Abstract
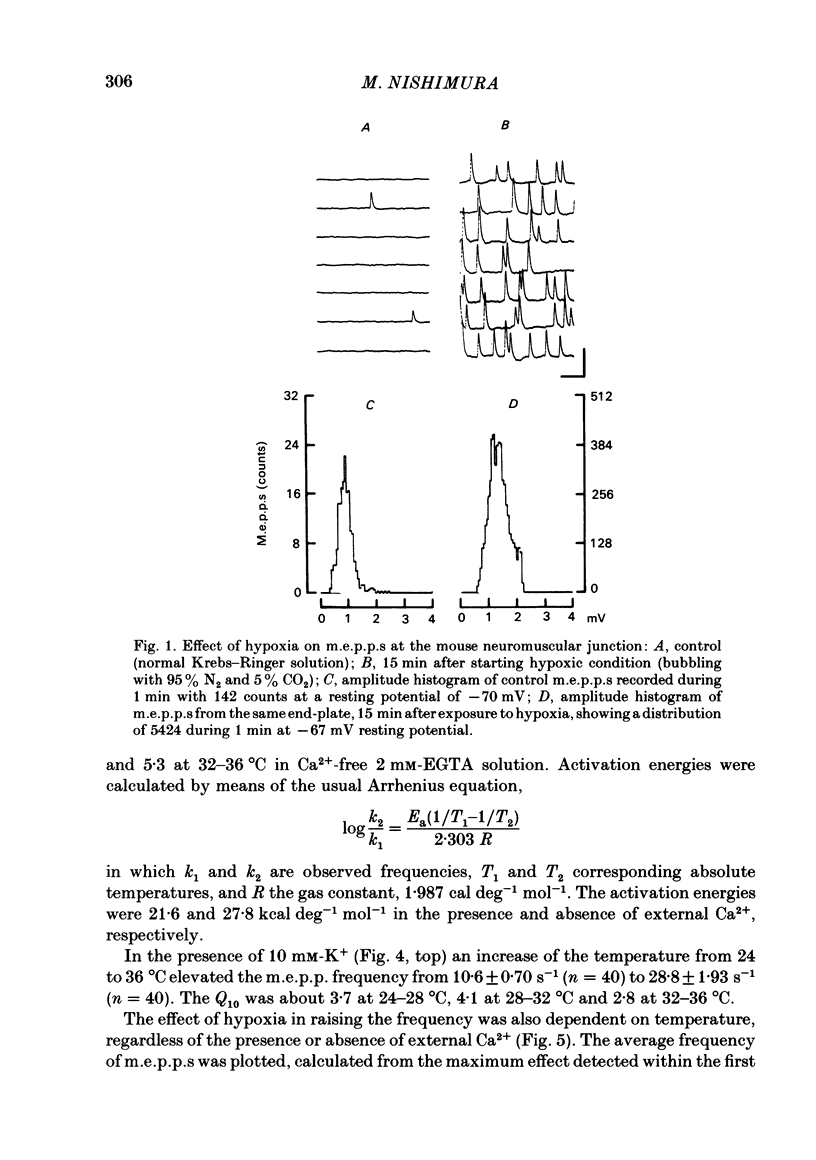
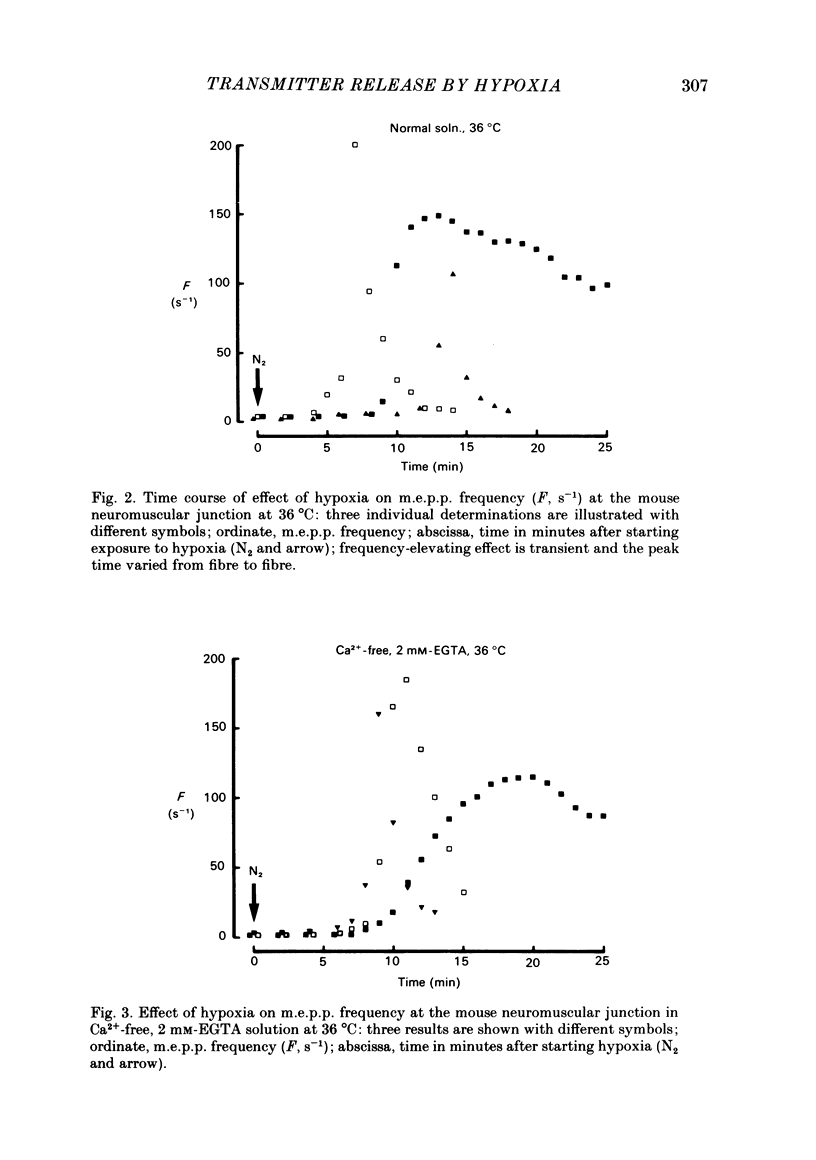
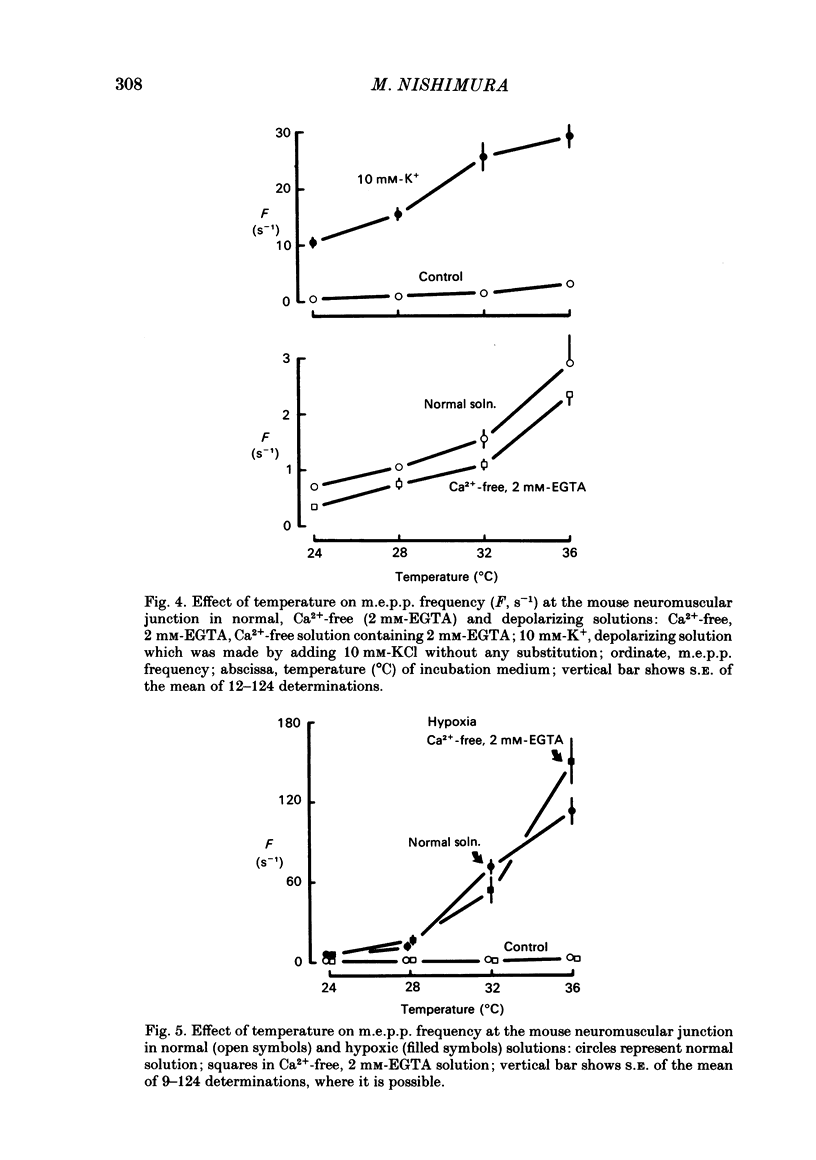
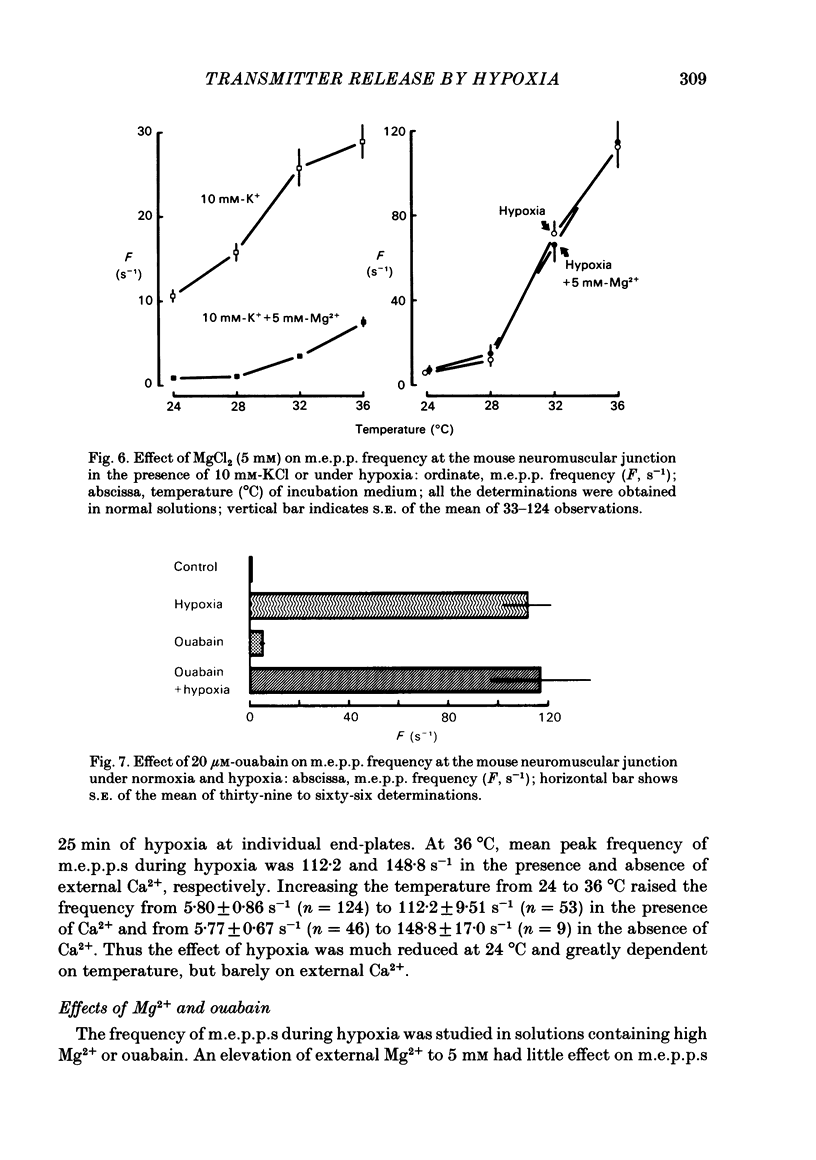
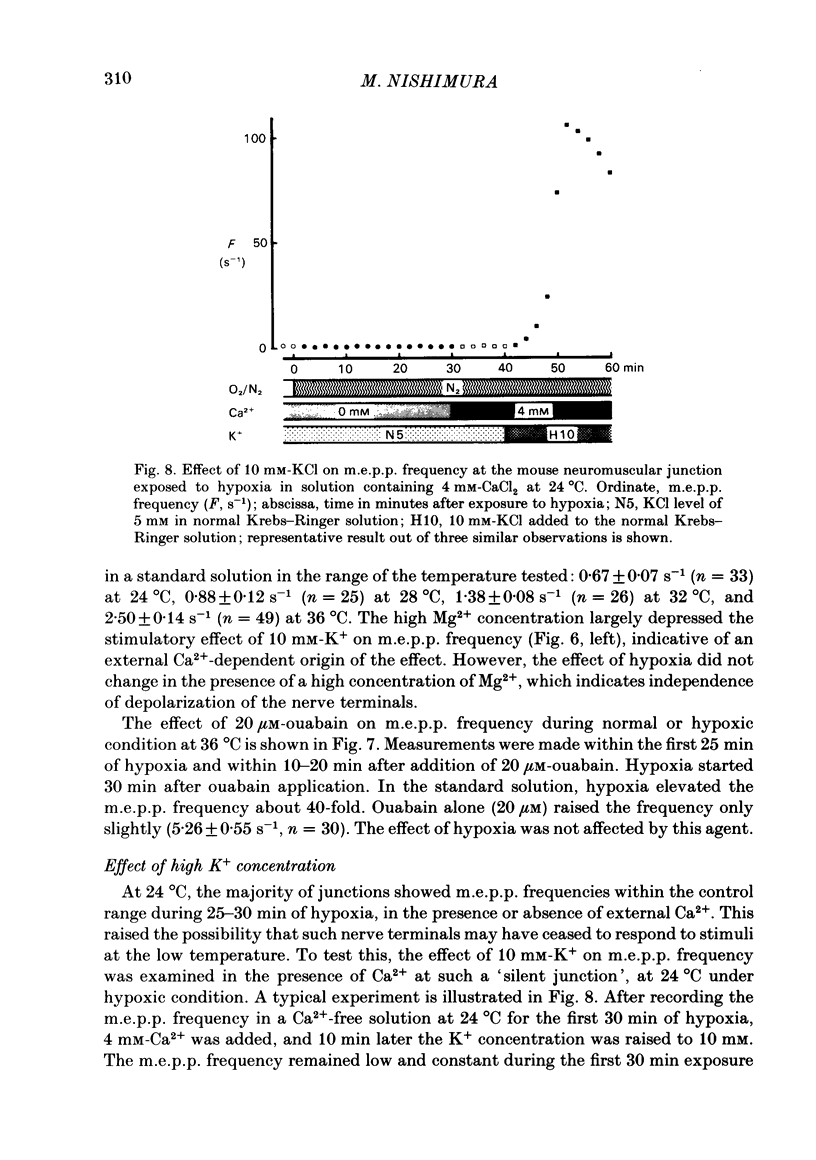
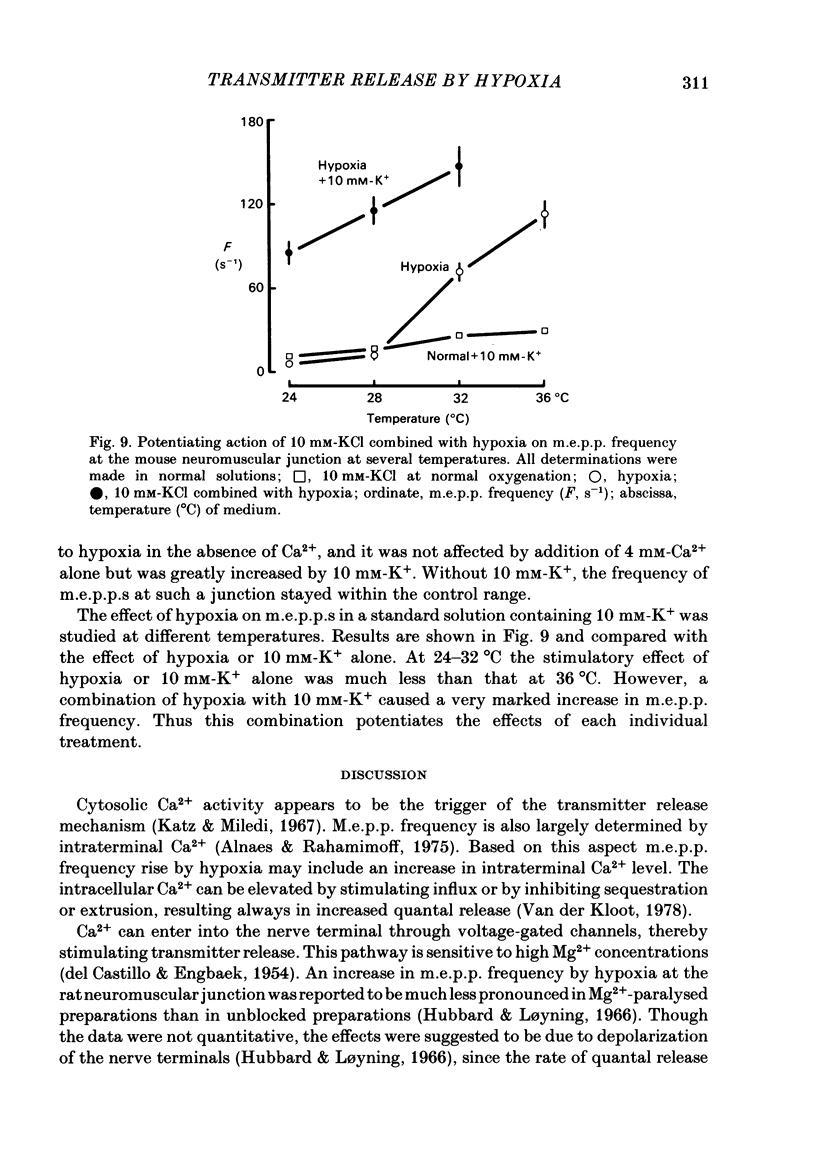
To test a possibility that the functional buffering of intracellular Ca2+ plays a primary role in the enhancement of spontaneous transmitter release during hypoxia, the frequency of miniature end-plate potentials (m.e.p.p.s) was examined under several conditions. At 36 degrees C, hypoxia (bubbling with 95% N2 and 5% CO2) increased the average frequency of m.e.p.p.s from about 3 s-1 to 100 s-1 or more, in a standard Krebs-Ringer solution. This effect declined with a decrease in the temperature and was much reduced at 24 degrees C. Removal of external Ca2+ (addition of 2 mM-EGTA), increase of Mg2+ levels to 5 mM, and treatment with 20 microM-ouabain, which gave a slight increase, did not reduce the rise in m.e.p.p. frequency during hypoxia. Pre-incubation of the tissue in a solution containing 10 mM-KCl at 24-32 degrees C and its subsequent exposure to hypoxia caused a very marked increase in m.e.p.p. frequency, while incubation in 10 mM-KCl alone caused a small rise in the frequency. These data indicate that this combination potentiates the individual effects of each treatment. These experiments suggest that the hypoxia-induced increase in spontaneous transmitter release is primarily due to an increase in intracellular Ca2+ levels, probably because of inhibition of mechanisms which control buffering and extrusion of intracellular Ca2+. The release and influx mechanisms which elevate intraterminal Ca2+ may also be involved passively in the effect of hypoxia.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alnaes E., Rahamimoff R. On the role of mitochondria in transmitter release from motor nerve terminals. J Physiol. 1975 Jun;248(2):285–306. doi: 10.1113/jphysiol.1975.sp010974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYD I. A., MARTIN A. R. Spontaneous subthreshold activity at mammalian neural muscular junctions. J Physiol. 1956 Apr 27;132(1):61–73. doi: 10.1113/jphysiol.1956.sp005502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F. Transport and metabolism of calcium ions in nerve. Prog Biophys Mol Biol. 1972;24:177–223. doi: 10.1016/0079-6107(72)90007-7. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Ratzlaff R. W., Schweitzer E. S. Control of intracellular calcium in presynaptic nerve terminals. Fed Proc. 1980 Aug;39(10):2790–2795. [PubMed] [Google Scholar]
- DEL CASTILLO J., ENGBAEK L. The nature of the neuromuscular block produced by magnesium. J Physiol. 1954 May 28;124(2):370–384. doi: 10.1113/jphysiol.1954.sp005114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. J., Statham H. E. Interacting effects of temperature and extracellular calcium on the spontaneous release of transmitter at the frog neuromuscular junction. J Physiol. 1977 Jun;268(2):319–333. doi: 10.1113/jphysiol.1977.sp011859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles R. M., Loyning Y., Oshima T. Effects of hypoxia on the monosynaptic reflex pathway in the cat spinal cord. J Neurophysiol. 1966 Mar;29(2):315–331. doi: 10.1152/jn.1966.29.2.315. [DOI] [PubMed] [Google Scholar]
- Elmqvist D., Feldman D. S. Calcium dependence of spontaneous acetylcholine release at mammalian motor nerve terminals. J Physiol. 1965 Dec;181(3):487–497. doi: 10.1113/jphysiol.1965.sp007777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- Hofmann W. W., Parsons R. L., Feigen G. A. Effects of temperature and drugs on mammalian motor nerve terminals. Am J Physiol. 1966 Jul;211(1):135–140. doi: 10.1152/ajplegacy.1966.211.1.135. [DOI] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. The effect of temperature change upon transmitter release, facilitation and post-tetanic potentiation. J Physiol. 1971 Aug;216(3):591–609. doi: 10.1113/jphysiol.1971.sp009542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Loyning Y. The effects of hypoxia on neuromuscular transmission in a mammalian preparation. J Physiol. 1966 Jul;185(1):205–223. doi: 10.1113/jphysiol.1966.sp007982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITO M., OSHIMA T. FURTHER STUDY ON THE ACTIVE TRANSPORT OF SODIUM ACROSS THE MOTONEURONAL MEMBRANE. Proc R Soc Lond B Biol Sci. 1964 Nov 17;161:132–141. doi: 10.1098/rspb.1964.0084. [DOI] [PubMed] [Google Scholar]
- JENKINSON D. H. The nature of the antagonism between calcium and magnesium ions at the neuromuscular junction. J Physiol. 1957 Oct 30;138(3):434–444. doi: 10.1113/jphysiol.1957.sp005860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin and neuromuscular transmission. Proc R Soc Lond B Biol Sci. 1967 Jan 31;167(1006):8–22. doi: 10.1098/rspb.1967.0010. [DOI] [PubMed] [Google Scholar]
- LILEY A. W. An investigation of spontaneous activity at the neuromuscular junction of the rat. J Physiol. 1956 Jun 28;132(3):650–666. doi: 10.1113/jphysiol.1956.sp005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M., Tsutsui I., Yagasaki O., Yanagiya I. Transmitter release at the mouse neuromuscular junction stimulated by cadmium ions. Arch Int Pharmacodyn Ther. 1984 Sep;271(1):106–121. [PubMed] [Google Scholar]
- SHANES A. M., BERMAN M. D. Kinetics of ion movement in the squid giant axon. J Gen Physiol. 1955 Nov 20;39(2):279–300. doi: 10.1085/jgp.39.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI N. The effect of temperature on the neuromuscular junction of the frog. Jpn J Physiol. 1958 Dec 20;8(4):391–404. doi: 10.2170/jjphysiol.8.391. [DOI] [PubMed] [Google Scholar]
- Ward D., Crowley W. J., Johns T. R. Effects of temperature at the neuromuscular junction. Am J Physiol. 1972 Jan;222(1):216–219. doi: 10.1152/ajplegacy.1972.222.1.216. [DOI] [PubMed] [Google Scholar]


