Abstract
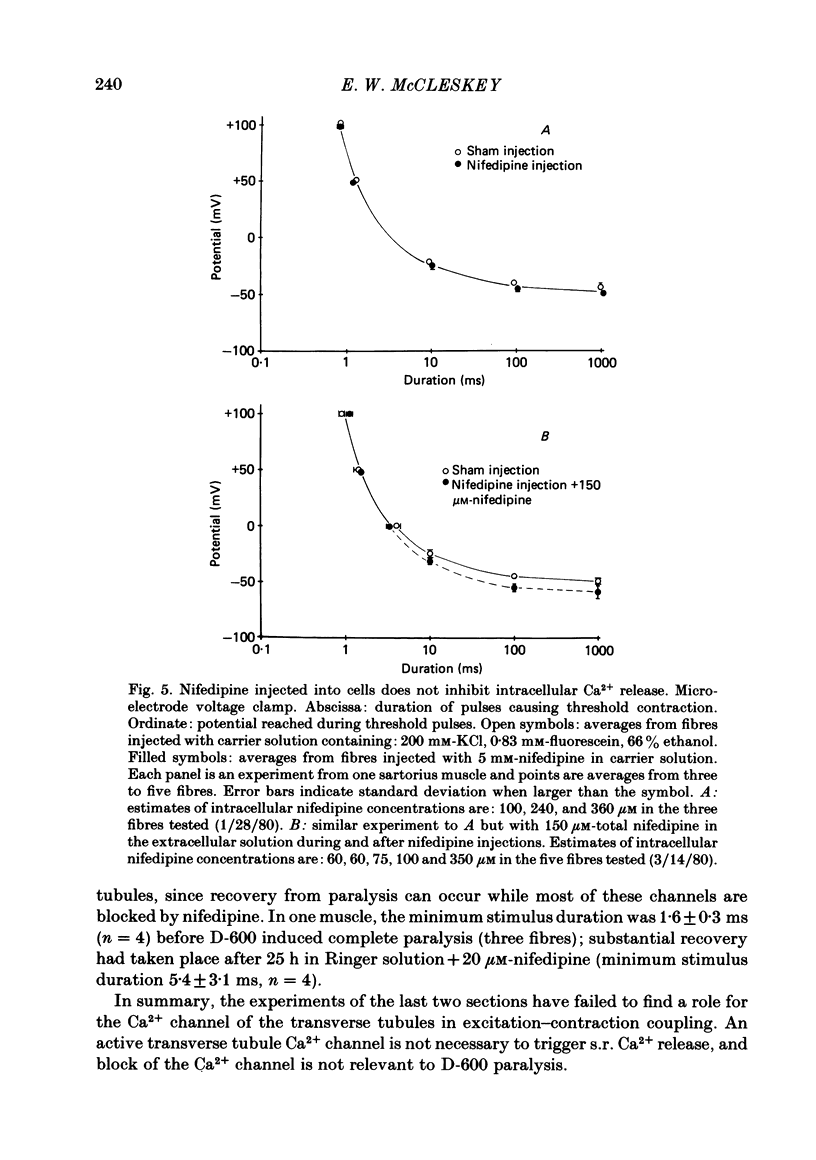
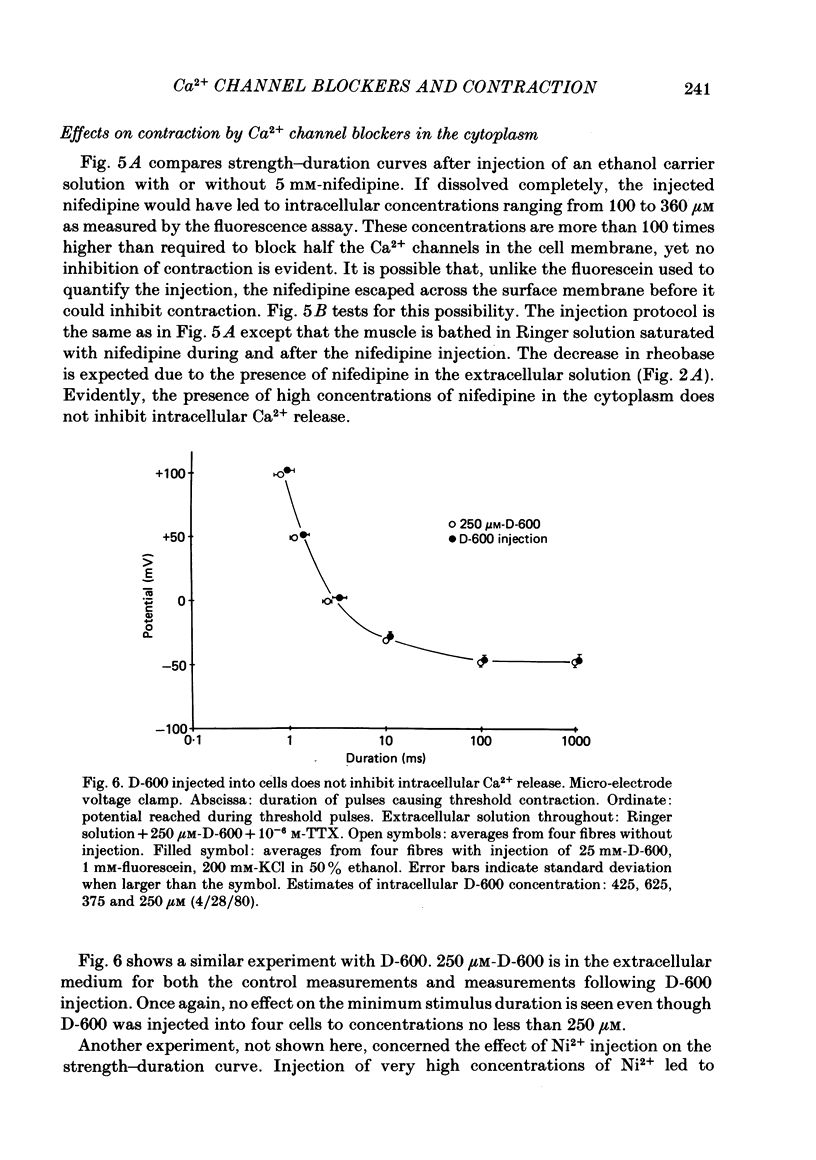
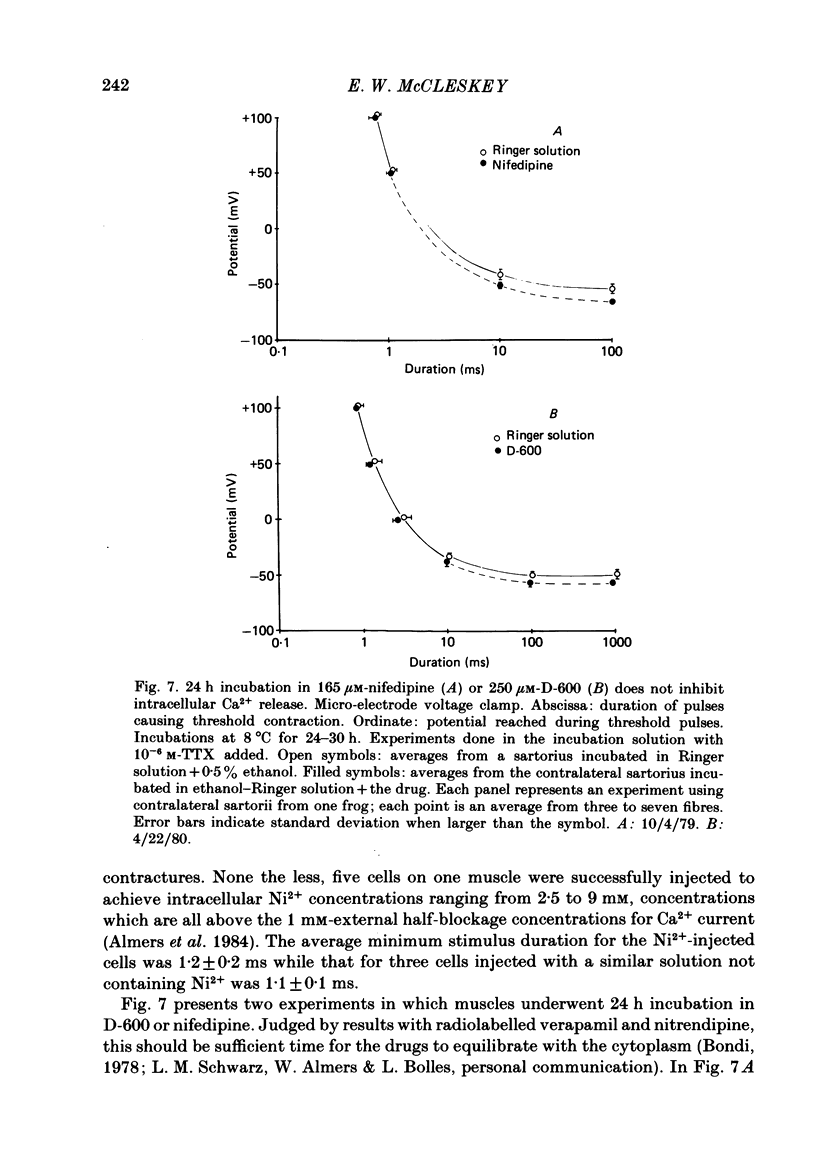
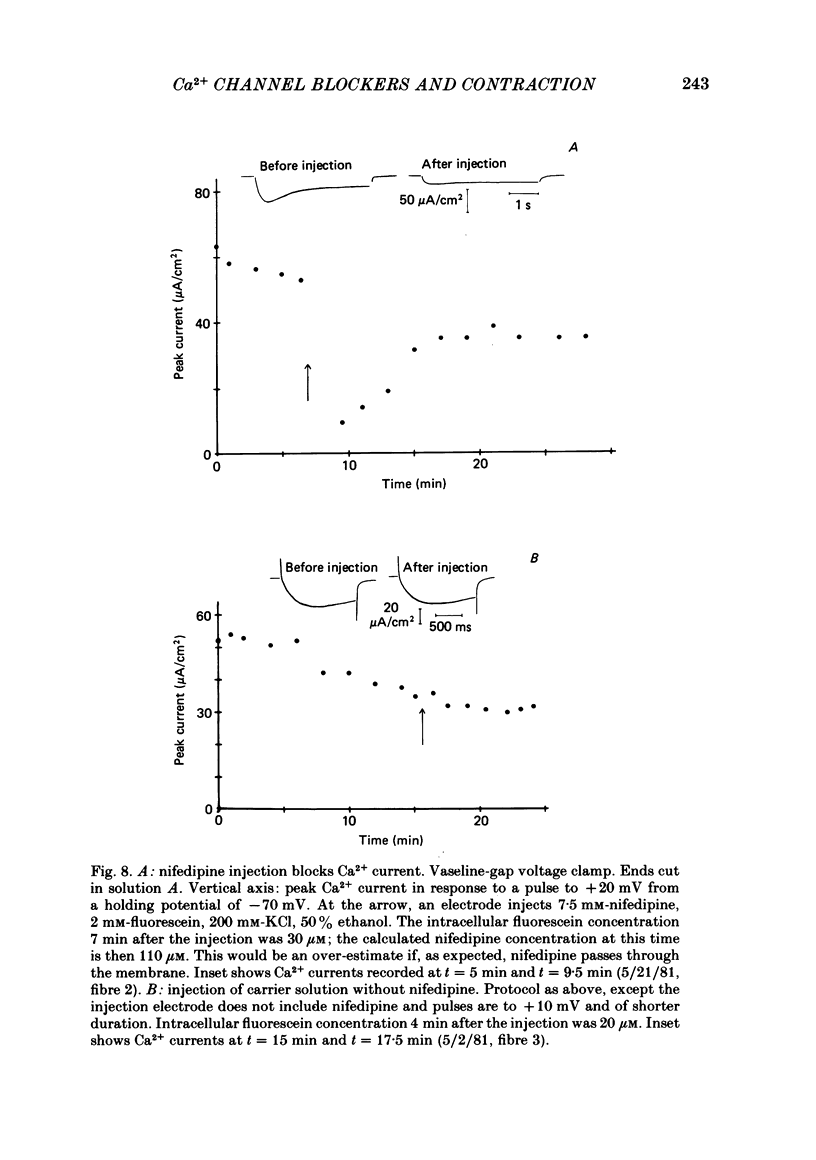
The pharmacology of Ca2+ channels and intracellular Ca2+ release from the sarcoplasmic reticulum (s.r.) were compared by injecting Ca2+ channel blockers into the cytoplasm and observing contraction under voltage clamp of frog skeletal muscle fibres, a preparation that contracts only in response to Ca2+ release from the s.r. A method for quantifying intracellular injections by co-injecting a fluorescent dye is described. Nifedipine injected into cells blocks Ca2+ current through the cell membrane showing that nifedipine is active when applied to the cytoplasmic side of the membrane in which Ca2+ channels are located. Neither the presence of Ca2+ channel blockers in the extracellular medium nor 24 h incubation in nifedipine and D-600 affect contraction. Nifedipine and D-600 injected to intracellular concentrations much greater than necessary to block Ca2+ channels do not affect contraction. The presence of 30 microM-D-600 during K+ contractures caused paralysis but 20 microM-nifedipine did not. Thus, contracture-dependent D-600 paralysis is not due to blockade of the transverse tubule Ca2+ channel. It is concluded that: (a) a functioning Ca2+ channel on the cell membrane is not necessary to trigger Ca2+ release from the s.r.; (b) s.r. Ca2+ release and Ca2+ channels are pharmacologically different.
Full text
PDF

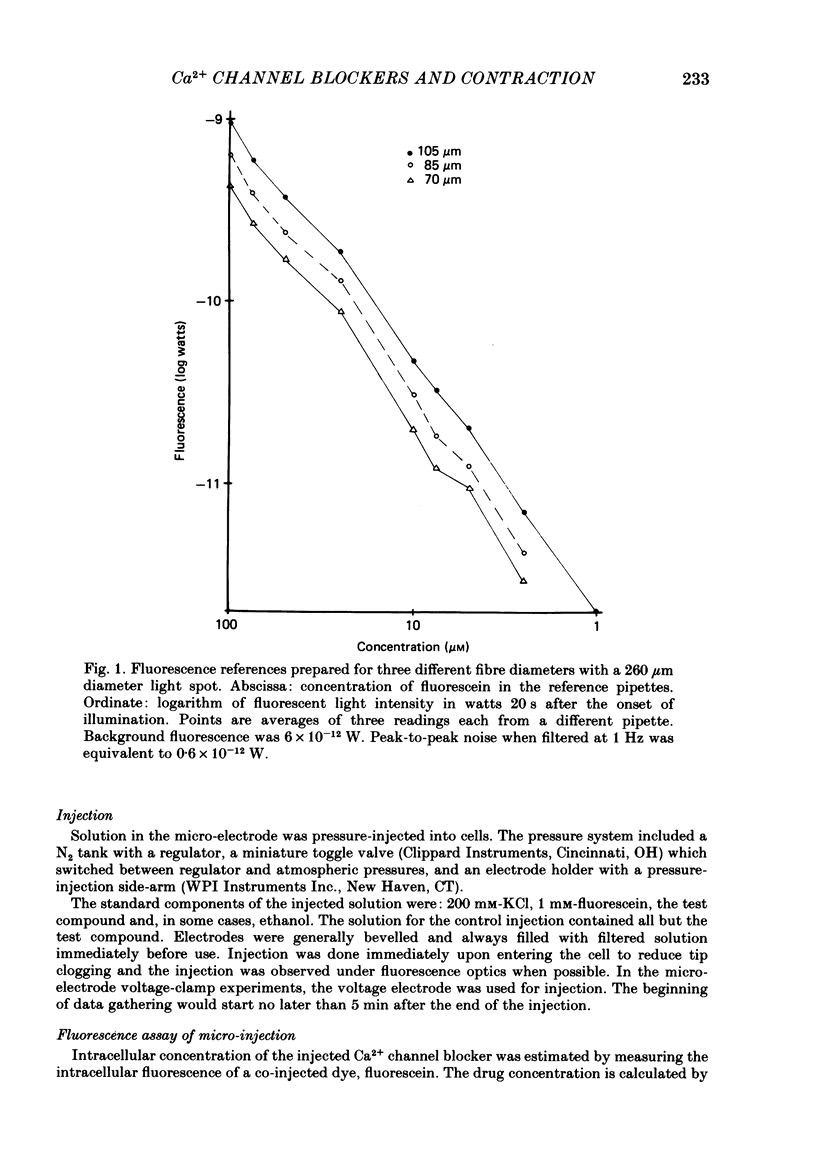


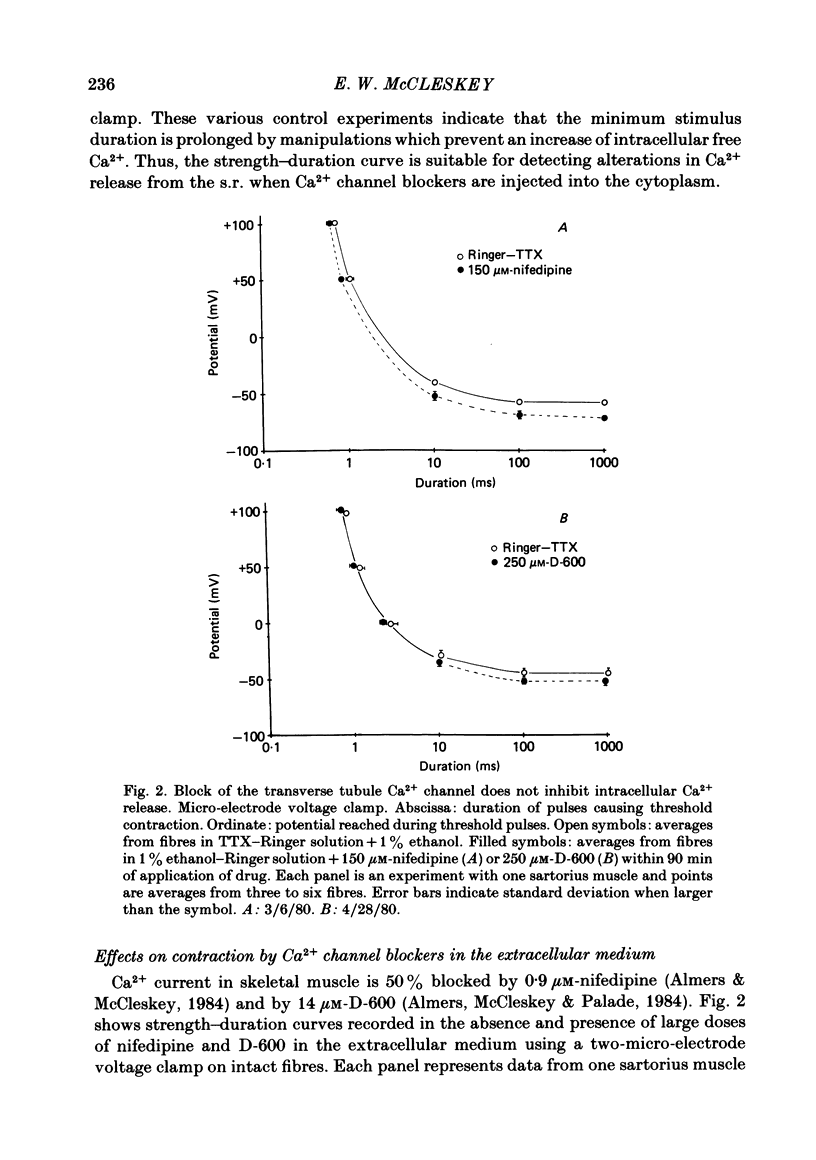
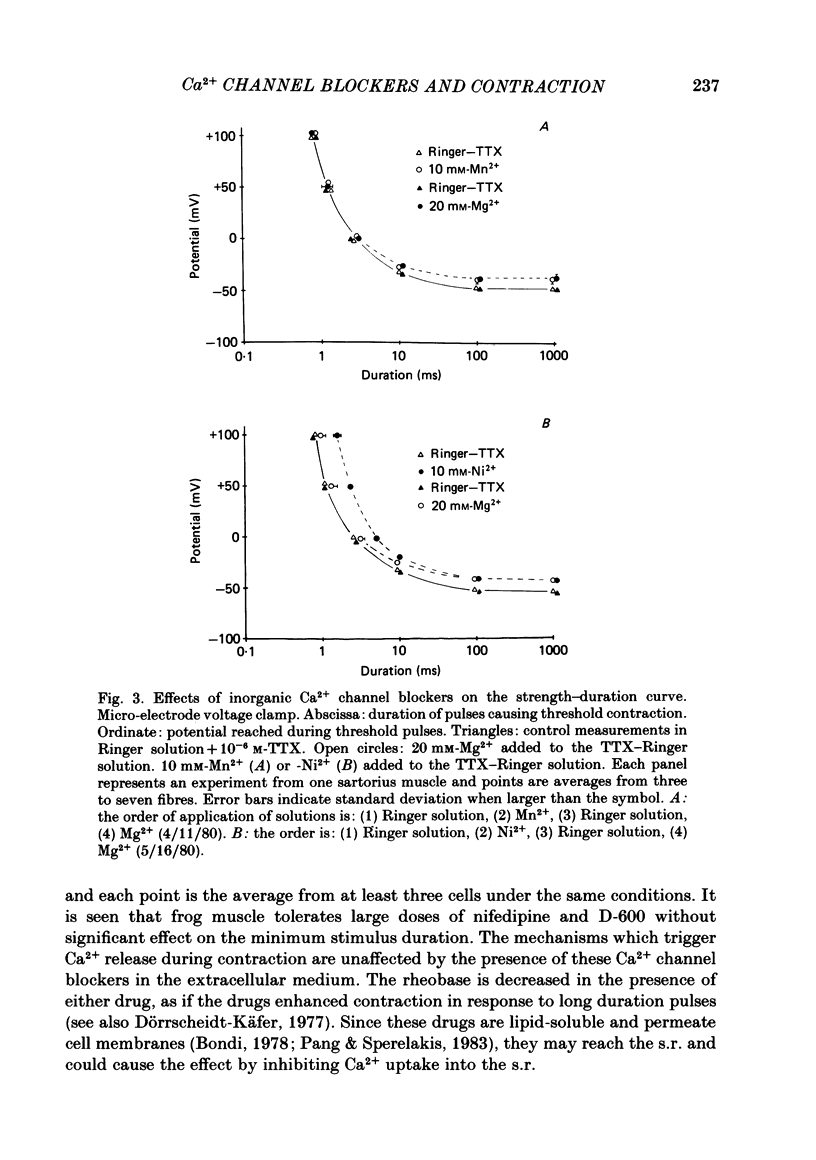

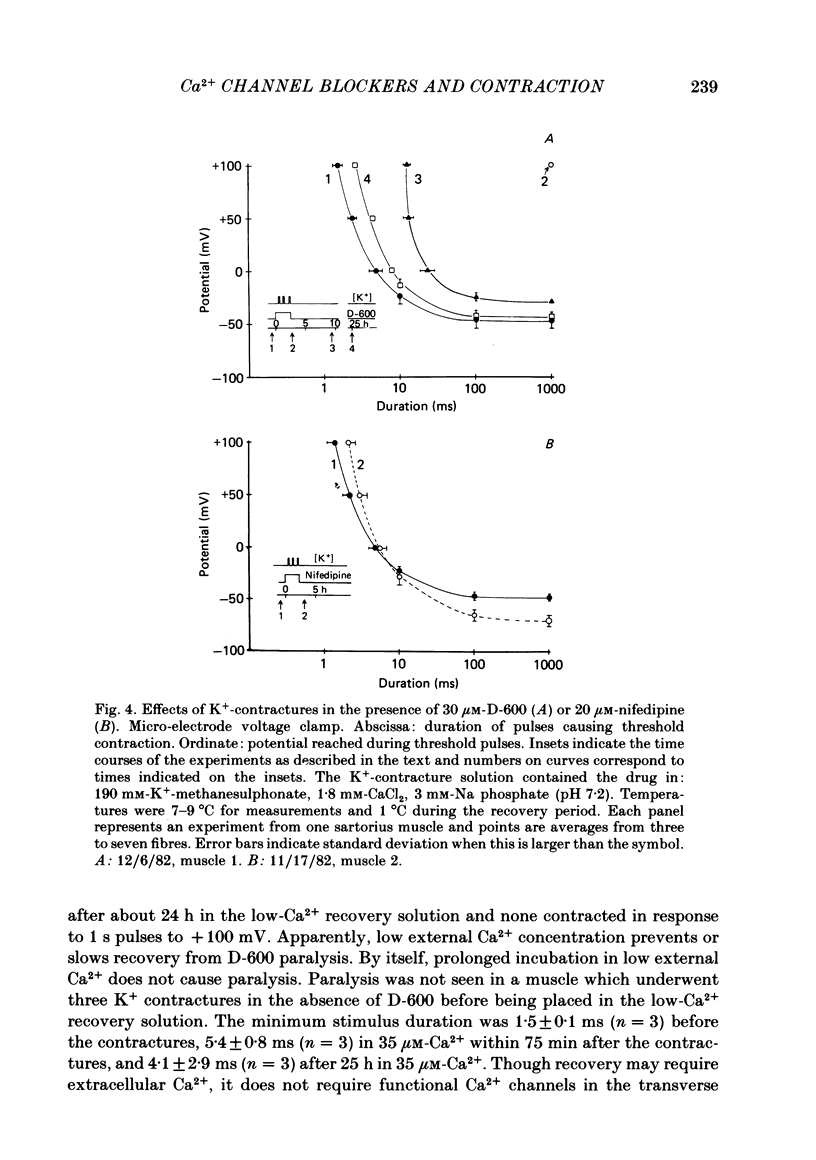






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Chandler W. K., Hodgkin A. L. The kinetics of mechanical activation in frog muscle. J Physiol. 1969 Sep;204(1):207–230. doi: 10.1113/jphysiol.1969.sp008909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W., Best P. M. Effects of tetracaine on displacement currents and contraction of frog skeletal muscle. J Physiol. 1976 Nov;262(3):583–611. doi: 10.1113/jphysiol.1976.sp011611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W. Differential effects of tetracaine on delayed potassium channels and displacement currents in frog skeletal muscle. J Physiol. 1976 Nov;262(3):613–637. doi: 10.1113/jphysiol.1976.sp011612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W., Fink R., Palade P. T. Calcium depletion in frog muscle tubules: the decline of calcium current under maintained depolarization. J Physiol. 1981 Mar;312:177–207. doi: 10.1113/jphysiol.1981.sp013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W., McCleskey E. W. Non-selective conductance in calcium channels of frog muscle: calcium selectivity in a single-file pore. J Physiol. 1984 Aug;353:585–608. doi: 10.1113/jphysiol.1984.sp015352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W., Palade P. T. Slow calcium and potassium currents across frog muscle membrane: measurements with a vaseline-gap technique. J Physiol. 1981 Mar;312:159–176. doi: 10.1113/jphysiol.1981.sp013622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W. Potassium conductance changes in skeletal muscle and the potassium concentration in the transverse tubules. J Physiol. 1972 Aug;225(1):33–56. doi: 10.1113/jphysiol.1972.sp009928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F. M., Horowicz P. Twitches in the presence of ethylene glycol bis( -aminoethyl ether)-N,N'-tetracetic acid. Biochim Biophys Acta. 1972 Jun 23;267(3):605–608. doi: 10.1016/0005-2728(72)90194-6. [DOI] [PubMed] [Google Scholar]
- Barrett N., Barrett E. F. Excitation-contraction coupling in skeletal muscle: blockade by high extracellular concentrations of calcium buffers. Science. 1978 Jun 16;200(4347):1270–1272. doi: 10.1126/science.96524. [DOI] [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Sarcoplasmic reticulum calcium release in frog skeletal muscle fibres estimated from Arsenazo III calcium transients. J Physiol. 1983 Nov;344:625–666. doi: 10.1113/jphysiol.1983.sp014959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellemann P., Ferry D., Lübbecke F., Glossman H. [3H]-Nitrendipine, a potent calcium antagonist, binds with high affinity to cardiac membranes. Arzneimittelforschung. 1981;31(12):2064–2067. [PubMed] [Google Scholar]
- Bolger G. T., Gengo P. J., Luchowski E. M., Siegel H., Triggle D. J., Janis R. A. High affinity binding of a calcium channel antagonist to smooth and cardiac muscle. Biochem Biophys Res Commun. 1982 Feb 26;104(4):1604–1609. doi: 10.1016/0006-291x(82)91436-x. [DOI] [PubMed] [Google Scholar]
- Bondi A. Y. Effects of verapamil on excitation-contraction coupling in frog sartorius muscle. J Pharmacol Exp Ther. 1978 Apr;205(1):49–57. [PubMed] [Google Scholar]
- Conti F., Hille B., Neumcke B., Nonner W., Stämpfli R. Measurement of the conductance of the sodium channel from current fluctuations at the node of Ranvier. J Physiol. 1976 Nov;262(3):699–727. doi: 10.1113/jphysiol.1976.sp011616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantin L. L. The effect o f calcium on contraction and conductance thresholds in frog skeletal muscle. J Physiol. 1968 Mar;195(1):119–132. doi: 10.1113/jphysiol.1968.sp008450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis B. M., Catterall W. A. Purification of the calcium antagonist receptor of the voltage-sensitive calcium channel from skeletal muscle transverse tubules. Biochemistry. 1984 May 8;23(10):2113–2118. doi: 10.1021/bi00305a001. [DOI] [PubMed] [Google Scholar]
- Eisenberg R. S., McCarthy R. T., Milton R. L. Paralysis of frog skeletal muscle fibres by the calcium antagonist D-600. J Physiol. 1983 Aug;341:495–505. doi: 10.1113/jphysiol.1983.sp014819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M. Entry of fluorescent dyes into the sarcotubular system of the frog muscle. J Physiol. 1966 Jul;185(1):224–238. doi: 10.1113/jphysiol.1966.sp007983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M., Tanaka M., Ogawa Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature. 1970 Oct 3;228(5266):34–36. doi: 10.1038/228034a0. [DOI] [PubMed] [Google Scholar]
- Fairhurst A. S., Thayer S. A., Colker J. E., Beatty D. A. A calcium antagonist drug binding site in skeletal muscle sarcoplasmic reticulum: evidence for a calcium channel. Life Sci. 1983 Mar 21;32(12):1331–1339. doi: 10.1016/0024-3205(83)90807-x. [DOI] [PubMed] [Google Scholar]
- Ferry D. R., Goll A., Glossmann H. Differential labelling of putative skeletal muscle calcium channels by [3H]-nifedipine, [3H]-nitrendipine, [3H]-nimodipine and [3H]-PN 200 110. Naunyn Schmiedebergs Arch Pharmacol. 1983 Jul;323(3):276–277. doi: 10.1007/BF00497674. [DOI] [PubMed] [Google Scholar]
- Ford L. E., Podolsky R. J. Regenerative calcium release within muscle cells. Science. 1970 Jan 2;167(3914):58–59. doi: 10.1126/science.167.3914.58. [DOI] [PubMed] [Google Scholar]
- Fosset M., Jaimovich E., Delpont E., Lazdunski M. [3H]nitrendipine receptors in skeletal muscle. J Biol Chem. 1983 May 25;258(10):6086–6092. [PubMed] [Google Scholar]
- Glossmann H., Ferry D. R., Boschek C. B. Purification of the putative calcium channel from skeletal muscle with the aid of [3H]-nimodipine binding. Naunyn Schmiedebergs Arch Pharmacol. 1983 Jun;323(1):1–11. doi: 10.1007/BF00498821. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Serratos H., Valle-Aguilera R., Lathrop D. A., Garcia M. C. Slow inward calcium currents have no obvious role in muscle excitation-contraction coupling. Nature. 1982 Jul 15;298(5871):292–294. doi: 10.1038/298292a0. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The effect of sudden changes in ionic concentrations on the membrane potential of single muscle fibres. J Physiol. 1960 Sep;153:370–385. doi: 10.1113/jphysiol.1960.sp006540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hescheler J., Pelzer D., Trube G., Trautwein W. Does the organic calcium channel blocker D600 act from inside or outside on the cardiac cell membrane? Pflugers Arch. 1982 Jun;393(4):287–291. doi: 10.1007/BF00581411. [DOI] [PubMed] [Google Scholar]
- Hille B., Campbell D. T. An improved vaseline gap voltage clamp for skeletal muscle fibers. J Gen Physiol. 1976 Mar;67(3):265–293. doi: 10.1085/jgp.67.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowicz P., Schneider M. F. Membrane charge moved at contraction thresholds in skeletal muscle fibres. J Physiol. 1981 May;314:595–633. doi: 10.1113/jphysiol.1981.sp013726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaumann A. J., Uchitel O. D. Reversible Inhibition of Potassium Contractures by optical isomers of verapamil and D 600 on slow muscle fibres of the frog. Naunyn Schmiedebergs Arch Pharmacol. 1976;292(1):21–27. doi: 10.1007/BF00506485. [DOI] [PubMed] [Google Scholar]
- Klitzner T., Morad M. The effects of Ni2+ on ionic currents and tension generation in frog ventricular muscle. Pflugers Arch. 1983 Sep;398(4):267–273. doi: 10.1007/BF00657236. [DOI] [PubMed] [Google Scholar]
- Kovács L., Ríos E., Schneider M. F. Calcium transients and intramembrane charge movement in skeletal muscle fibres. Nature. 1979 May 31;279(5712):391–396. doi: 10.1038/279391a0. [DOI] [PubMed] [Google Scholar]
- LUETTGAU H. C. THE ACTION OF CALCIUM IONS ON POTASSIUM CONTRACTURES OF SINGLE MUSCLE FIBRES. J Physiol. 1963 Oct;168:679–697. doi: 10.1113/jphysiol.1963.sp007215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. Mechanism of calcium channel blockade by verapamil, D600, diltiazem and nitrendipine in single dialysed heart cells. Nature. 1983 Apr 28;302(5911):790–794. doi: 10.1038/302790a0. [DOI] [PubMed] [Google Scholar]
- Lüttgau H. C., Oetliker H. The action of caffeine on the activation of the contractile mechanism in straited muscle fibres. J Physiol. 1968 Jan;194(1):51–74. doi: 10.1113/jphysiol.1968.sp008394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Weinstock M. M. Nickel and calcium ions modify the characteristics of the acetylcholine receptor-channel complex at the frog neuromuscular junction. J Physiol. 1980 Feb;299:203–218. doi: 10.1113/jphysiol.1980.sp013120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Parker I., Schalow G. Measurement of calcium transients in frog muscle by the use of arsenazo III. Proc R Soc Lond B Biol Sci. 1977 Aug 22;198(1131):201–210. doi: 10.1098/rspb.1977.0094. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I., Zhu P. H. Calcium transients studied under voltage-clamp control in frog twitch muscle fibres. J Physiol. 1983 Jul;340:649–680. doi: 10.1113/jphysiol.1983.sp014785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. Voltage-gated cation conductance channel from fragmented sarcoplasmic reticulum: steady-state electrical properties. J Membr Biol. 1978 Apr 20;40(1):1–23. doi: 10.1007/BF01909736. [DOI] [PubMed] [Google Scholar]
- Murphy K. M., Gould R. J., Snyder S. H. Autoradiographic visualization of [3H]nitrendipine binding sites in rat brain: localization to synaptic zones. Eur J Pharmacol. 1982 Jul 16;81(3):517–519. doi: 10.1016/0014-2999(82)90120-0. [DOI] [PubMed] [Google Scholar]
- Nachshen D. A., Blaustein M. P. The effects of some organic "calcium antagonists" on calcium influx in presynaptic nerve terminals. Mol Pharmacol. 1979 Sep;16(2):576–586. [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Racker E., Eytan E. A coupling factor from sarcoplasmic reticulum required for the translocation of Ca2+ ions in a reconstituted Ca2+ATPase pump. J Biol Chem. 1975 Sep 25;250(18):7533–7534. [PubMed] [Google Scholar]
- Sarmiento J. G., Janis R. A., Colvin R. A., Triggle D. J., Katz A. M. Binding of the calcium channel blocker nitrendipine to its receptor in purified sarcolemma from canine cardiac ventricle. J Mol Cell Cardiol. 1983 Feb;15(2):135–137. doi: 10.1016/0022-2828(83)90289-4. [DOI] [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973 Mar 23;242(5395):244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- Siri L. N., Sánchez J. A., Stefani E. Effect of glycerol treatment on the calcium current of frog skeletal muscle. J Physiol. 1980 Aug;305:87–96. doi: 10.1113/jphysiol.1980.sp013351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield P. R. A calcium dependent inward current in frog skeletal muscle fibres. Pflugers Arch. 1977 Apr 25;368(3):267–270. doi: 10.1007/BF00585206. [DOI] [PubMed] [Google Scholar]
- Walden J., Witte O. W., Speckmann E. J., Elger C. E. Reduction of calcium currents in identified neurons of Helix pomatia: intracellular injection of D890. Comp Biochem Physiol C. 1984;77(2):211–217. doi: 10.1016/0742-8413(84)90004-5. [DOI] [PubMed] [Google Scholar]


