Abstract
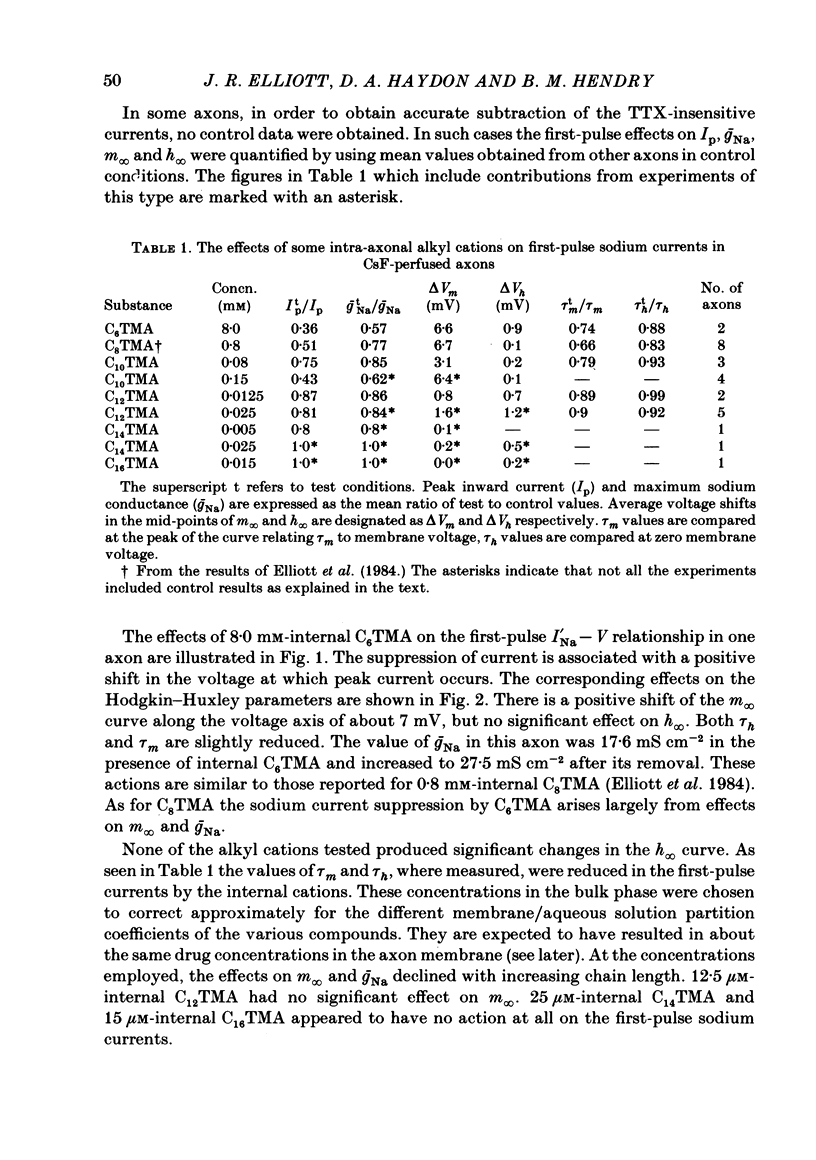
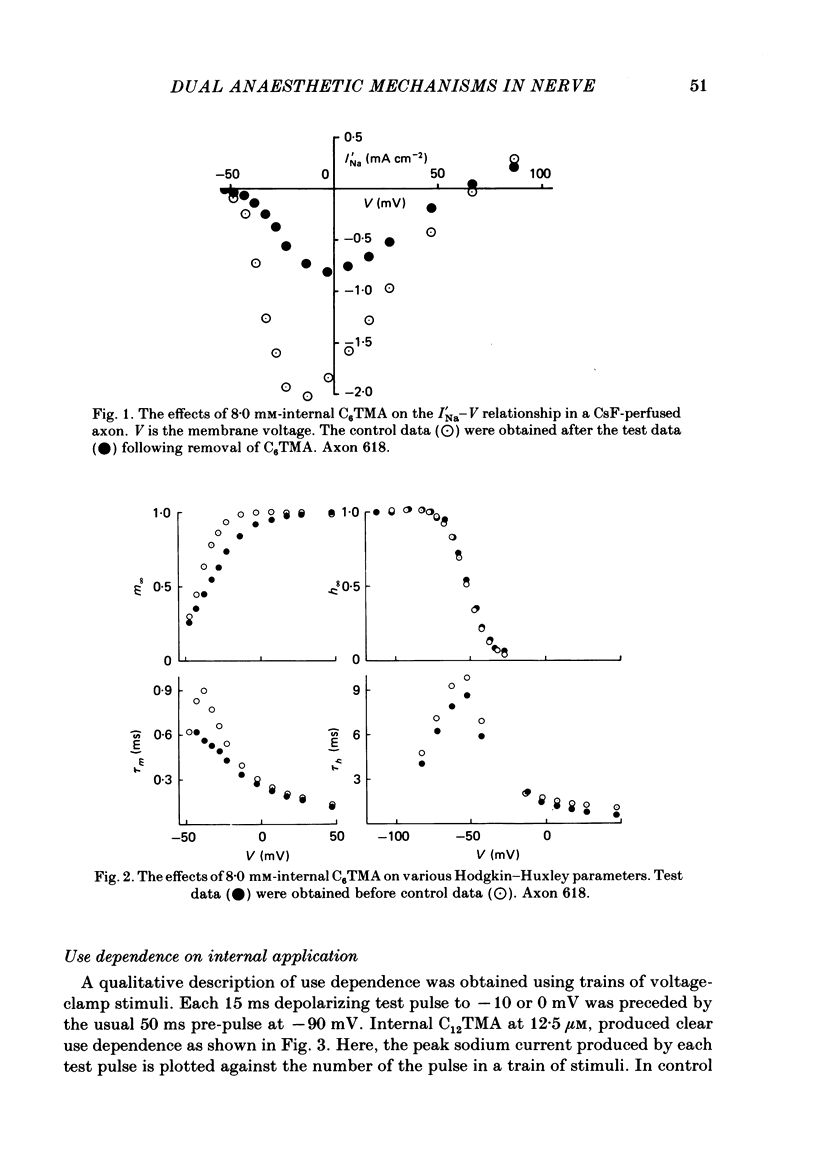
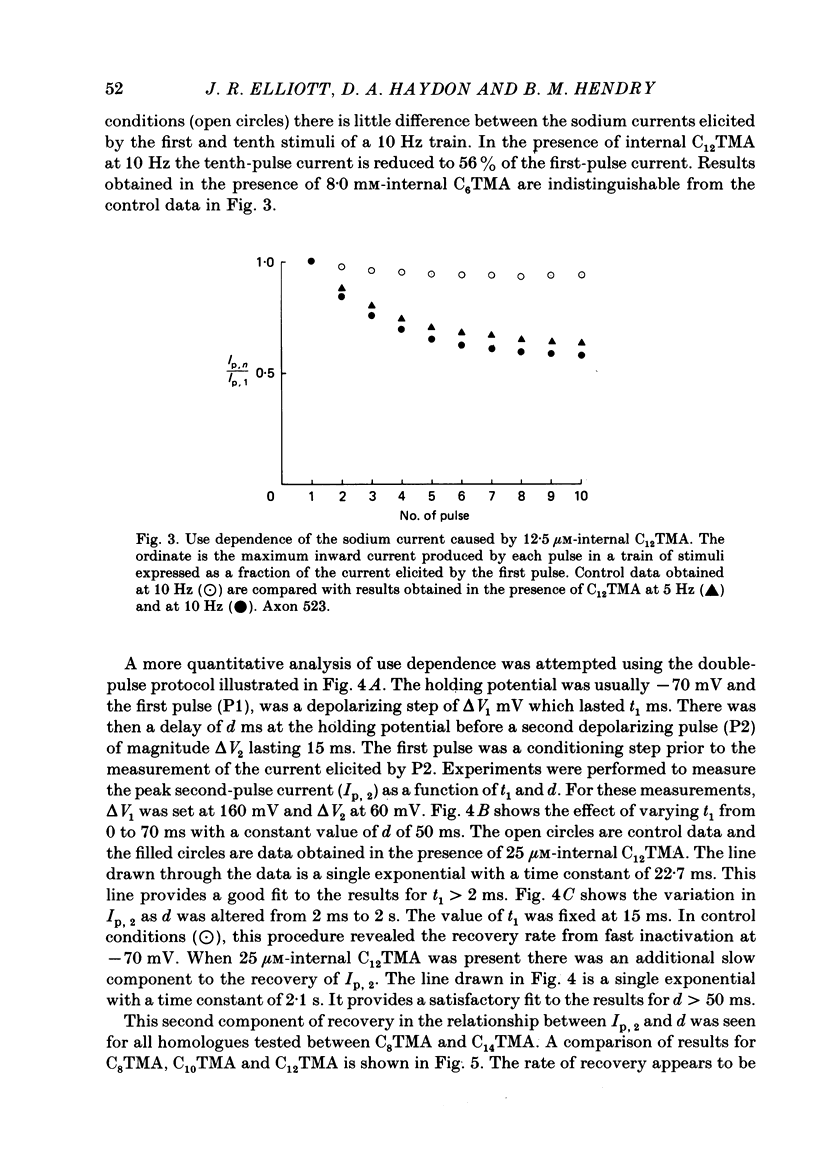
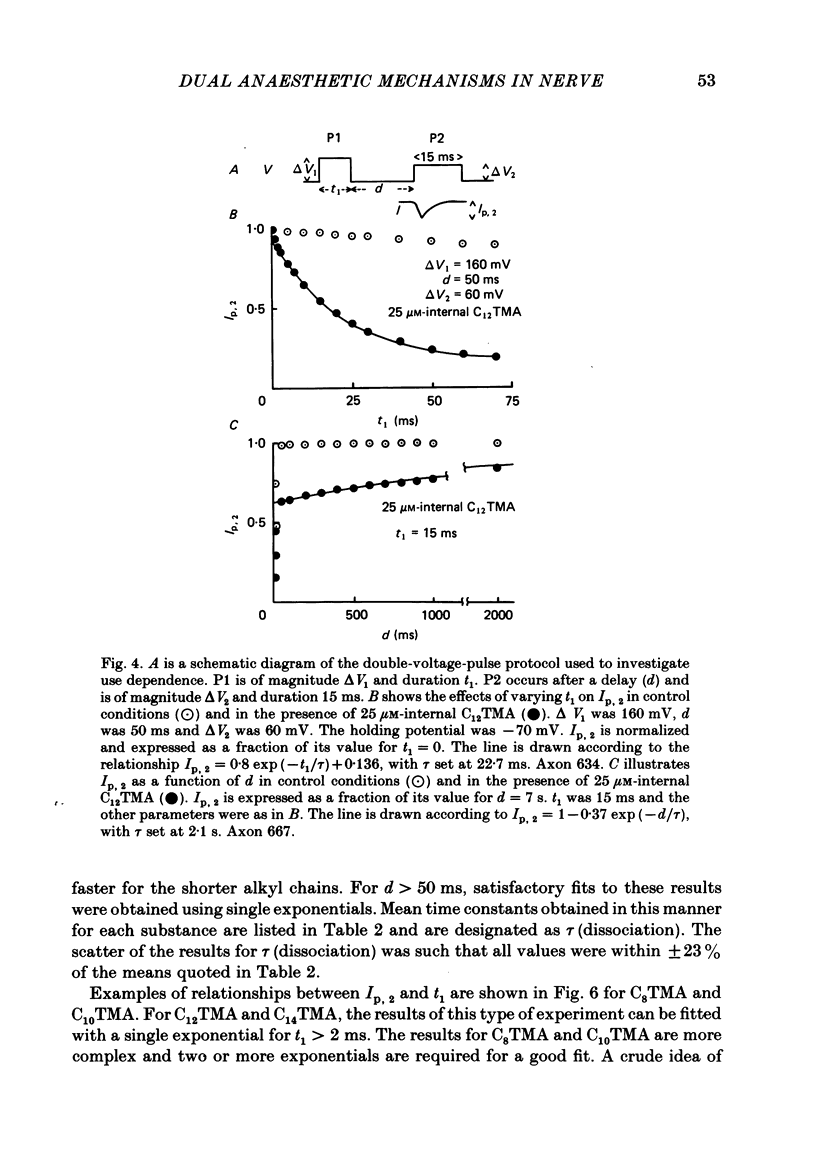
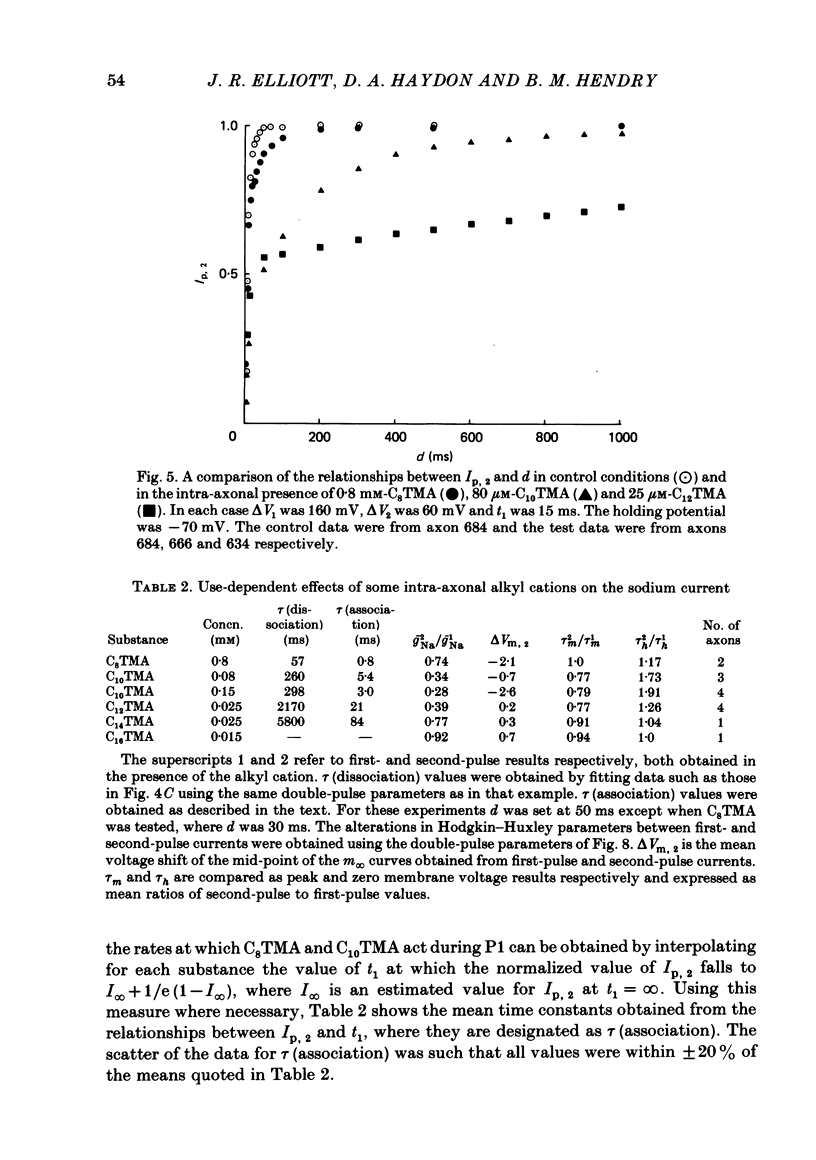
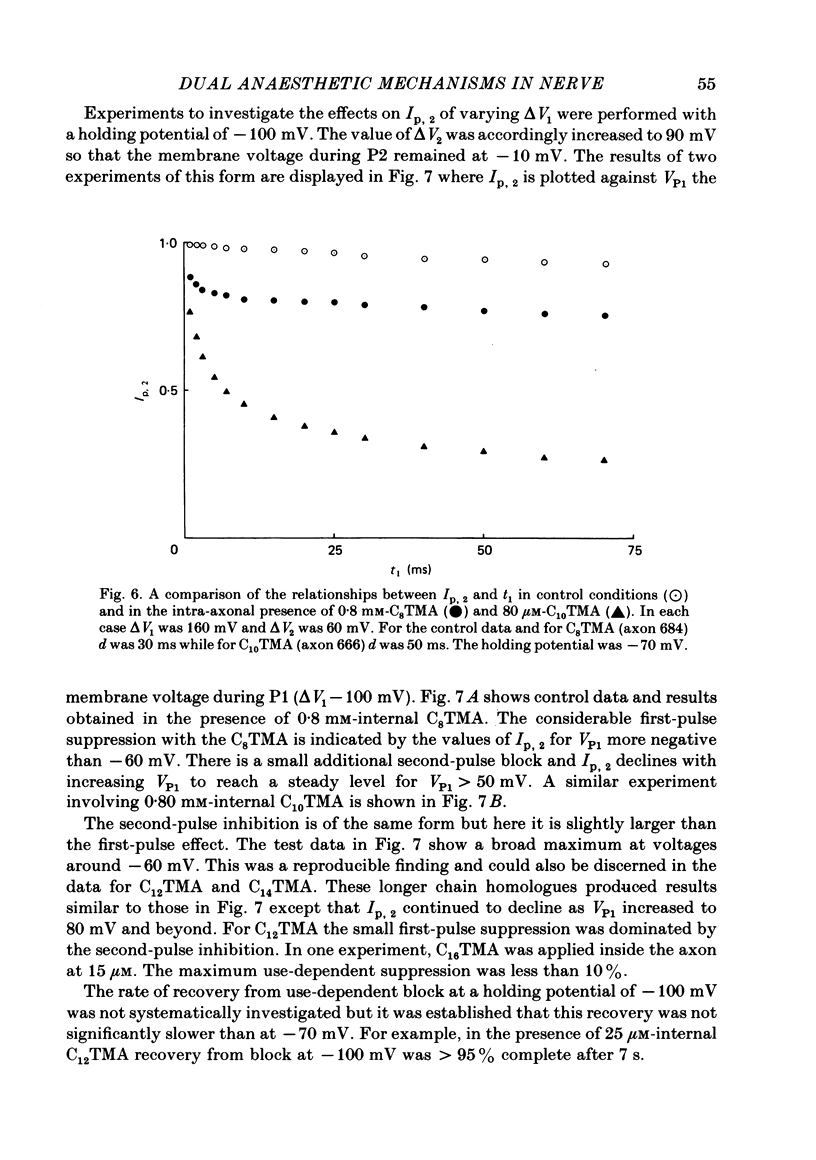
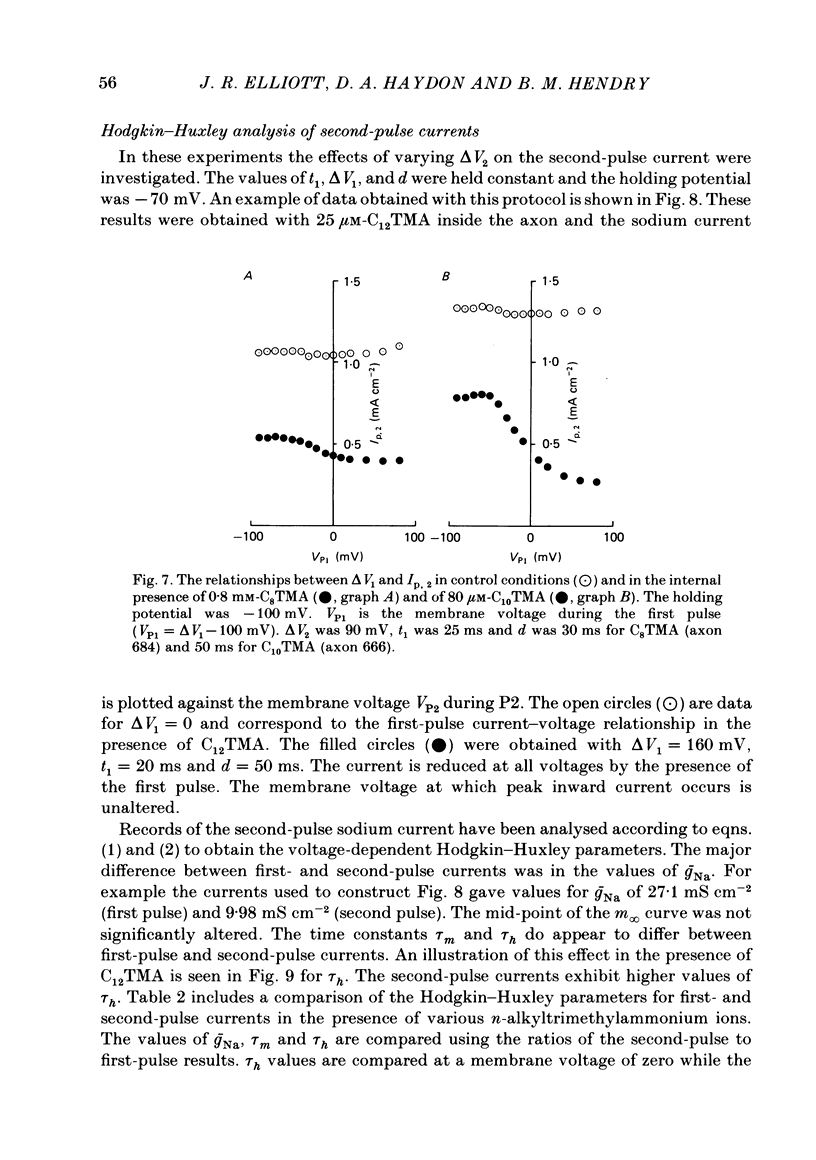
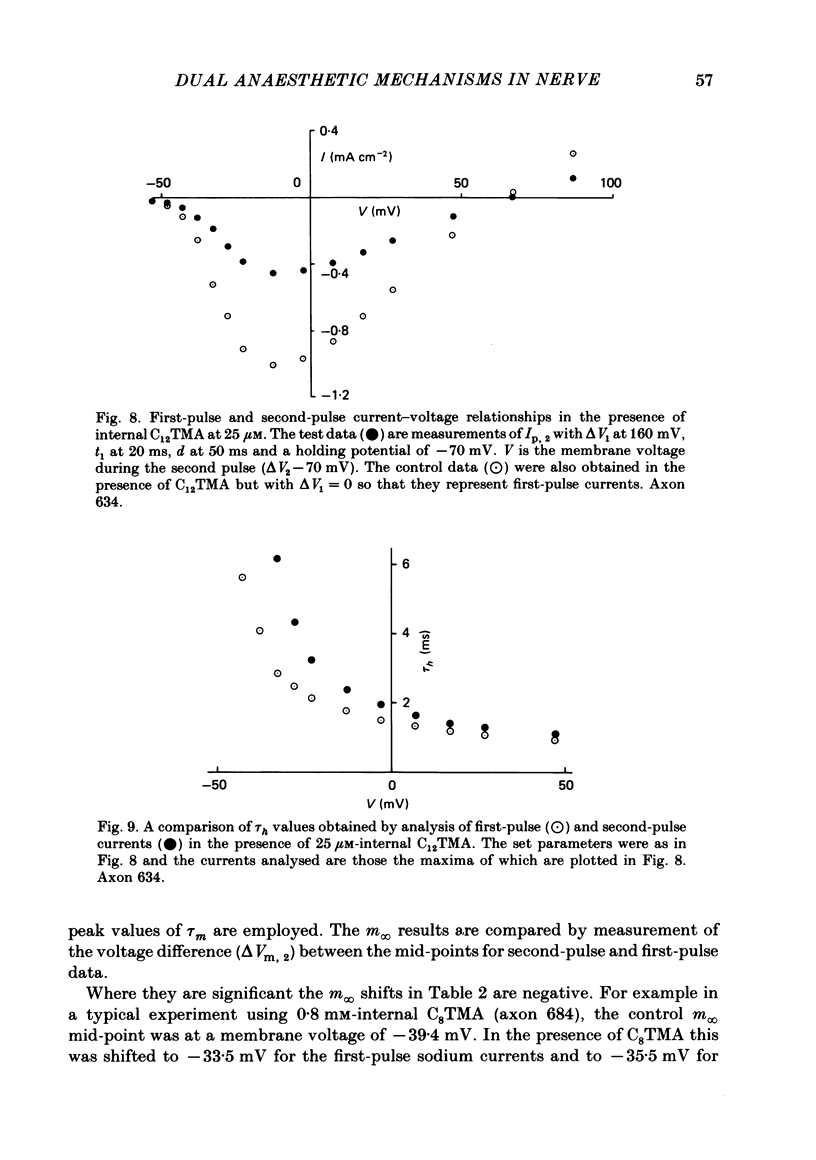
The actions of members of the homologous series of alkyl cations CH3 (CH2)n-1 N+ (CH3)3 (Cn TMA) on the sodium current in giant axons of Loligo forbesi have been investigated. The substances tested correspond to n = 6, 8, 10, 12, 14 and 16. These cations only produced significant sodium current suppression when applied inside the axon. Actions on first-pulse sodium currents and use-dependent effects were separately studied. The shorter members of the series (C6TMA and C8TMA) produced suppression of first-pulse sodium currents without causing significant use dependence. The first-pulse suppression arose partly from a positive shift along the voltage axis of the steady-state activation parameter (m infinity) and partly from a reduction in the maximum sodium conductance (gNa). C12TMA and C14TMA produced little first-pulse suppression but caused clear use dependence. C10TMA showed intermediate properties while C16TMA was inactive. The use-dependent actions have been quantitatively investigated using a double-pulse protocol. The results are consistent with a model in which the cations enter a blocking site on the ion-channel via the intra-axonal aqueous phase. The cations appear able to bind to inactivated sodium channels at significant rates. The possible molecular locations of the sites responsible for m infinity shifts and use dependence are discussed. It is argued that the existence of two separate sites may help to explain certain distinctions between the actions of neutral general anaesthetics and clinical local anaesthetics on the sodium channel.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M. Inactivation of the potassium conductance and related phenomena caused by quaternary ammonium ion injection in squid axons. J Gen Physiol. 1969 Nov;54(5):553–575. doi: 10.1085/jgp.54.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRINK F., POSTERNAK J. M. Thermodynamic analysis of the relative effectiveness of narcotics. J Cell Physiol. 1948 Oct;32(2):211–233. doi: 10.1002/jcp.1030320208. [DOI] [PubMed] [Google Scholar]
- Cahalan M. D. Local anesthetic block of sodium channels in normal and pronase-treated squid giant axons. Biophys J. 1978 Aug;23(2):285–311. doi: 10.1016/S0006-3495(78)85449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Evidence for two types of sodium conductance in axons perfused with sodium fluoride solution. J Physiol. 1970 Dec;211(3):653–678. doi: 10.1113/jphysiol.1970.sp009298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney K. R. Mechanism of frequency-dependent inhibition of sodium currents in frog myelinated nerve by the lidocaine derivative GEA. J Pharmacol Exp Ther. 1975 Nov;195(2):225–236. [PubMed] [Google Scholar]
- Courtney K. R. Structure-activity relations for frequency-dependent sodium channel block in nerve by local anesthetics. J Pharmacol Exp Ther. 1980 Apr;213(1):114–119. [PubMed] [Google Scholar]
- Elliott J. R., Haydon D. A., Hendry B. M. The asymmetrical effects of some ionized n-octyl derivatives on the sodium current of the giant axon of Loligo forbesi. J Physiol. 1984 May;350:429–445. doi: 10.1113/jphysiol.1984.sp015210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Hendry B. M., Levinson S. R., Requena J. Anaesthesia by the n-alkanes. A comparative study of nerve impulse blockage and the properties of black lipid bilayer membranes. Biochim Biophys Acta. 1977 Oct 3;470(1):17–34. doi: 10.1016/0005-2736(77)90058-x. [DOI] [PubMed] [Google Scholar]
- Haydon D. A., Hendry B. M. Nerve impulse blockage in squid axons by n-alkanes: the effect of axon diameter. J Physiol. 1982 Dec;333:393–403. doi: 10.1113/jphysiol.1982.sp014460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Kimura J. E. Some effects of n-pentane on the sodium and potassium currents of the squid giant axon. J Physiol. 1981 Mar;312:57–70. doi: 10.1113/jphysiol.1981.sp013615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Requena J., Urban B. W. Some effects of aliphatic hydrocarbons on the electrical capacity and ionic currents of the squid giant axon membrane. J Physiol. 1980 Dec;309:229–245. doi: 10.1113/jphysiol.1980.sp013506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Urban B. W. The action of alcohols and other non-ionic surface active substances on the sodium current of the squid giant axon. J Physiol. 1983 Aug;341:411–427. doi: 10.1113/jphysiol.1983.sp014813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Urban B. W. The action of hydrocarbons and carbon tetrachloride on the sodium current of the squid giant axon. J Physiol. 1983 May;338:435–450. doi: 10.1113/jphysiol.1983.sp014682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Urban B. W. The effects of some inhalation anaesthetics on the sodium current of the squid giant axon. J Physiol. 1983 Aug;341:429–439. doi: 10.1113/jphysiol.1983.sp014814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977 Apr;69(4):497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura J. E., Meves H. The effect of temperature on the asymmetrical charge movement in squid giant axons. J Physiol. 1979 Apr;289:479–500. doi: 10.1113/jphysiol.1979.sp012748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle M. J., Brown K. B., Miller K. W. Can the lipid theories of anesthesia account for the cutoff in anesthetic potency in homologous series of alcohols? Mol Pharmacol. 1981 Jan;19(1):49–55. [PubMed] [Google Scholar]
- Rojas E., Rudy B. Destruction of the sodium conductance inactivation by a specific protease in perfused nerve fibres from Loligo. J Physiol. 1976 Nov;262(2):501–531. doi: 10.1113/jphysiol.1976.sp011608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strichartz G. R. The inhibition of sodium currents in myelinated nerve by quaternary derivatives of lidocaine. J Gen Physiol. 1973 Jul;62(1):37–57. doi: 10.1085/jgp.62.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]


