Abstract
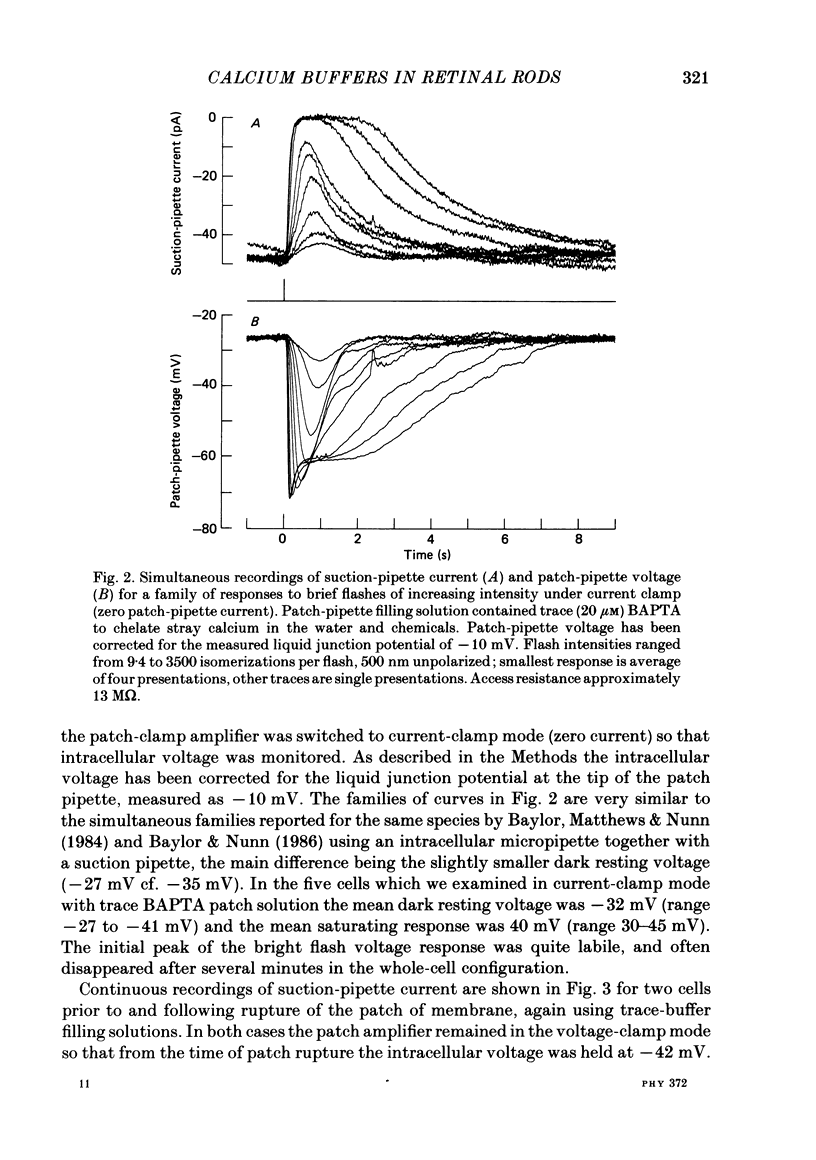
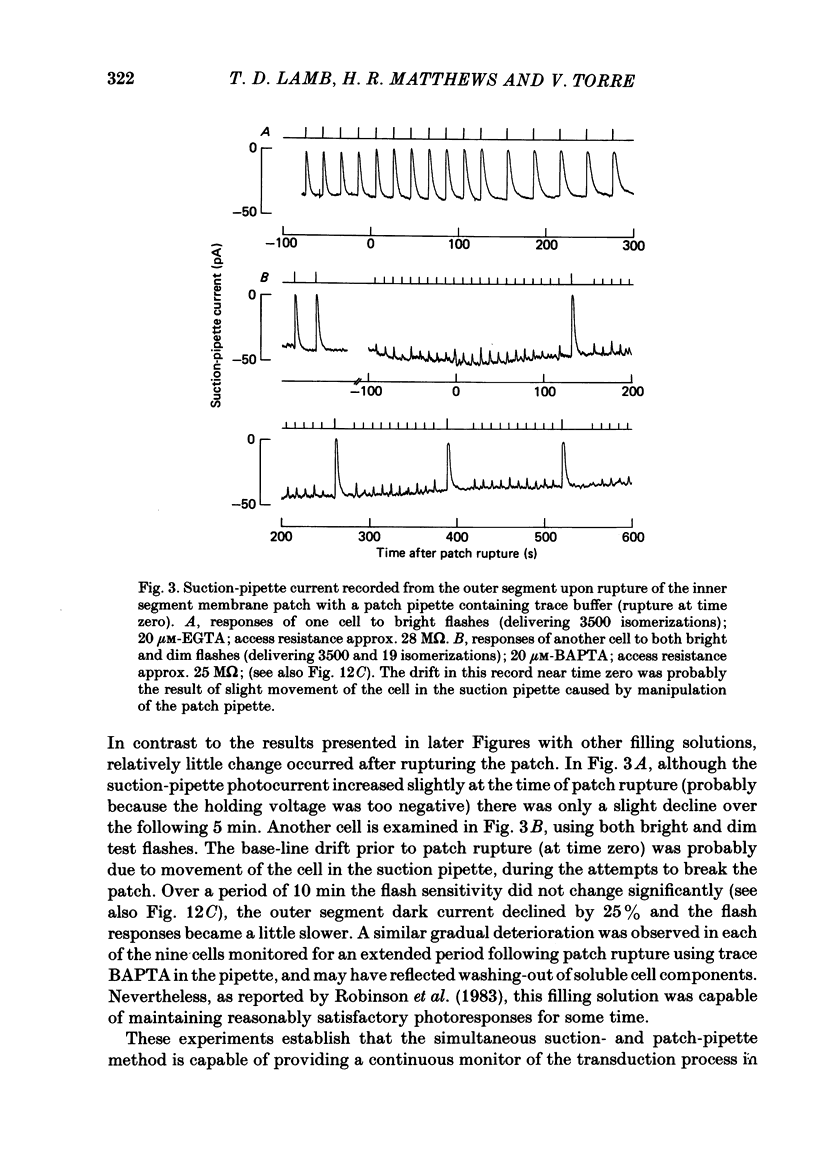
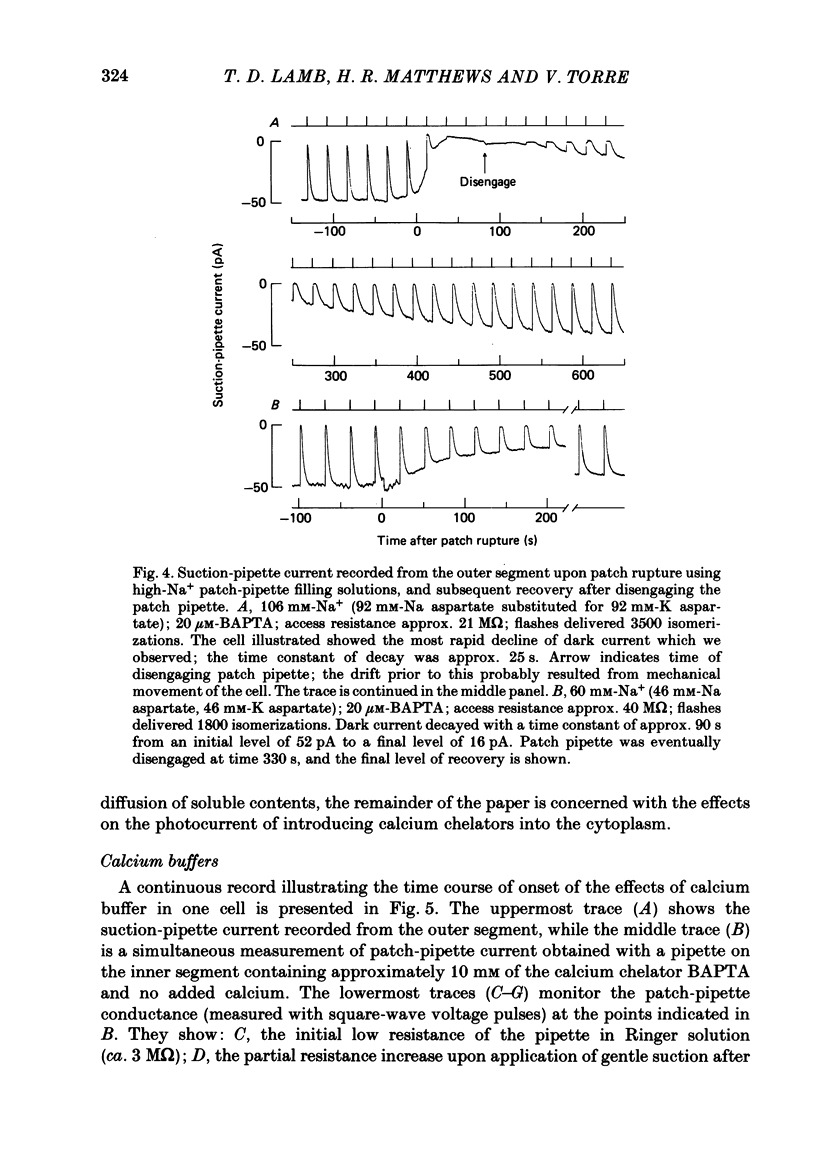
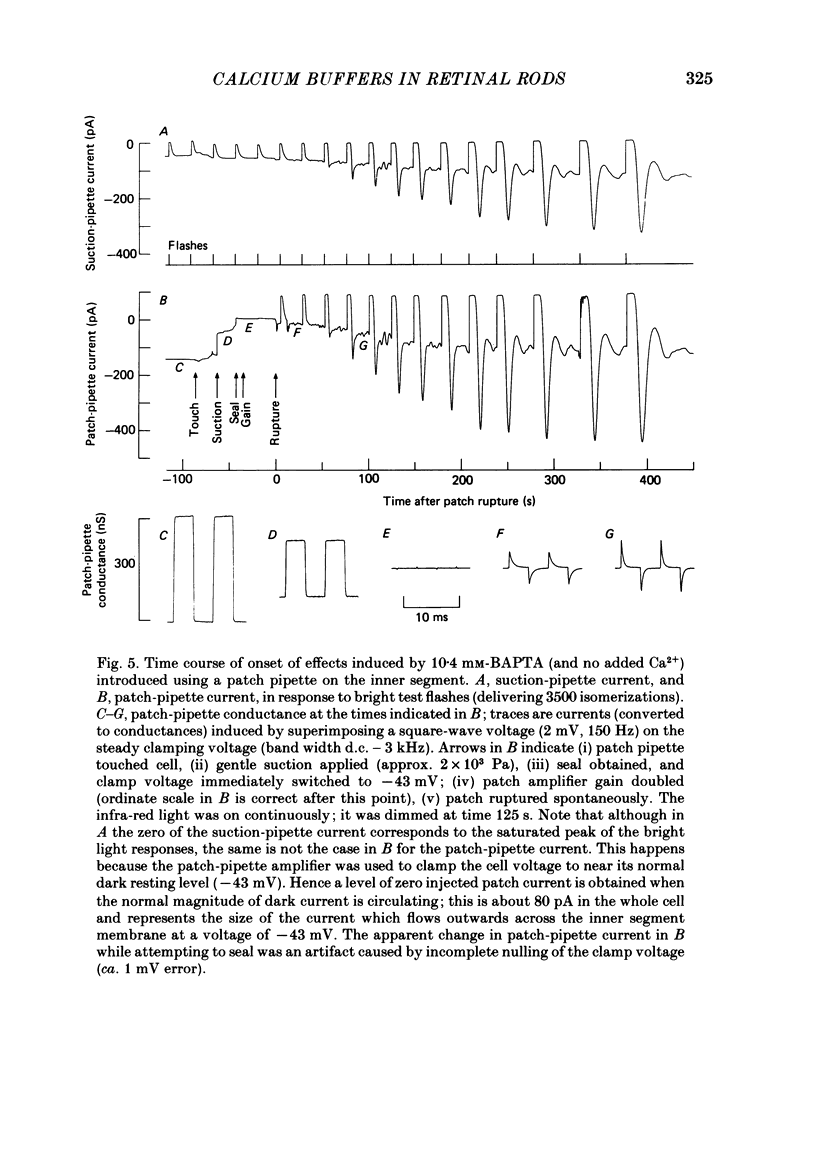
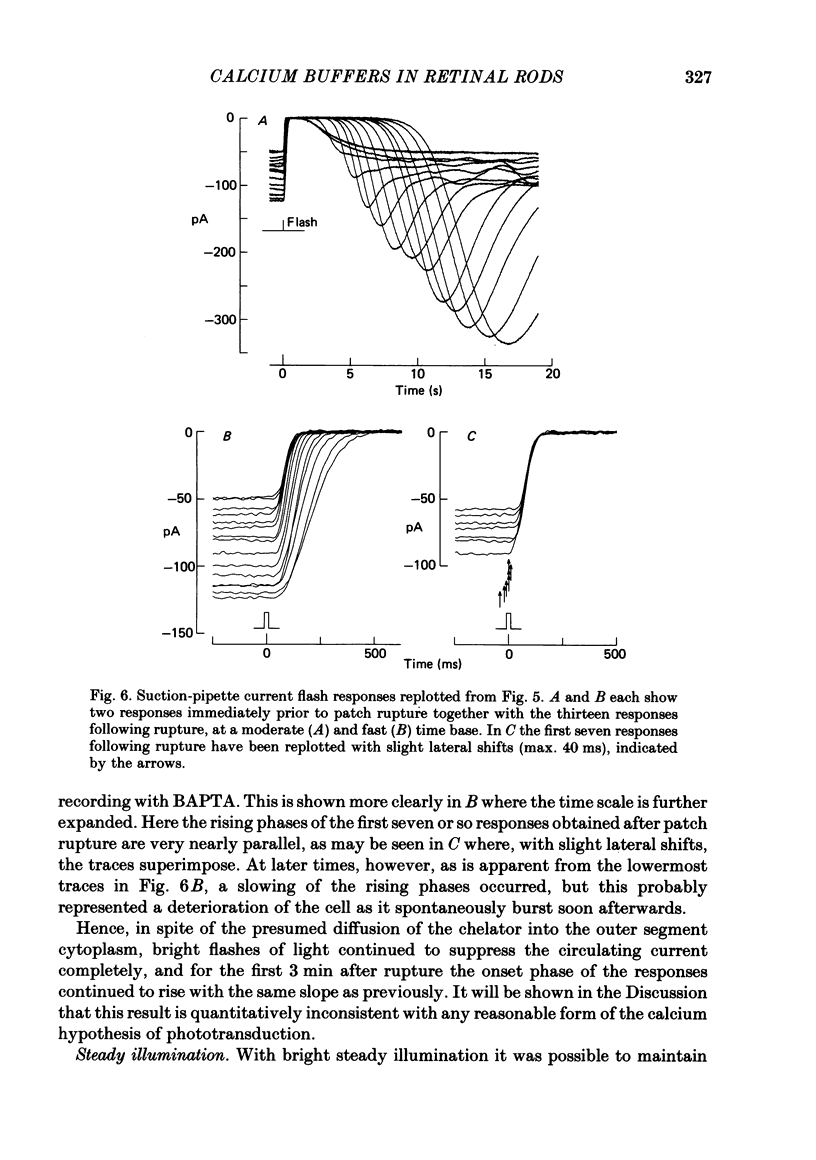
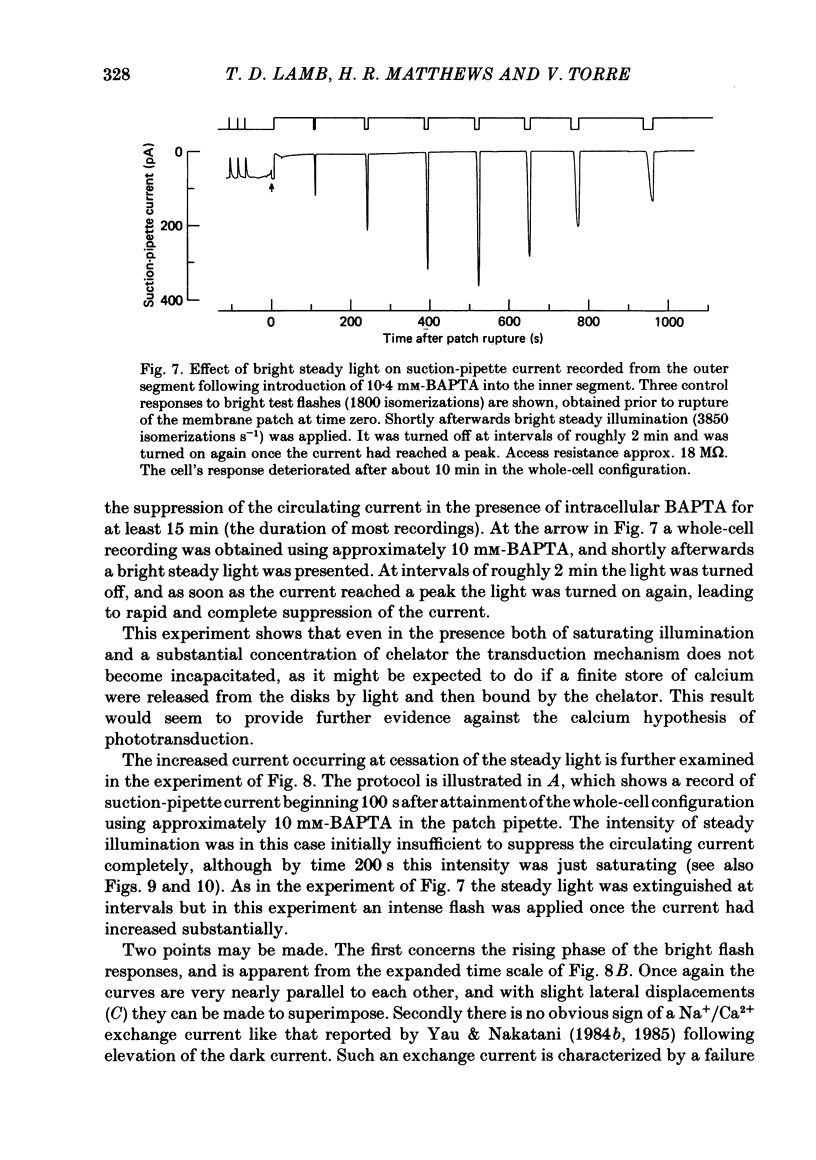
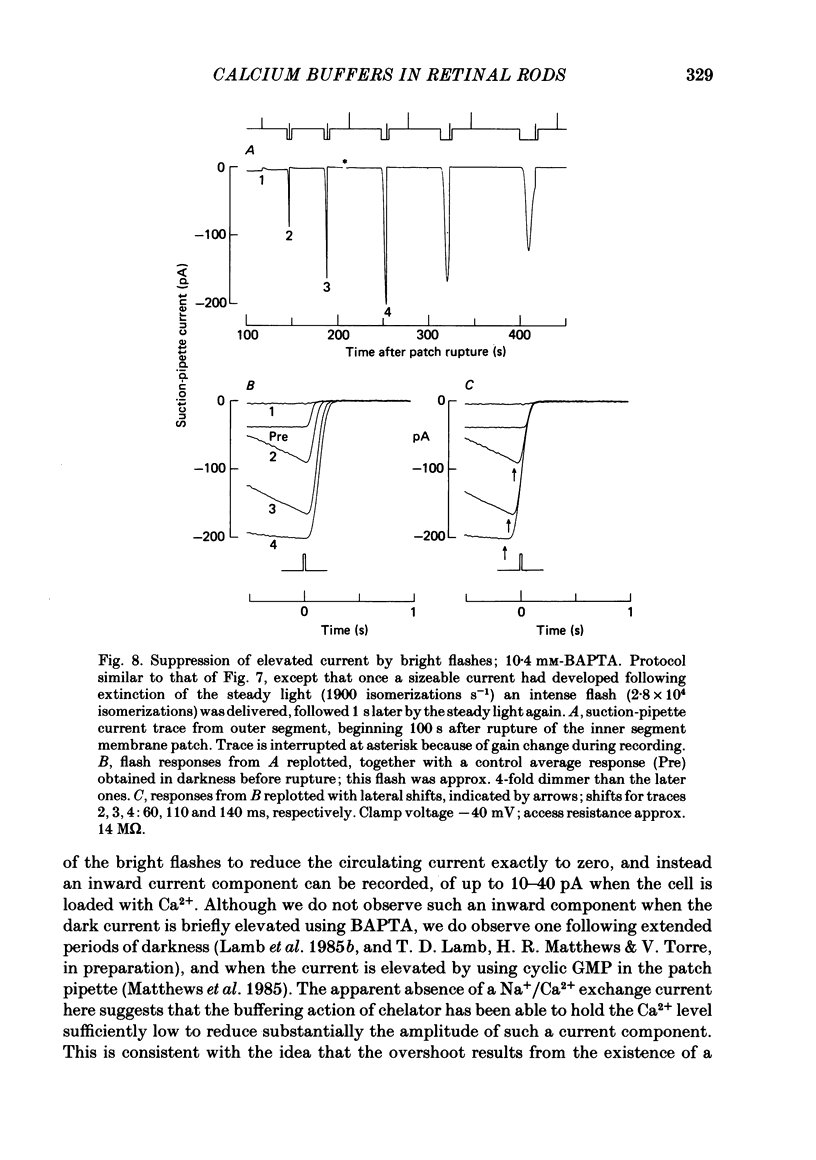
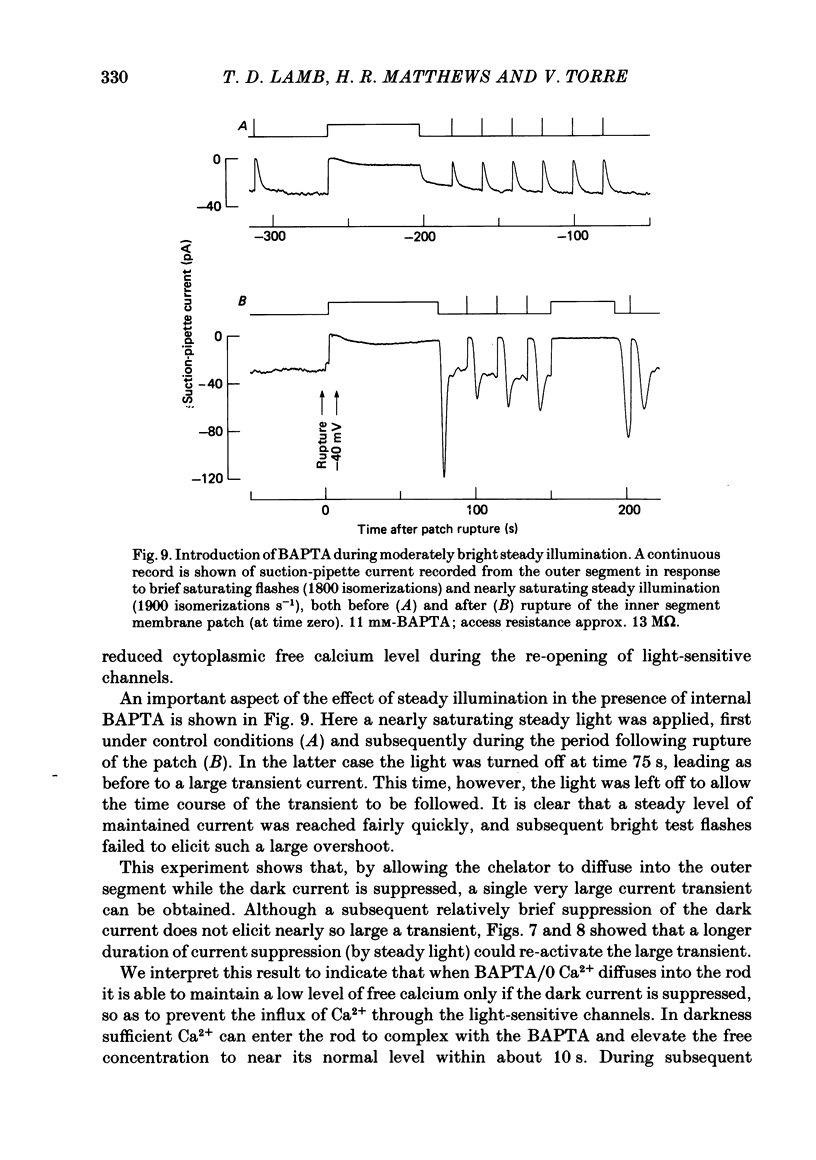
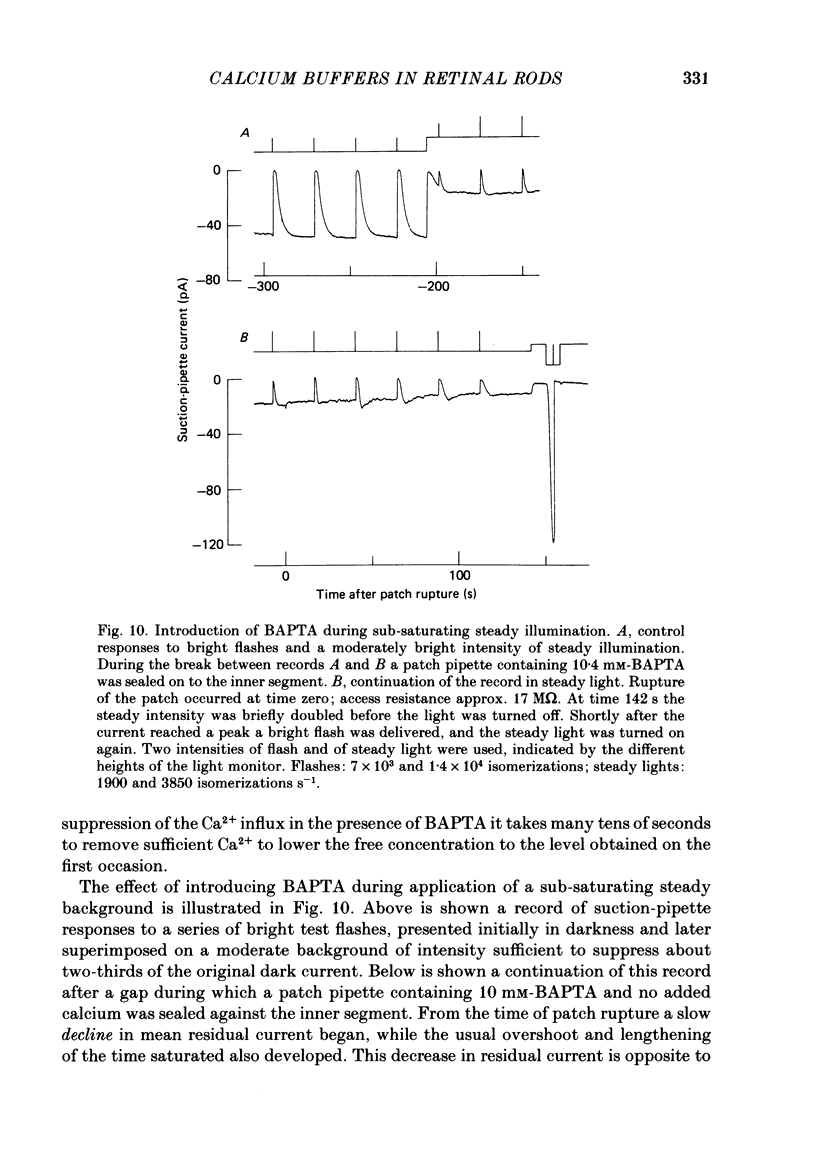
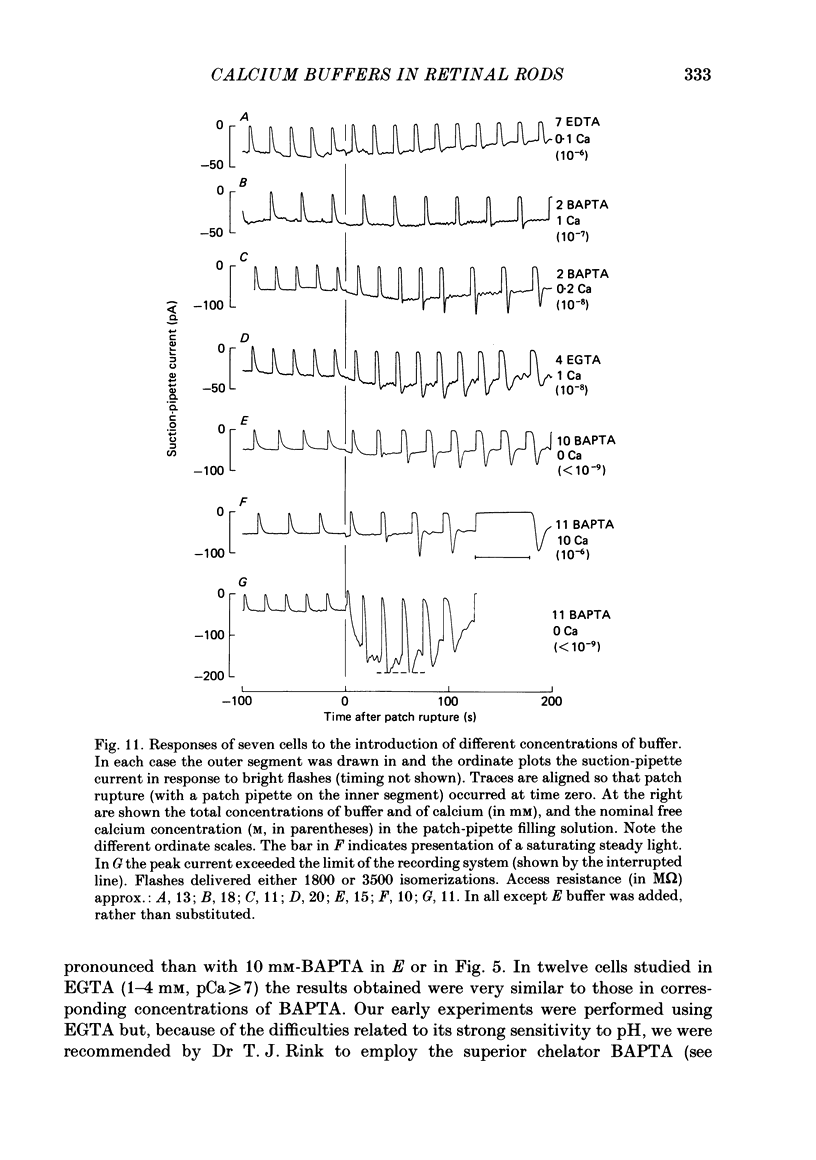
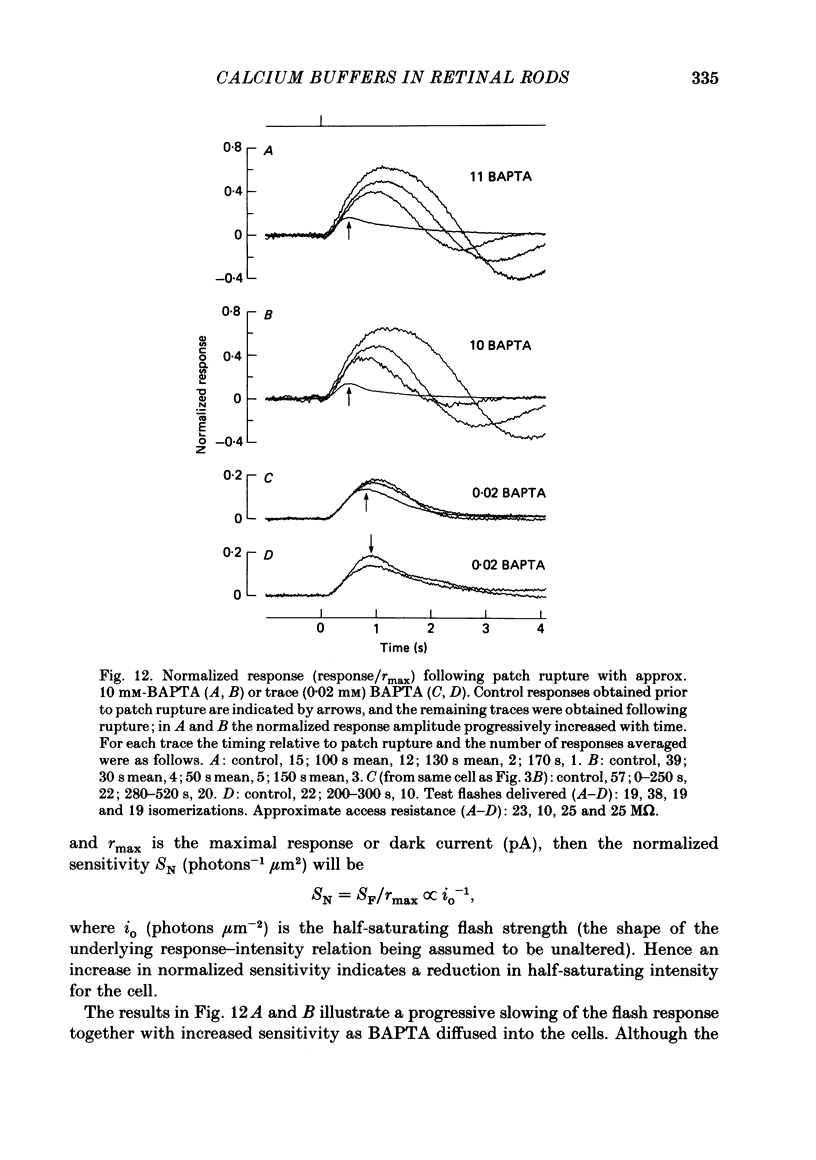
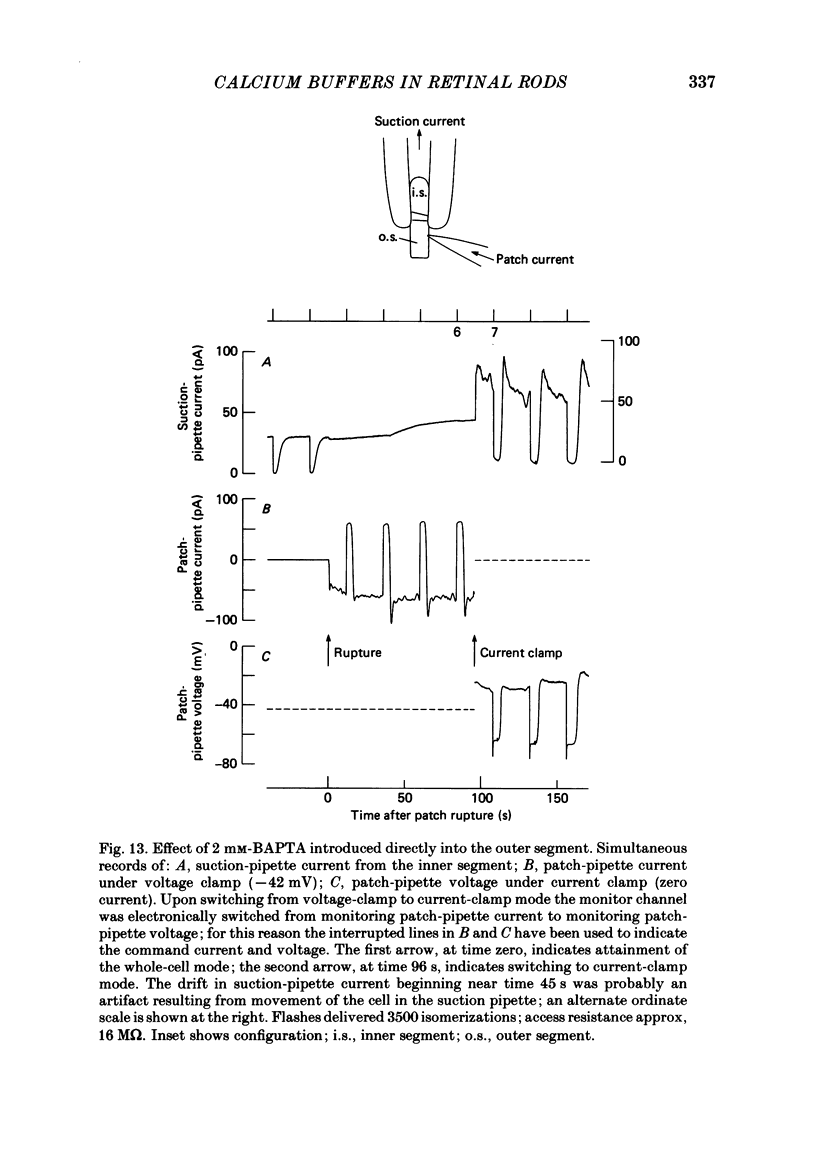
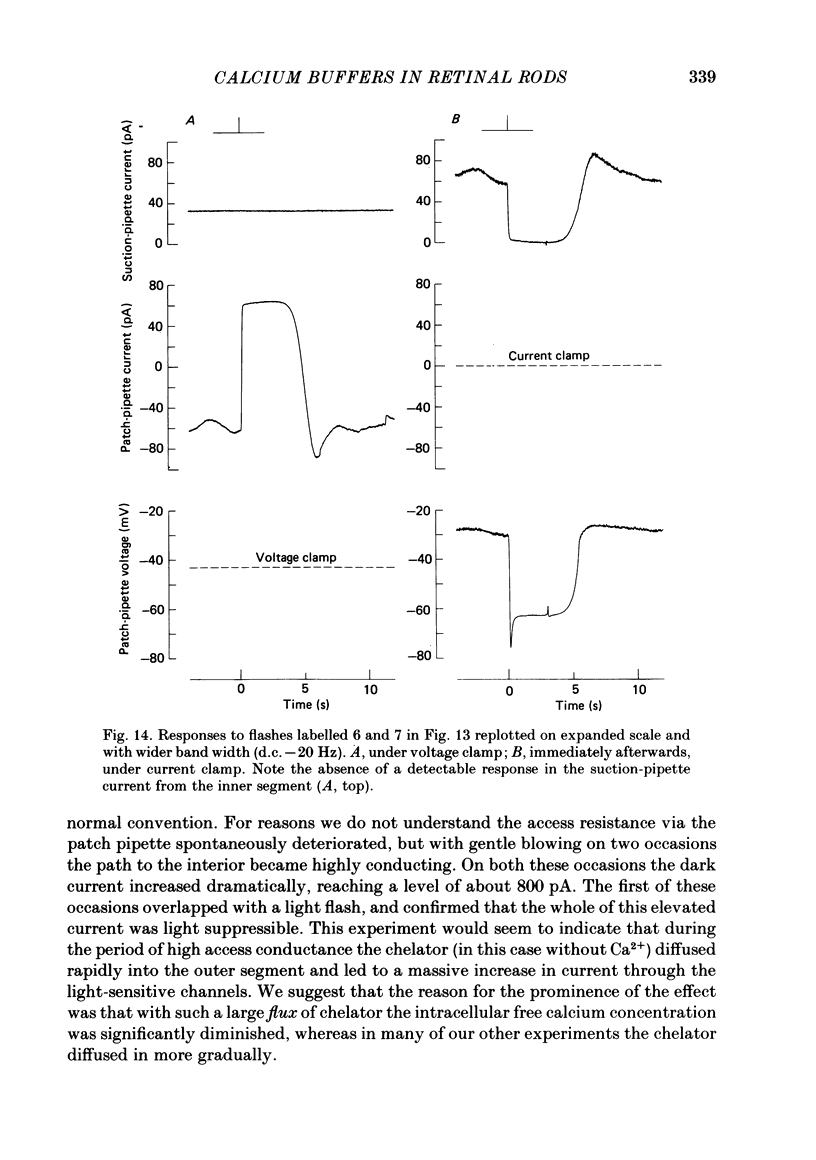
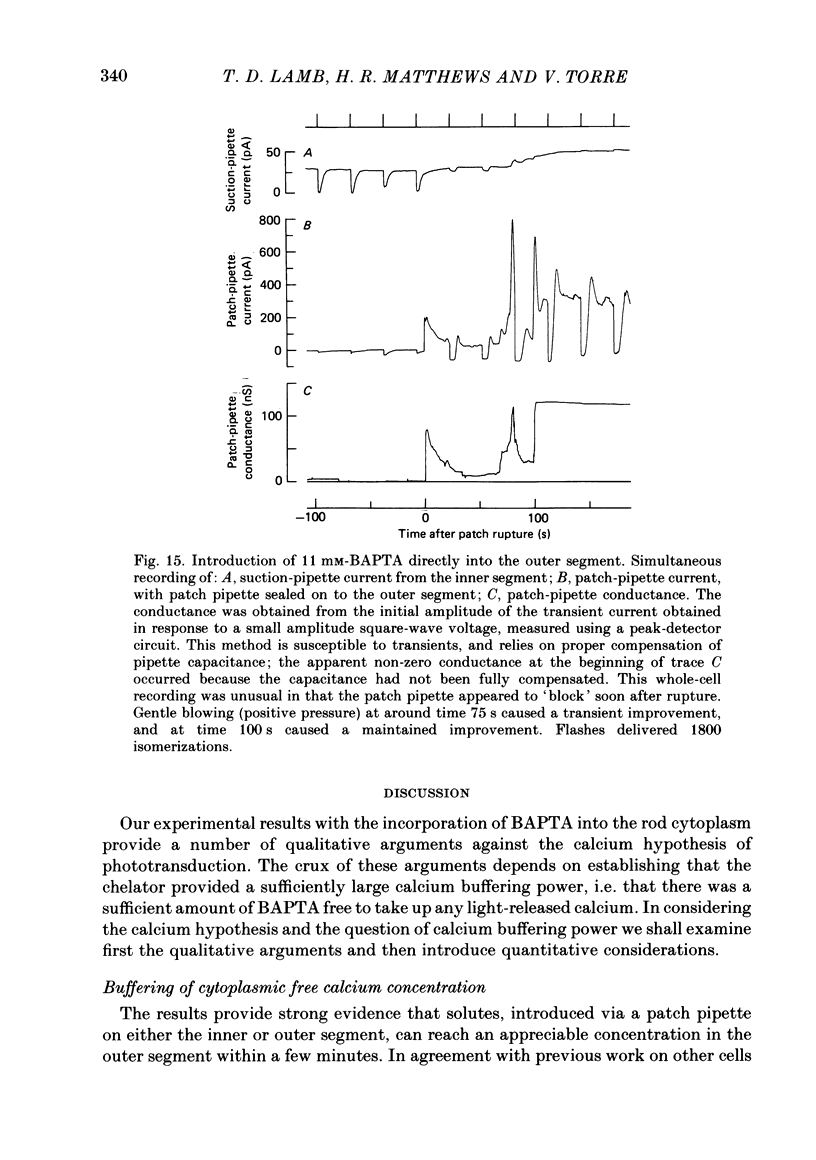
The suction-electrode technique was used to monitor the photocurrent of isolated retinal rods from the tiger salamander, by drawing in the light-sensitive outer segment, or sometimes the inner segment. Calcium buffers or other agents were then introduced into the rod cytoplasm by the 'whole-cell patch-clamp' technique. A patch pipette was sealed against the region of the rod protruding from the suction pipette (usually the inner segment), and the membrane patch was ruptured to obtain a whole-cell recording. Several lines of evidence indicated that the pipette contents diffused into the outer segment, and showed that the cell could be adequately voltage clamped. With only trace quantities of chelator in the patch pipette (to bind stray calcium), a gradual decline of the dark current and slowing of responses was usually observed over a period of 10-20 min after rupture of the patch. When the patch pipette contained no added calcium and 10 mM of the calcium chelator BAPTA (1,2-bis(o-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid) (free Ca2+ ca. 10(-9) M) rupture of the patch led, over a period of a few minutes, to an increase in mean dark current, an increased duration of responses, a substantial increase in flash sensitivity, and a pronounced overshoot in the recovery phase, but with virtually no change in the rising phase of the response to bright flashes. Similar results were obtained when EGTA was used in place of BAPTA, and also in the few cases when successful rupture of the outer segment membrane was obtained. With the free calcium concentration in the patch pipette buffered to the higher level of 1 microM (with 10 mM-Ca2+/11 mM-BAPTA) the results were qualitatively similar to those obtained with BAPTA alone, except that the mean dark current did not increase. This is consistent with a resting free calcium concentration in darkness in the region of 1 microM. In the presence of bright steady illumination with BAPTA in the cell the suppression of outer segment current could be maintained for at least 15 min. Upon extinction of the light a very large current transient developed (similar to the overshoot with flashes) which was light suppressible. With backgrounds of moderate intensity the incorporation of buffer led to a gradual reduction of the residual current.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastian B. L., Fain G. L. The effects of sodium replacement on the responses of toad rods. J Physiol. 1982 Sep;330:331–347. doi: 10.1113/jphysiol.1982.sp014344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Changes in time scale and sensitivity in turtle photoreceptors. J Physiol. 1974 Nov;242(3):729–758. doi: 10.1113/jphysiol.1974.sp010732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L., Lamb T. D. The electrical response of turtle cones to flashes and steps of light. J Physiol. 1974 Nov;242(3):685–727. doi: 10.1113/jphysiol.1974.sp010731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. Responses of retinal rods to single photons. J Physiol. 1979 Mar;288:613–634. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. The membrane current of single rod outer segments. J Physiol. 1979 Mar;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Matthews G., Nunn B. J. Location and function of voltage-sensitive conductances in retinal rods of the salamander, Ambystoma tigrinum. J Physiol. 1984 Sep;354:203–223. doi: 10.1113/jphysiol.1984.sp015372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Nunn B. J. Electrical properties of the light-sensitive conductance of rods of the salamander Ambystoma tigrinum. J Physiol. 1986 Feb;371:115–145. doi: 10.1113/jphysiol.1986.sp015964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand D., Fuortes M. G., Pochobradsky J. Actions of EGTA and high calcium on the cones in the turtle retina. J Physiol. 1978 Feb;275:419–437. doi: 10.1113/jphysiol.1978.sp012198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodoia R. D., Detwiler P. B. Patch-clamp recordings of the light-sensitive dark noise in retinal rods from the lizard and frog. J Physiol. 1985 Oct;367:183–216. doi: 10.1113/jphysiol.1985.sp015820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bownds M. D. Biochemical steps in visual transduction: roles for nucleotides and calcium ions. Photochem Photobiol. 1980 Oct;32(4):487–490. doi: 10.1111/j.1751-1097.1980.tb03792.x. [DOI] [PubMed] [Google Scholar]
- Brown J. E., Coles J. A., Pinto L. H. Effects of injections of calcium and EGTA into the outer segments of retinal rods of Bufo marinus. J Physiol. 1977 Aug;269(3):707–722. doi: 10.1113/jphysiol.1977.sp011924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Pinto L. H. Ionic mechanism for the photoreceptor potential of the retina of Bufo marinus. J Physiol. 1974 Feb;236(3):575–591. doi: 10.1113/jphysiol.1974.sp010453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly L., Moody W. J. Intracellular calcium ions and calcium currents in perfused neurones of the snail, Lymnaea stagnalis. J Physiol. 1984 Jul;352:637–652. doi: 10.1113/jphysiol.1984.sp015314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capovilla M., Caretta A., Cervetto L., Torre V. Ionic movements through light-sensitive channels of toad rods. J Physiol. 1983 Oct;343:295–310. doi: 10.1113/jphysiol.1983.sp014893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capovilla M., Cervetto L., Pasino E., Torre V. The sodium current underlying the responses of toad rods to light. J Physiol. 1981 Aug;317:223–242. doi: 10.1113/jphysiol.1981.sp013822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervetto L. Influence of sodium, potassium and chloride ions on the intracellular responses of turtle photoreceptors. Nature. 1973 Feb 9;241(5389):401–403. doi: 10.1038/241401a0. [DOI] [PubMed] [Google Scholar]
- Cobbs W. H., Pugh E. N., Jr Cyclic GMP can increase rod outer-segment light-sensitive current 10-fold without delay of excitation. Nature. 1985 Feb 14;313(6003):585–587. doi: 10.1038/313585a0. [DOI] [PubMed] [Google Scholar]
- Fatt P. Decline of the calcium hypothesis of visual transduction. Nature. 1979 Aug 2;280(5721):355–356. doi: 10.1038/280355a0. [DOI] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985 Jan 24;313(6000):310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- Hagins W. A. The visual process: Excitatory mechanisms in the primary receptor cells. Annu Rev Biophys Bioeng. 1972;1:131–158. doi: 10.1146/annurev.bb.01.060172.001023. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J. The ionic selectivity and calcium dependence of the light-sensitive pathway in toad rods. J Physiol. 1985 Jan;358:447–468. doi: 10.1113/jphysiol.1985.sp015561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J., Yau K. W. Effect of ions on retinal rods from Bufo marinus. J Physiol. 1984 May;350:649–680. doi: 10.1113/jphysiol.1984.sp015223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbell W. L., Bownds M. D. Visual transduction in vertebrate photoreceptors. Annu Rev Neurosci. 1979;2:17–34. doi: 10.1146/annurev.ne.02.030179.000313. [DOI] [PubMed] [Google Scholar]
- Lamb T. D., McNaughton P. A., Yau K. W. Spatial spread of activation and background desensitization in toad rod outer segments. J Physiol. 1981;319:463–496. doi: 10.1113/jphysiol.1981.sp013921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman P. A., Pugh E. N., Jr The control of phosphodiesterase in rod disk membranes: kinetics, possible mechanisms and significance for vision. Vision Res. 1979;19(4):375–380. doi: 10.1016/0042-6989(79)90097-x. [DOI] [PubMed] [Google Scholar]
- Lolley R. N., Racz E. Calcium modulation of cyclic GMP synthesis in rat visual cells. Vision Res. 1982;22(12):1481–1486. doi: 10.1016/0042-6989(82)90213-9. [DOI] [PubMed] [Google Scholar]
- MacLeish P. R., Schwartz E. A., Tachibana M. Control of the generator current in solitary rods of the Ambystoma tigrinum retina. J Physiol. 1984 Mar;348:645–664. doi: 10.1113/jphysiol.1984.sp015131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G. Membrane current noise in toad retinal rods exposed to low external calcium. J Physiol. 1985 Apr;361:205–217. doi: 10.1113/jphysiol.1985.sp015641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H. R., Torre V., Lamb T. D. Effects on the photoresponse of calcium buffers and cyclic GMP incorporated into the cytoplasm of retinal rods. Nature. 1985 Feb 14;313(6003):582–585. doi: 10.1038/313582a0. [DOI] [PubMed] [Google Scholar]
- Miki N., Baraban J. M., Keirns J. J., Boyce J. J., Bitensky M. W. Purification and properties of the light-activated cyclic nucleotide phosphodiesterase of rod outer segments. J Biol Chem. 1975 Aug 25;250(16):6320–6327. [PubMed] [Google Scholar]
- Owen W. G., Torre V. High-pass filtering of small signals by retinal rods. Ionic studies. Biophys J. 1983 Mar;41(3):325–339. doi: 10.1016/S0006-3495(83)84444-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder W. H., Fain G. L. Light-dependent calcium release from photoreceptors measured by laser micro-mass analysis. Nature. 1984 May 17;309(5965):268–270. doi: 10.1038/309268a0. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Walz B. Elemental distribution in Rana pipiens retinal rods: quantitative electron probe analysis. J Physiol. 1985 Jan;358:183–195. doi: 10.1113/jphysiol.1985.sp015547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Werblin F. S. Transmission along and between rods in the tiger salamander retina. J Physiol. 1978 Jul;280:449–470. doi: 10.1113/jphysiol.1978.sp012394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau K. W., McNaughton P. A., Hodgkin A. L. Effect of ions on the light-sensitive current in retinal rods. Nature. 1981 Aug 6;292(5823):502–505. doi: 10.1038/292502a0. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Cation selectivity of light-sensitive conductance in retinal rods. Nature. 1984 May 24;309(5966):352–354. doi: 10.1038/309352a0. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Electrogenic Na-Ca exchange in retinal rod outer segment. Nature. 1984 Oct 18;311(5987):661–663. doi: 10.1038/311661a0. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Light-induced reduction of cytoplasmic free calcium in retinal rod outer segment. Nature. 1985 Feb 14;313(6003):579–582. doi: 10.1038/313579a0. [DOI] [PubMed] [Google Scholar]
- Yoshikami S., George J. S., Hagins W. A. Light-induced calcium fluxes from outer segment layer of vertebrate retinas. Nature. 1980 Jul 24;286(5771):395–398. doi: 10.1038/286395a0. [DOI] [PubMed] [Google Scholar]



