Abstract
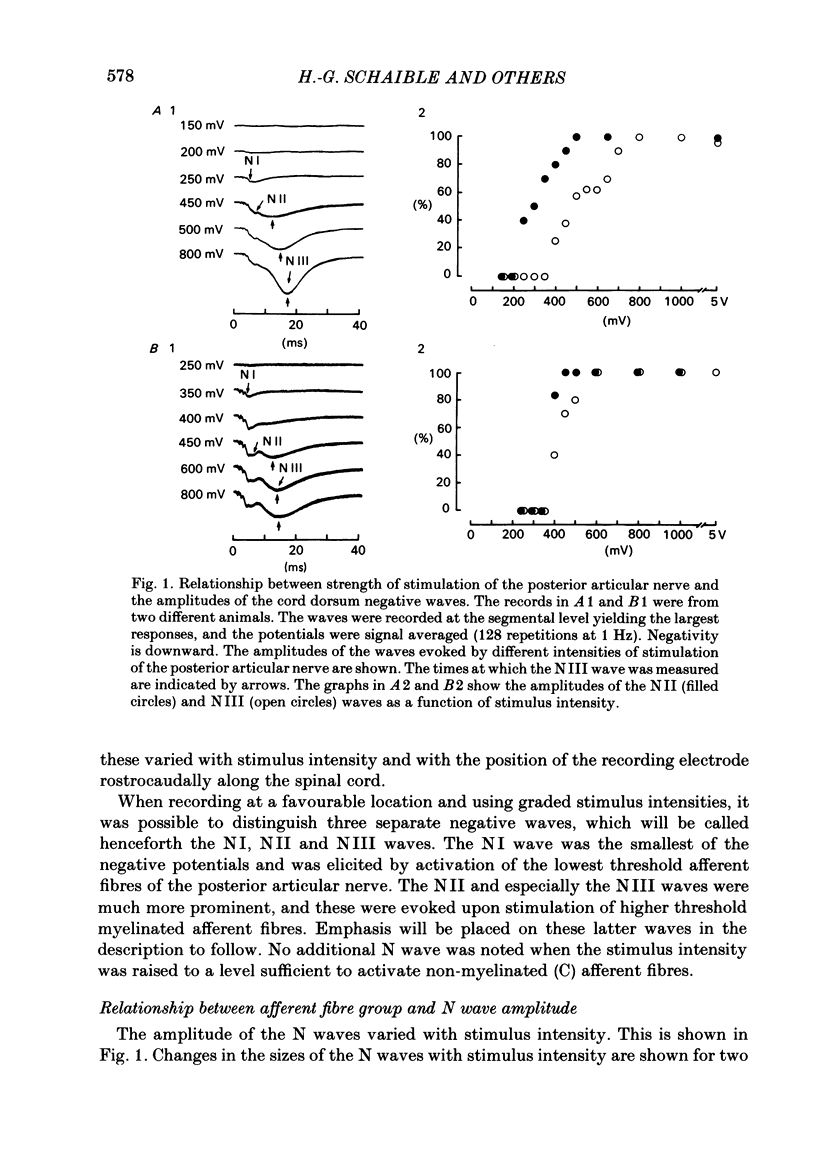
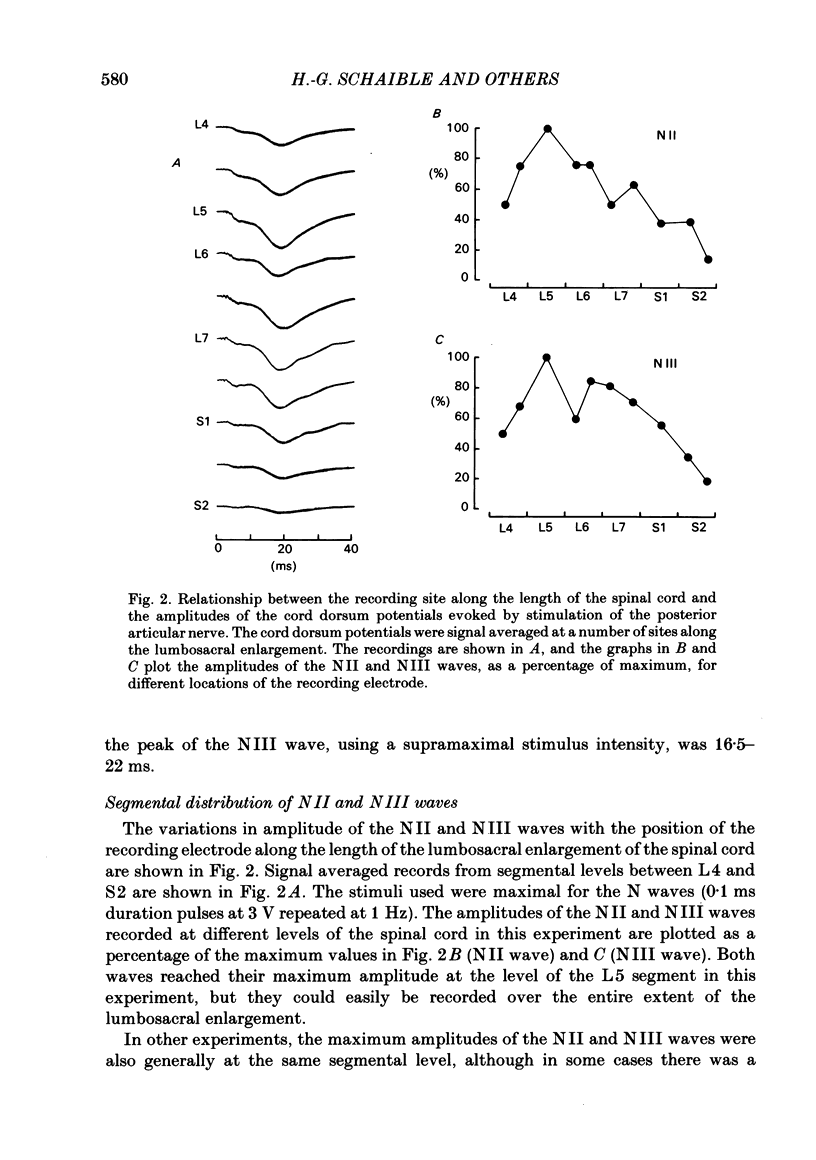
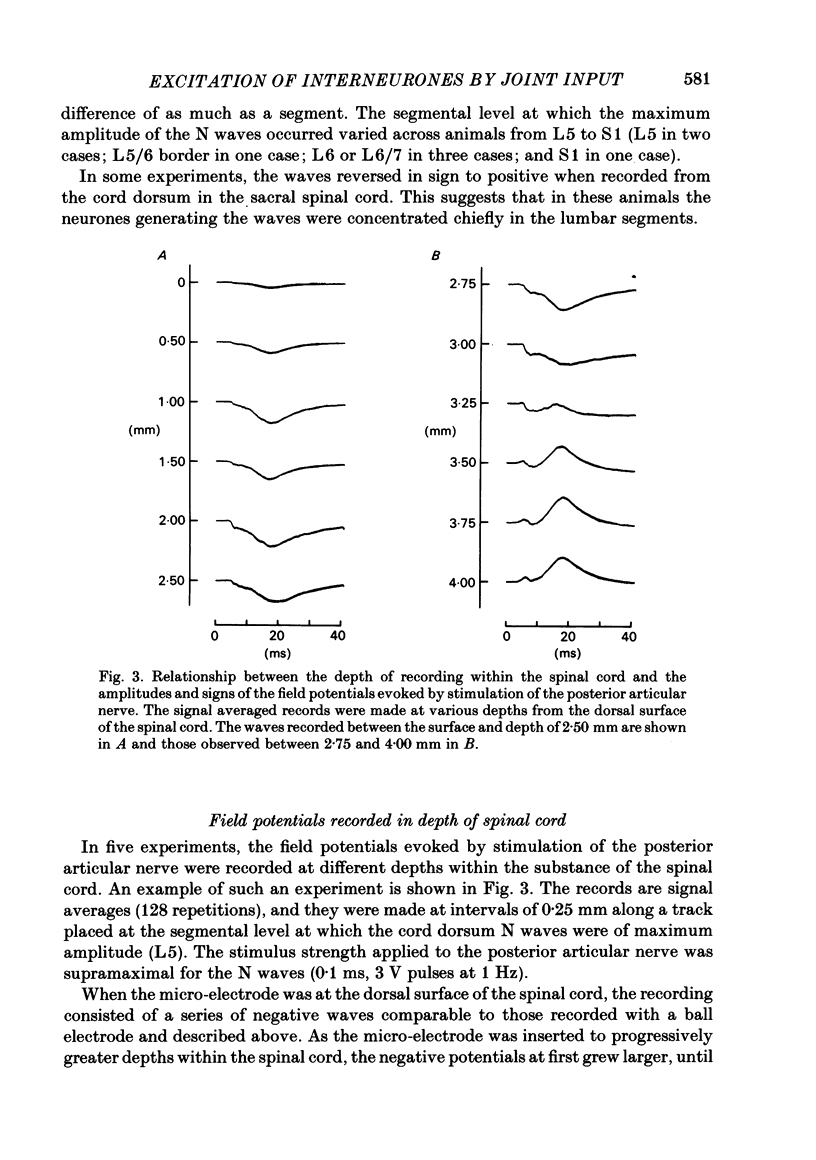
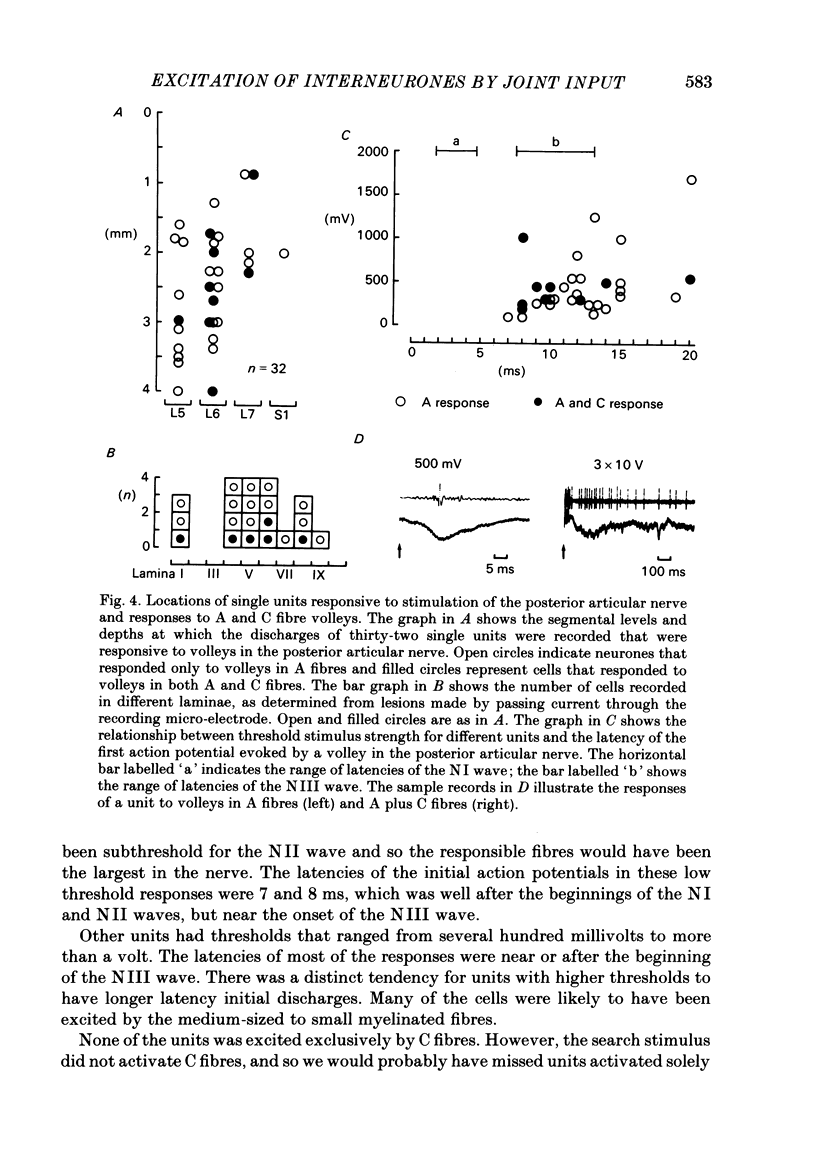
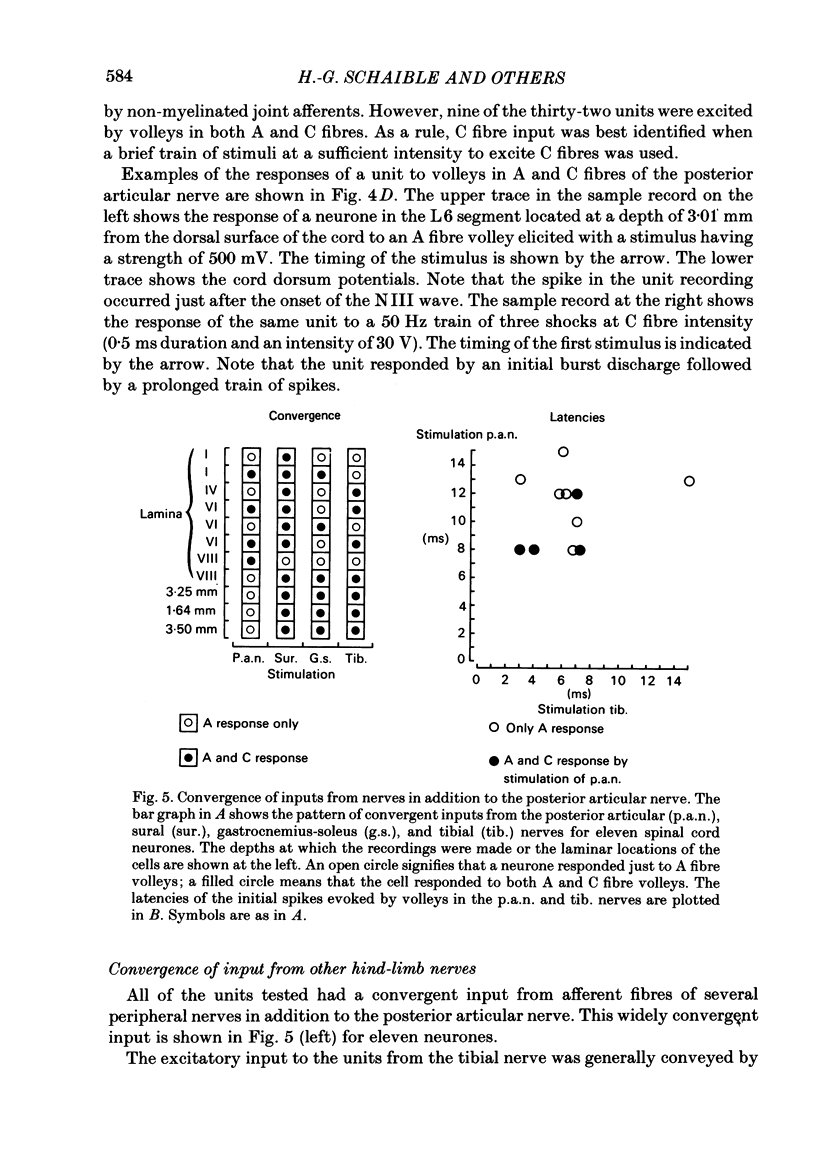
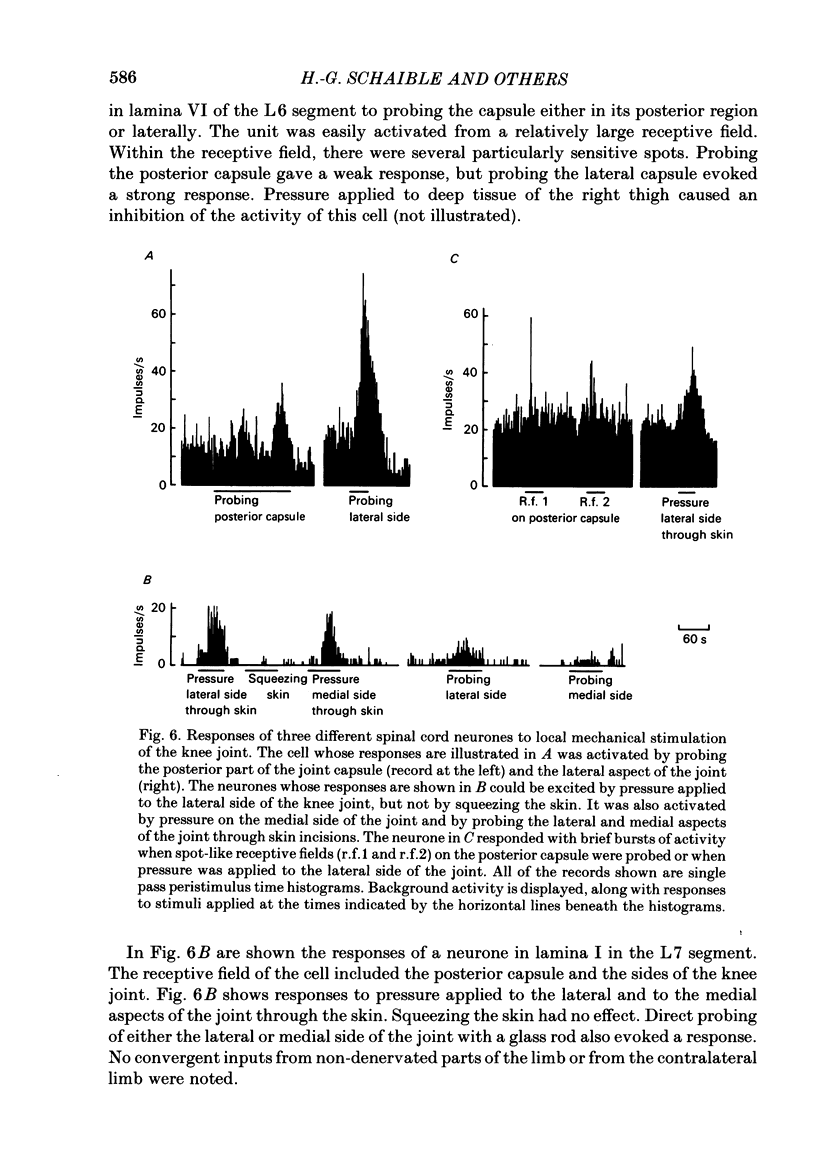
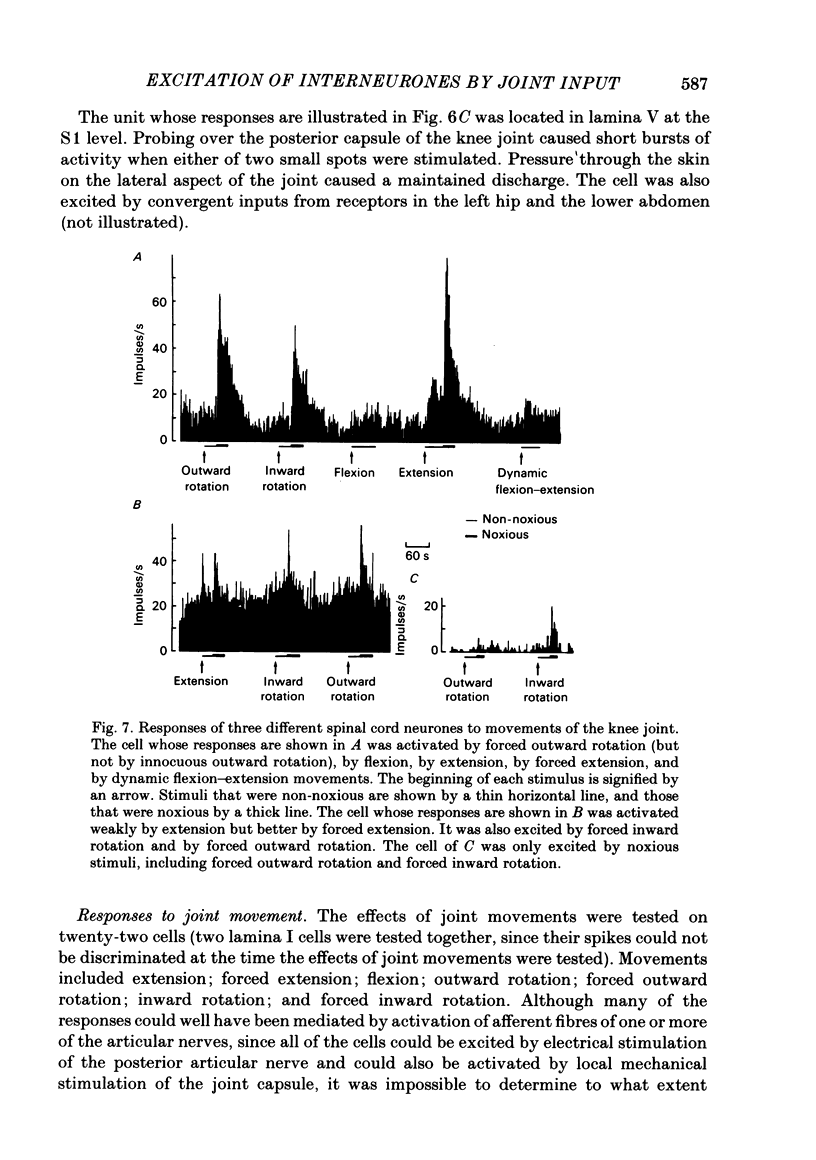
Responses of spinal cord neurones to excitatory input from myelinated and non-myelinated afferent fibres of the knee joint were investigated in spinalized cats that were anaesthetized with alpha-chloralose. The hind limb was largely denervated except for the knee joint. The action of joint afferent volleys on populations of spinal cord neurones was assessed by recordings of cord dorsum potentials and of field potentials within the substance of the spinal cord. Three different negative cord dorsum potentials (NI, NII and NIII waves) were produced by volleys in progressively smaller sized myelinated afferent fibres of the posterior articular nerve of the knee. Volleys in non-myelinated joint afferent fibres did not evoke a detectable cord dorsum potential. The NII and NIII waves could be recorded at segmental levels L4-S2. Field potentials corresponding to the cord dorsum negative waves were recorded with a tungsten micro-electrode inserted into the substance of the cord. The potentials were negative when the recording electrode was in the dorsal horn, but reversed to become positive in the ventral horn. There were differences in the depths at which the different components of the field potential sequence reversed in sign. Single unit recordings revealed that afferent volleys in the posterior articular nerve could excite neurones in segments L5-S1 and at depths from less than 1 to 4 mm below the dorsal surface of the cord. Most marked recording sites were in laminae I, IV-VI or VIII. Many units were activated just by the A fibre component of the joint afferent volley, but others could also be excited by the C fibre component. None were excited just by C fibres. All units tested had a convergent excitatory input from fibres belonging to cutaneous, muscle and mixed nerves. Neurones excited by joint afferent volleys were tested for a receptive field using several forms of local mechanical stimulation of the knee joint and joint movements. Most cells could be activated by one or more of these stimuli. Although many of the effective mechanical stimuli were innocuous, noxious stimuli could often excite the cells more effectively, and some neurones were only activated by noxious stimuli. A few cells with receptive fields in the knee joint did not respond to joint movements.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYD I. A., ROBERTS T. D. Proprioceptive discharges from stretch-receptors in the knee-joint of the cat. J Physiol. 1953 Oct;122(1):38–58. doi: 10.1113/jphysiol.1953.sp004977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYD I. A. The histological structure of the receptors in the knee-joint of the cat correlated with their physiological response. J Physiol. 1954 Jun 28;124(3):476–488. doi: 10.1113/jphysiol.1954.sp005122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxendale R. H., Ferrell W. R. The effect of knee joint afferent discharge on transmission in flexion reflex pathways in decerebrate cats. J Physiol. 1981 Jun;315:231–242. doi: 10.1113/jphysiol.1981.sp013744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall J. E., Applebaum A. E., Foreman R. D., Willis W. D. Spinal cord potentials evoked by cutaneous afferents in the monkey. J Neurophysiol. 1977 Mar;40(2):199–211. doi: 10.1152/jn.1977.40.2.199. [DOI] [PubMed] [Google Scholar]
- Burgess P. R., Clark F. J. Characteristics of knee joint receptors in the cat. J Physiol. 1969 Aug;203(2):317–335. doi: 10.1113/jphysiol.1969.sp008866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., CURTIS D. R., LANDGREN S. Spinal cord potentials generated by impulses in muscle and cutaneous afferent fibres. J Neurophysiol. 1956 Sep;19(5):452–467. doi: 10.1152/jn.1956.19.5.452. [DOI] [PubMed] [Google Scholar]
- Cervero F., Iggo A., Ogawa H. Nociceptor-driven dorsal horn neurones in the lumbar spinal cord of the cat. Pain. 1976 Mar;2(1):5–24. doi: 10.1016/0304-3959(76)90042-7. [DOI] [PubMed] [Google Scholar]
- Cervero F. Somatic and visceral inputs to the thoracic spinal cord of the cat: effects of noxious stimulation of the biliary system. J Physiol. 1983 Apr;337:51–67. doi: 10.1113/jphysiol.1983.sp014611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen B. N., Perl E. R. Spinal neurons specifically excited by noxious or thermal stimuli: marginal zone of the dorsal horn. J Neurophysiol. 1970 Mar;33(2):293–307. doi: 10.1152/jn.1970.33.2.293. [DOI] [PubMed] [Google Scholar]
- Clark F. J., Burgess P. R. Slowly adapting receptors in cat knee joint: can they signal joint angle? J Neurophysiol. 1975 Nov;38(6):1448–1463. doi: 10.1152/jn.1975.38.6.1448. [DOI] [PubMed] [Google Scholar]
- Clark F. J. Information signaled by sensory fibers in medial articular nerve. J Neurophysiol. 1975 Nov;38(6):1464–1472. doi: 10.1152/jn.1975.38.6.1464. [DOI] [PubMed] [Google Scholar]
- Coggeshall R. E., Hong K. A., Langford L. A., Schaible H. G., Schmidt R. F. Discharge characteristics of fine medial articular afferents at rest and during passive movements of inflamed knee joints. Brain Res. 1983 Aug 1;272(1):185–188. doi: 10.1016/0006-8993(83)90379-7. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Magni F., Willis W. D. Depolarization of central terminals of Group I afferent fibres from muscle. J Physiol. 1962 Jan;160(1):62–93. doi: 10.1113/jphysiol.1962.sp006835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell W. R. The adequacy of stretch receptors in the cat knee joint for signalling joint angle throughout a full range of movement. J Physiol. 1980 Feb;299:85–99. doi: 10.1113/jphysiol.1980.sp013112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman R. D., Kenshalo D. R., Jr, Schmidt R. F., Willis W. D. Field potentials and excitation of primate spinothalamic neurones in response to volleys in muscle afferents. J Physiol. 1979 Jan;286:197–213. doi: 10.1113/jphysiol.1979.sp012614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu T. C., Santini M., Schomburg E. D. Characteristics and distribution of spinal focal synaptic potentials generated by group II muscle afferents. Acta Physiol Scand. 1974 Jul;91(3):298–313. doi: 10.1111/j.1748-1716.1974.tb05686.x. [DOI] [PubMed] [Google Scholar]
- GARDNER E., LATIMER F., STILWELL D. Central connections for afferent fibers from the knee joint of the cat. Am J Physiol. 1949 Nov;159(2):195–198. doi: 10.1152/ajplegacy.1949.159.2.195. [DOI] [PubMed] [Google Scholar]
- Grigg P. Mechanical factors influencing response of joint afferent neurons from cat knee. J Neurophysiol. 1975 Nov;38(6):1473–1484. doi: 10.1152/jn.1975.38.6.1473. [DOI] [PubMed] [Google Scholar]
- Jiménez I., Rudomin P., Solodkin M., Vyklicky L. Specific and potassium components in the depolarization of the la afferents in the spinal cord of the cat. Brain Res. 1983 Aug 1;272(1):179–184. doi: 10.1016/0006-8993(83)90378-5. [DOI] [PubMed] [Google Scholar]
- Langford L. A., Schmidt R. F. Afferent and efferent axons in the medial and posterior articular nerves of the cat. Anat Rec. 1983 May;206(1):71–78. doi: 10.1002/ar.1092060109. [DOI] [PubMed] [Google Scholar]
- Lundberg A. Multisensory control of spinal reflex pathways. Prog Brain Res. 1979;50:11–28. doi: 10.1016/S0079-6123(08)60803-1. [DOI] [PubMed] [Google Scholar]
- McCall W. D., Jr, Farias M. C., Williams W. J., BeMent S. L. Static and dynamic responses of slowly adapting joint receptors. Brain Res. 1974 Apr 19;70(2):221–243. doi: 10.1016/0006-8993(74)90314-x. [DOI] [PubMed] [Google Scholar]
- Mendell L. M. Physiological properties of unmyelinated fiber projection to the spinal cord. Exp Neurol. 1966 Nov;16(3):316–332. doi: 10.1016/0014-4886(66)90068-9. [DOI] [PubMed] [Google Scholar]
- Meyers D. E., Snow P. J. The responses to somatic stimuli of deep spinothalamic tract cells in the lumbar spinal cord of the cat. J Physiol. 1982 Aug;329:355–371. doi: 10.1113/jphysiol.1982.sp014307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKOGLUND S. Anatomical and physiological studies of knee joint innervation in the cat. Acta Physiol Scand Suppl. 1956;36(124):1–101. [PubMed] [Google Scholar]
- Schaible H. G., Schmidt R. F. Activation of groups III and IV sensory units in medial articular nerve by local mechanical stimulation of knee joint. J Neurophysiol. 1983 Jan;49(1):35–44. doi: 10.1152/jn.1983.49.1.35. [DOI] [PubMed] [Google Scholar]
- Schaible H. G., Schmidt R. F. Responses of fine medial articular nerve afferents to passive movements of knee joints. J Neurophysiol. 1983 May;49(5):1118–1126. doi: 10.1152/jn.1983.49.5.1118. [DOI] [PubMed] [Google Scholar]
- Schmidt R. F. Presynaptic inhibition in the vertebrate central nervous system. Ergeb Physiol. 1971;63:20–101. doi: 10.1007/BFb0047741. [DOI] [PubMed] [Google Scholar]
- Wall P. D. The laminar organization of dorsal horn and effects of descending impulses. J Physiol. 1967 Feb;188(3):403–423. doi: 10.1113/jphysiol.1967.sp008146. [DOI] [PMC free article] [PubMed] [Google Scholar]


