Abstract
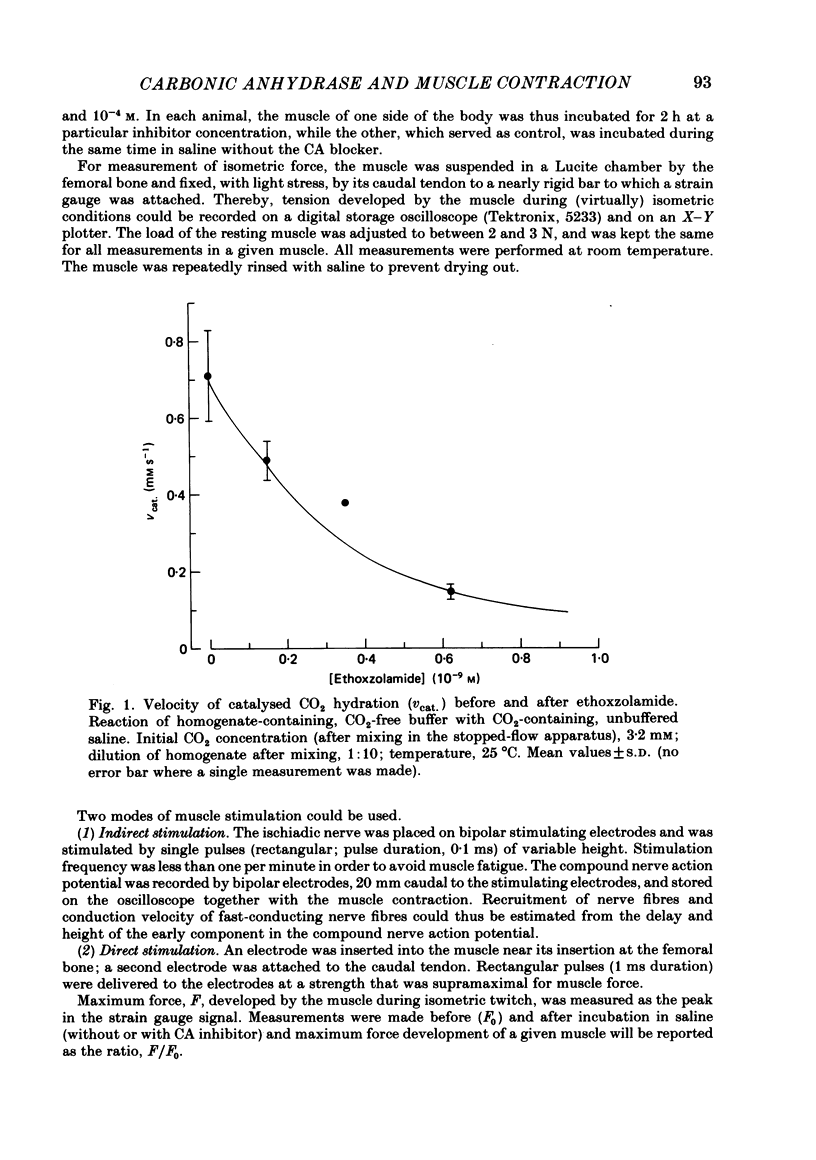
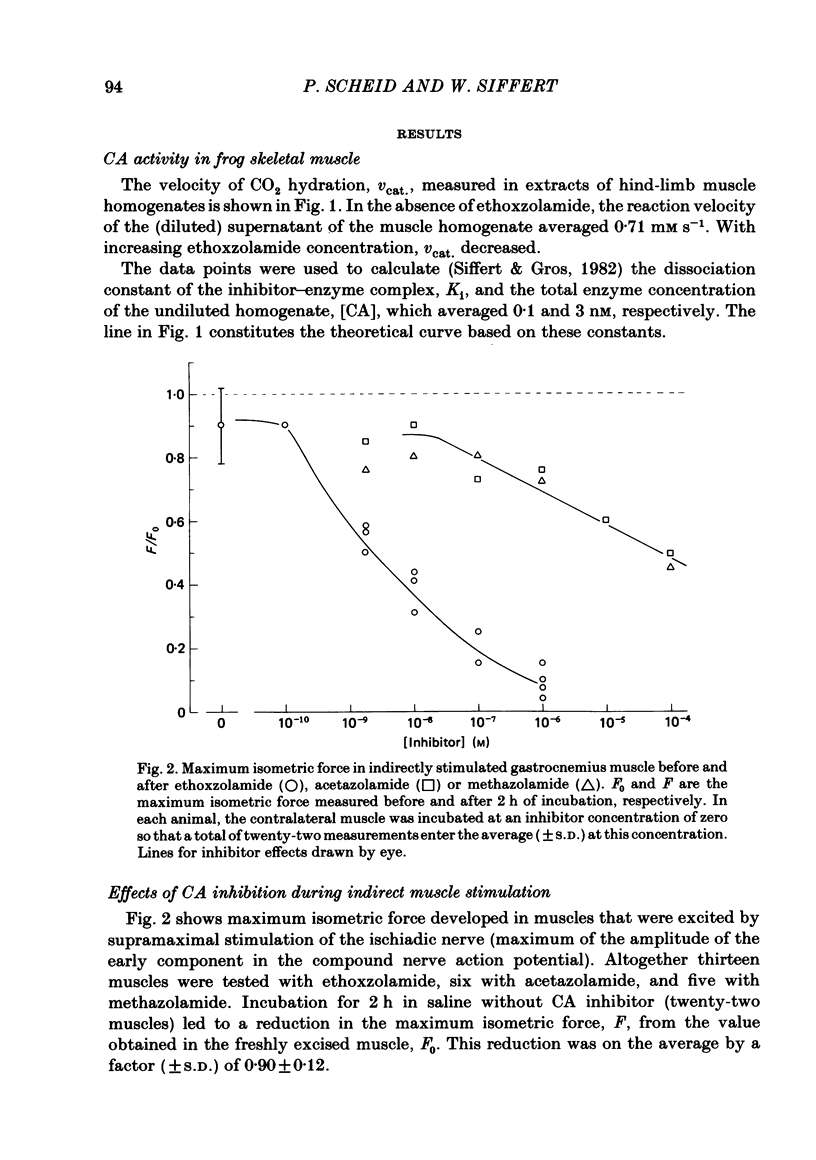
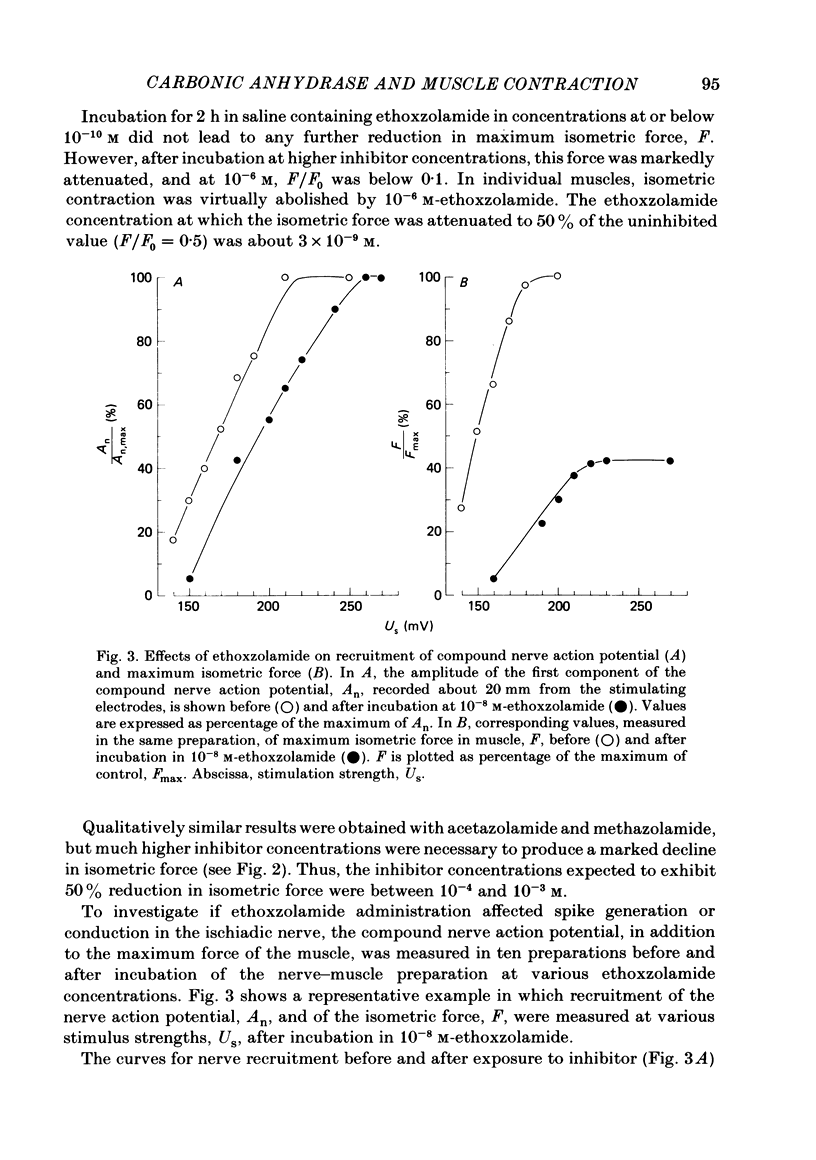
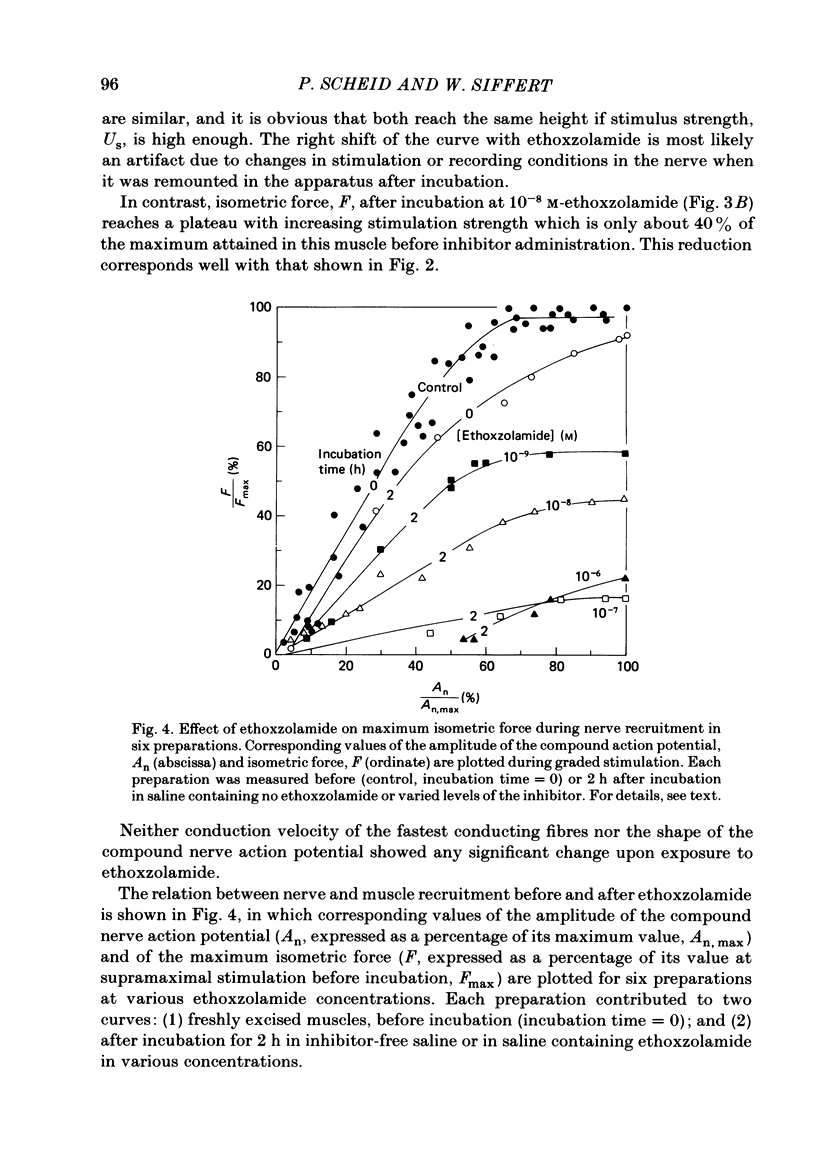
Carbonic anhydrase (CA) activity was determined in a homogenate of frog skeletal muscle by measuring the kinetics of CO2 hydration in a pH stopped-flow apparatus. The results suggest that frog skeletal muscle contains a high-activity CA with properties similar to those of the isoenzyme CA II found in white skeletal muscle tissue of the rabbit. In an attempt to assess the functional significance of CA in skeletal muscle, the maximal isometric force of frog gastrocnemius muscle was measured in response to direct or indirect (ischiadic nerve) single-pulse electrical stimulation before (control) and after exposing the muscle to various concentrations of the specific carbonic anhydrase inhibitors, ethoxzolamide, acetazolamide, and methazolamide. In the range of ethoxzolamide concentration between 10(-9) and 10(-6) M, maximal isometric force with indirect supramaximal stimulation declined progressively with inhibitor concentration to less than 10% of the control value. Acetazolamide and methazolamide were less effective in that concentrations of above 10(-4) M were necessary to inhibit maximum isometric force by 50%. Even at the highest ethoxzolamide concentration used (10(-6) M), no effect was observed either on the amplitude of the compound nerve action potential or on the conduction velocity of group I fibres in the ischiadic nerve, suggesting that ethoxzolamide did not affect the mechanisms responsible for spike generation or conduction in the motor fibres. With direct supramaximal stimulation of the gastrocnemius muscle, no effects on maximal isometric force were observed of CA inhibition by any of the inhibitors used. The results suggest that CA acts on the neuromuscular transmission. The exact site and mechanism of action are unknown.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carmignani M., Scoppetta C., Ranelletti F. O., Tonali P. Adverse interaction between acetazolamide and anticholinesterase drugs at the normal and myasthenic neuromuscular junction level. Int J Clin Pharmacol Ther Toxicol. 1984 Mar;22(3):140–144. [PubMed] [Google Scholar]
- Carter M. J. Carbonic anhydrase: isoenzymes, properties, distribution, and functional significance. Biol Rev Camb Philos Soc. 1972 Nov;47(4):465–513. doi: 10.1111/j.1469-185x.1972.tb01079.x. [DOI] [PubMed] [Google Scholar]
- Dalakas M. C., Engel W. K. Treatment of "permanent" muscle weakness in familial Hypokalemic Periodic Paralysis. Muscle Nerve. 1983 Mar-Apr;6(3):182–186. doi: 10.1002/mus.880060303. [DOI] [PubMed] [Google Scholar]
- Gros G., Forster R. E., Lin L. The carbamate reaction of glycylglycine, plasma, and tissue extracts evaluated by a pH stopped flow apparatus. J Biol Chem. 1976 Jul 25;251(14):4398–4407. [PubMed] [Google Scholar]
- Holmes R. S. Purification, molecular properties and ontogeny of carbonic anhydrase isozymes. Evidence for A, B and C isozymes in avian and mammalian tissues. Eur J Biochem. 1977 Sep;78(2):511–520. doi: 10.1111/j.1432-1033.1977.tb11764.x. [DOI] [PubMed] [Google Scholar]
- Kawashiro T., Scheid P. Measurement of Krogh's diffusion constant of CO2 in respiring muscle at various CO2 levels: evidence for facilitated diffusion. Pflugers Arch. 1976 Mar 30;362(2):127–133. doi: 10.1007/BF00583638. [DOI] [PubMed] [Google Scholar]
- Koester M. K., Pullan L. M., Noltmann E. A. The p-nitrophenyl phosphatase activity of muscle carbonic anhydrase. Arch Biochem Biophys. 1981 Oct 15;211(2):632–642. doi: 10.1016/0003-9861(81)90499-9. [DOI] [PubMed] [Google Scholar]
- Lönnerholm G. Carbonic anhydrase in rat liver and rabbit skeletal muscle: further evidence for the specificity of the histochemical cobalt-phosphate method of Hansson. J Histochem Cytochem. 1980 May;28(5):427–433. doi: 10.1177/28.5.6769996. [DOI] [PubMed] [Google Scholar]
- Maren T. H. Carbonic anhydrase: chemistry, physiology, and inhibition. Physiol Rev. 1967 Oct;47(4):595–781. doi: 10.1152/physrev.1967.47.4.595. [DOI] [PubMed] [Google Scholar]
- Maren T. H., Jankowska L., Sanyal G., Edelhauser H. F. The transcorneal permeability of sulfonamide carbonic anhydrase inhibitors and their effect on aqueous humor secretion. Exp Eye Res. 1983 Apr;36(4):457–479. doi: 10.1016/0014-4835(83)90041-6. [DOI] [PubMed] [Google Scholar]
- Maren T. H., Sanyal G. The activity of sulfonamides and anions against the carbonic anhydrases of animals, plants, and bacteria. Annu Rev Pharmacol Toxicol. 1983;23:439–459. doi: 10.1146/annurev.pa.23.040183.002255. [DOI] [PubMed] [Google Scholar]
- Micro-electrode measurement of the intracellular pH and buffering power of mouse soleus muscle fibres. J Physiol. 1977 Jun;267(3):791–810. doi: 10.1113/jphysiol.1977.sp011838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Register A. M., Koester M. K., Noltmann E. A. Discovery of carbonic anhydrase in rabbit skeletal muscle and evidence for its identity with "basic muscle protein". J Biol Chem. 1978 Jun 25;253(12):4143–4152. [PubMed] [Google Scholar]
- Renaud J. M., Stevens E. D. Effect of acclimation temperature and pH on contraction of frog sartorius muscle. Am J Physiol. 1981 May;240(5):R301–R309. doi: 10.1152/ajpregu.1981.240.5.R301. [DOI] [PubMed] [Google Scholar]
- Renaud J. M., Stevens E. D. Effects of step changes in pH on isometric tetanic tension of toad sartorius muscle. Can J Physiol Pharmacol. 1983 Aug;61(8):830–835. doi: 10.1139/y83-127. [DOI] [PubMed] [Google Scholar]
- Riley D. A., Ellis S., Bain J. Carbonic anhydrase activity in skeletal muscle fiber types, axons, spindles, and capillaries of rat soleus and extensor digitorum longus muscles. J Histochem Cytochem. 1982 Dec;30(12):1275–1288. doi: 10.1177/30.12.6218195. [DOI] [PubMed] [Google Scholar]
- Sanyal G., Swenson E. R., Pessah N. I., Maren T. H. The carbon dioxide hydration activity of skeletal muscle carbonic anhydrase. Inhibition by sulfonamides and anions. Mol Pharmacol. 1982 Jul;22(1):211–220. [PubMed] [Google Scholar]
- Siffert W., Gros G. Carbonic anhydrase C in white-skeletal-muscle tissue. Biochem J. 1982 Sep 1;205(3):559–566. doi: 10.1042/bj2050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. The effect of carbon dioxide on the intracellular pH and buffering power of snail neurones. J Physiol. 1976 Mar;255(3):715–735. doi: 10.1113/jphysiol.1976.sp011305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vänänen H. K., Kumpulainen T., Korhonen L. K. Carbonic anhydrase in the type I skeletal muscle fibers of the rat. An immunohistochemical study. J Histochem Cytochem. 1982 Nov;30(11):1109–1113. doi: 10.1177/30.11.6216280. [DOI] [PubMed] [Google Scholar]
- Whitney P. L., Briggle T. V. Membrane-associated carbonic anhydrase purified from bovine lung. J Biol Chem. 1982 Oct 25;257(20):12056–12059. [PubMed] [Google Scholar]
- Zborowska-Sluis D. T., L'Abbate A., Klassen G. A. Evidence of carbonic anhydrase activity in skeletal muscle: a role for facilitative carbon dioxide transport. Respir Physiol. 1974 Sep;21(3):341–350. doi: 10.1016/0034-5687(74)90064-4. [DOI] [PubMed] [Google Scholar]


