from the solution applied to the cut ends. Optical artifacts due to fibre movement were minimized or eliminated by stretching the fibres to sarcomere lengths at which there was little or no overlap of thick and thin contractile filaments. Remaining movement-independent optical changes intrinsic to the fibre and unrelated to the dye were monitored at 850 nm, where free and dye-bound APIII have no absorbance. These 850 nm signals scaled by lambda -1.2 were used to remove intrinsic components from the signals at 700 or 720 nm, wave-lengths at which the APIII absorbance increases when calcium is bound. The corrected 700 or 720 nm signals were used to calculate delta [Ca2+]. The decay of delta [Ca2+] following fibre repolarization at the termination of a depolarizing pulse was well described by a single exponential plus a constant. The exponential rate constant for the decay of delta [Ca2+] decreased and the final 'steady' level that delta [Ca2+] appeared to be approaching increased with increasing amplitude and/or duration of the depolarizing pulse. Both the decreasing decay rate and the build up of the 'steady' level can be accounted for using a two-component model for the removal of free calcium from the myoplasm. One component consists of a set number of a single type of saturable calcium binding site in the myoplasm. The second component is a non-saturable, first-order uptake mechanism operating in parallel with the saturable binding sites. The removal model parameter values were adjusted to fit simultaneously the decay of delta [Ca2+] after pulses of various amplitudes and durations in a given fibre. The basic procedure was to track delta [Ca2+] during each pulse when an undetermined calcium release was occurring, but to calculate the decay of delta [Ca2+] starting 14 ms after repolarization when release was assumed to be negligible. After appropriate selection of parameter values, the model reproduced most aspects of the decay of delta [Ca2+].(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Blinks J. R., Prendergast F. G. Aequorin luminescence: relation of light emission to calcium concentration--a calcium-independent component. Science. 1977 Mar 11;195(4282):996–998. doi: 10.1126/science.841325. [DOI] [PubMed] [Google Scholar]
- Ashley C. C. Calcium ion regulation in barnacle muscle fibers and its relation to force development. Ann N Y Acad Sci. 1978 Apr 28;307:308–329. doi: 10.1111/j.1749-6632.1978.tb41959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Optical measurements of intracellular pH and magnesium in frog skeletal muscle fibres. J Physiol. 1982 Oct;331:105–137. doi: 10.1113/jphysiol.1982.sp014367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Sarcoplasmic reticulum calcium release in frog skeletal muscle fibres estimated from Arsenazo III calcium transients. J Physiol. 1983 Nov;344:625–666. doi: 10.1113/jphysiol.1983.sp014959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Use of metallochromic dyes to measure changes in myoplasmic calcium during activity in frog skeletal muscle fibres. J Physiol. 1982 Oct;331:139–177. doi: 10.1113/jphysiol.1982.sp014368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R., Rüdel R., Taylor S. R. Calcium transients in isolated amphibian skeletal muscle fibres: detection with aequorin. J Physiol. 1978 Apr;277:291–323. doi: 10.1113/jphysiol.1978.sp012273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M. B., Allen D. G. Model of calcium movements during activation in the sarcomere of frog skeletal muscle. Biophys J. 1984 May;45(5):913–925. doi: 10.1016/S0006-3495(84)84238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C. The effect of caffeine and tetracaine on the time course of potassium contractures of single muscle fibres. J Physiol. 1976 Feb;255(1):191–207. doi: 10.1113/jphysiol.1976.sp011275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland W. W. The statistical analysis of enzyme kinetic data. Adv Enzymol Relat Areas Mol Biol. 1967;29:1–32. doi: 10.1002/9780470122747.ch1. [DOI] [PubMed] [Google Scholar]
- Gillis J. M., Thomason D., Lefèvre J., Kretsinger R. H. Parvalbumins and muscle relaxation: a computer simulation study. J Muscle Res Cell Motil. 1982 Dec;3(4):377–398. doi: 10.1007/BF00712090. [DOI] [PubMed] [Google Scholar]
- Harafuji H., Ogawa Y. Re-examination of the apparent binding constant of ethylene glycol bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid with calcium around neutral pH. J Biochem. 1980 May;87(5):1305–1312. doi: 10.1093/oxfordjournals.jbchem.a132868. [DOI] [PubMed] [Google Scholar]
- Horowicz P., Schneider M. F. Membrane charge movement in contracting and non-contracting skeletal muscle fibres. J Physiol. 1981 May;314:565–593. doi: 10.1113/jphysiol.1981.sp013725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs L., Rios E., Schneider M. F. Measurement and modification of free calcium transients in frog skeletal muscle fibres by a metallochromic indicator dye. J Physiol. 1983 Oct;343:161–196. doi: 10.1113/jphysiol.1983.sp014887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács L., Ríos E., Schneider M. F. Calcium transients and intramembrane charge movement in skeletal muscle fibres. Nature. 1979 May 31;279(5712):391–396. doi: 10.1038/279391a0. [DOI] [PubMed] [Google Scholar]
- Kovács L., Schneider M. F. Contractile activation by voltage clamp depolarization of cut skeletal muscle fibres. J Physiol. 1978 Apr;277:483–506. doi: 10.1113/jphysiol.1978.sp012286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács L., Schneider M. F. Increased optical transparency associated with excitation--contraction coupling in voltage-clamped cut skeletal muscle fibres. Nature. 1977 Feb 10;265(5594):556–560. doi: 10.1038/265556a0. [DOI] [PubMed] [Google Scholar]
- Melzer W., Rios E., Schneider M. F. Time course of calcium release and removal in skeletal muscle fibers. Biophys J. 1984 Mar;45(3):637–641. doi: 10.1016/S0006-3495(84)84203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Parker I., Zhu P. H. Calcium transients evoked by action potentials in frog twitch muscle fibres. J Physiol. 1982 Dec;333:655–679. doi: 10.1113/jphysiol.1982.sp014474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter J. D., Gergely J. The calcium and magnesium binding sites on troponin and their role in the regulation of myofibrillar adenosine triphosphatase. J Biol Chem. 1975 Jun 25;250(12):4628–4633. [PubMed] [Google Scholar]
- Robertson S. P., Johnson J. D., Potter J. D. The time-course of Ca2+ exchange with calmodulin, troponin, parvalbumin, and myosin in response to transient increases in Ca2+. Biophys J. 1981 Jun;34(3):559–569. doi: 10.1016/S0006-3495(81)84868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. D., Liesegang G. W., Berger R. L., Czerlinski G., Podolsky R. J. A stopped-flow investigation of calcium ion binding by ethylene glycol bis(beta-aminoethyl ether)-N,N'-tetraacetic acid. Anal Biochem. 1984 Nov 15;143(1):188–195. doi: 10.1016/0003-2697(84)90575-x. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Gonzalez-Serratos H. G., Shuman H., McClellan G., Somlyo A. P. Calcium release and ionic changes in the sarcoplasmic reticulum of tetanized muscle: an electron-probe study. J Cell Biol. 1981 Sep;90(3):577–594. doi: 10.1083/jcb.90.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]


