Abstract
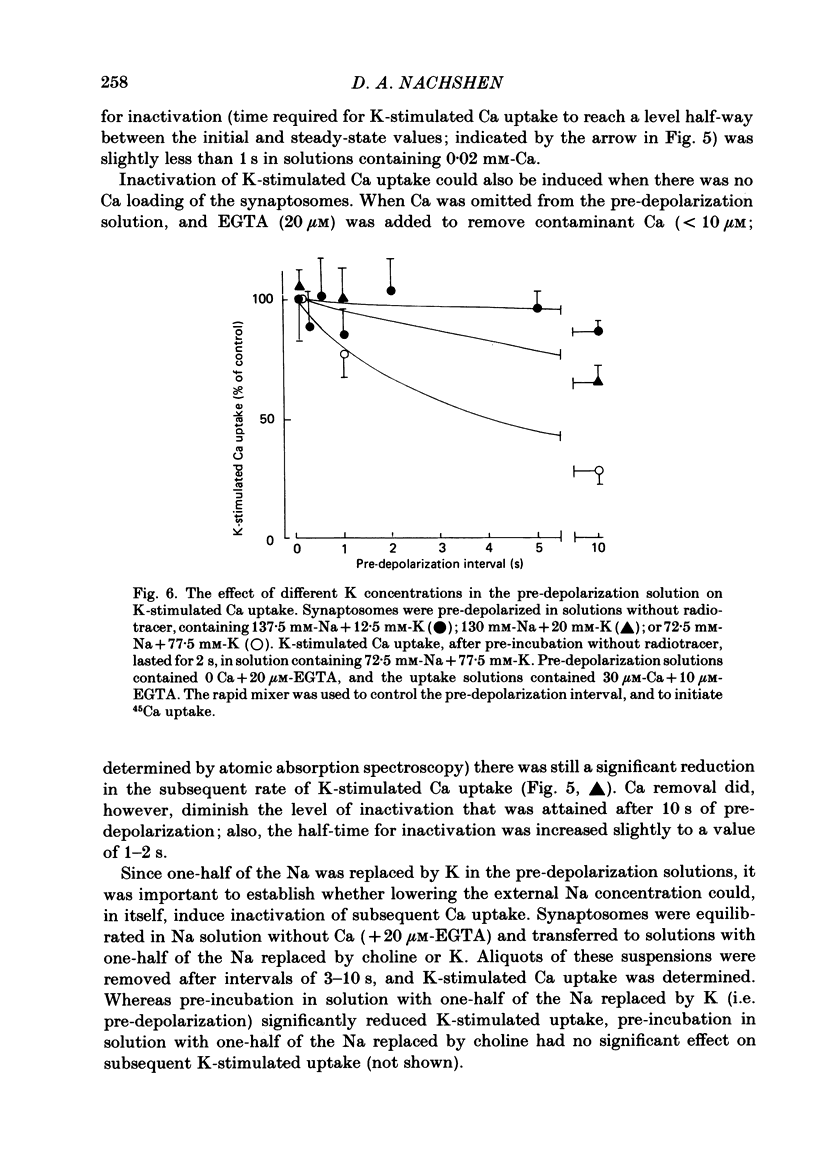
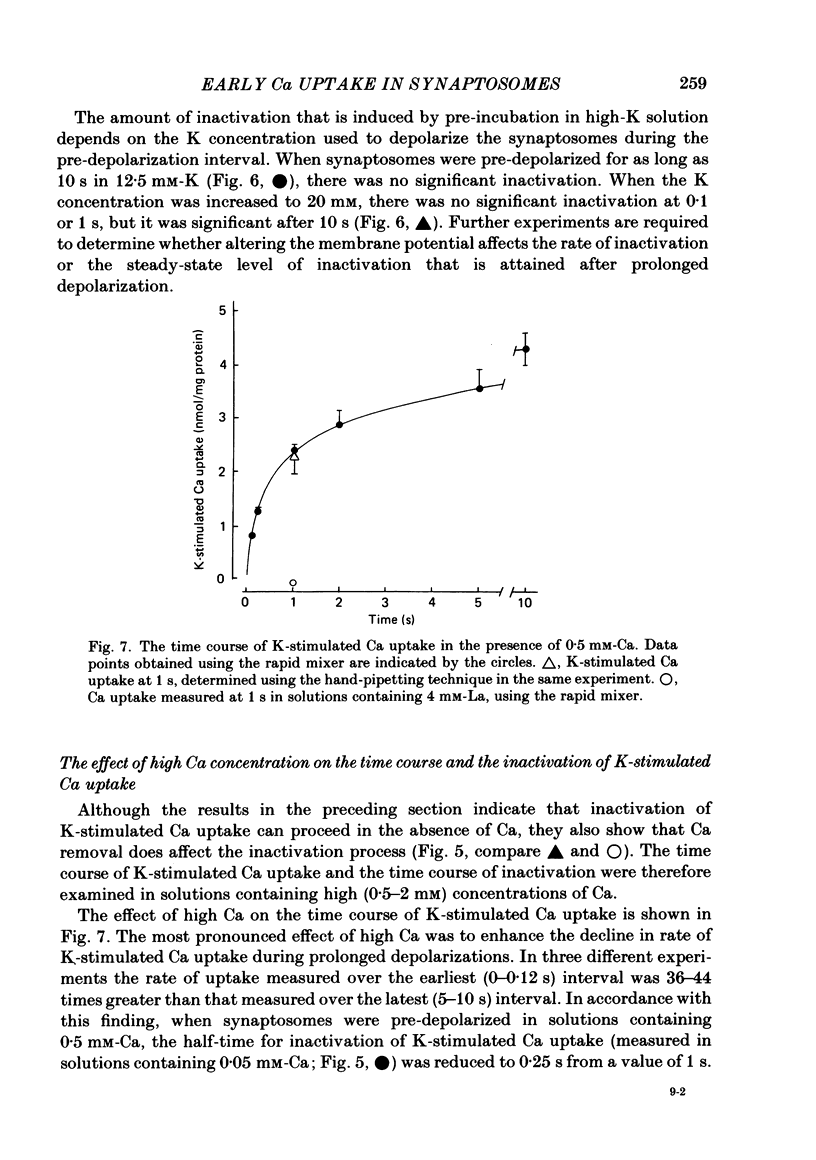
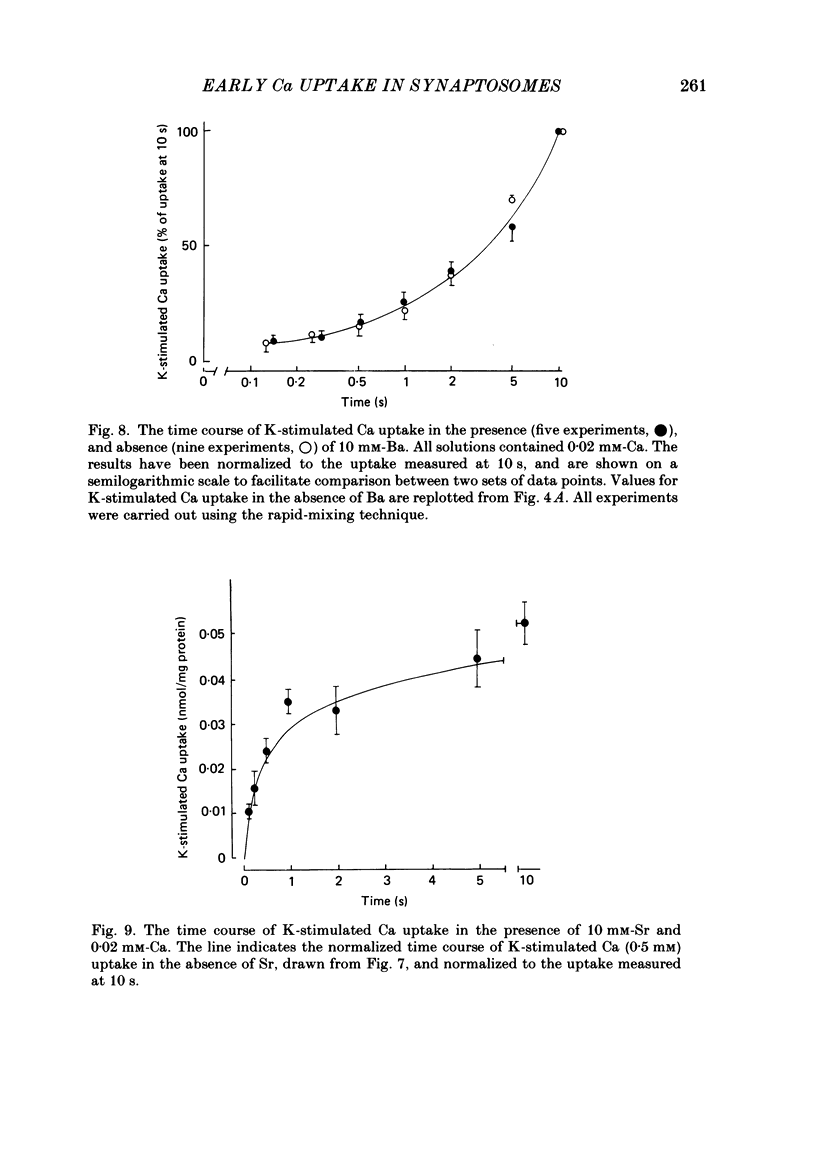
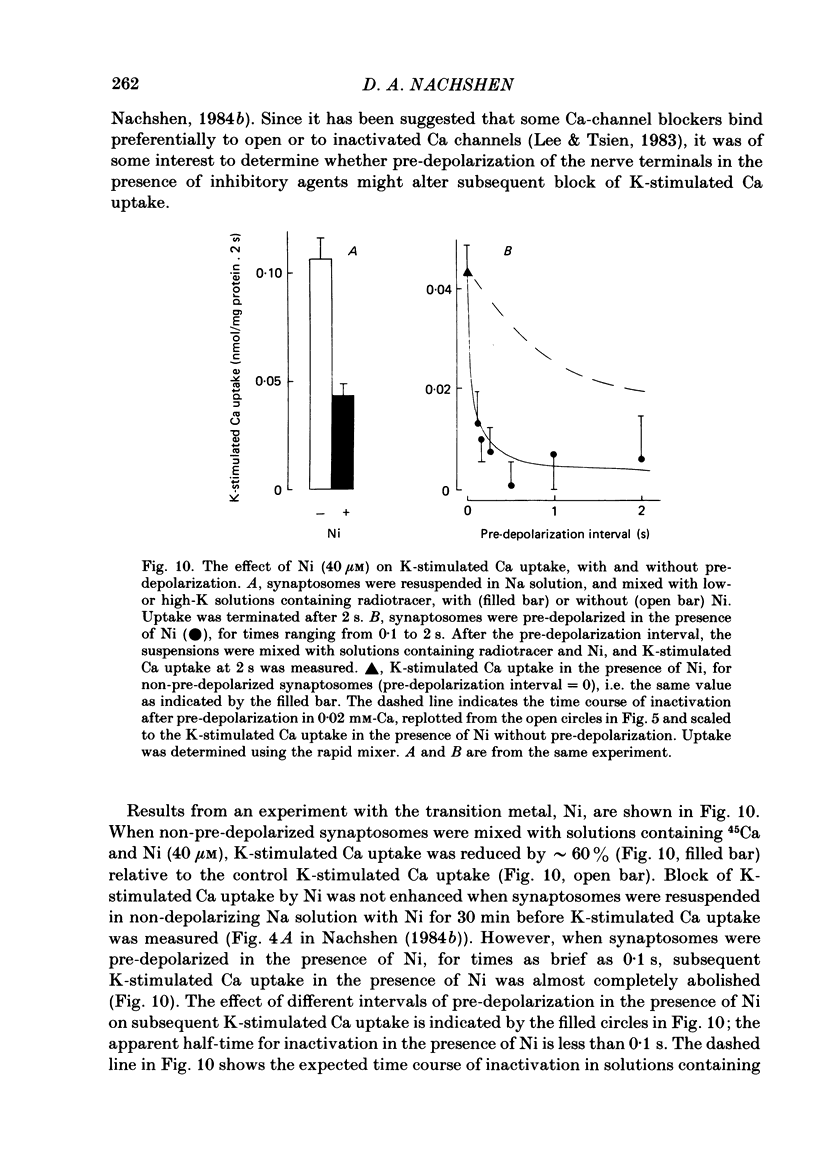
K-stimulated (voltage-dependent) 45Ca uptake in rat brain synaptosomes was measured at times ranging from 0.1 to 10 s, in experiments that employed a rapid-mixing device to initiate and terminate radiotracer uptake. The rapid mixing did not disrupt the functional integrity of the synaptosomes, as judged by their ability to take up Ca. In solutions containing a low (0.02 mM) concentration of Ca, the rate of K-stimulated Ca uptake measured after 0-0.12 s depolarization was 8 times greater than that measured after 5-10 s of depolarization. The decline in rate of K-stimulated Ca uptake was not due to tracer backflux from the synaptosomes, nor to Ca loading of the nerve terminals, since it also occurred after synaptosomes were depolarized in solutions without Ca. It is suggested that this decline in rate of Ca uptake after depolarization was due to inactivation of voltage-dependent Ca channels in the nerve terminals. This inactivation appeared to be voltage rather than Ca dependent. The extent to which K-stimulated Ca uptake declined after depolarization in high-K solution depended on the K concentration that was used to depolarize the synaptosomes. Whereas pre-incubation in solution with one-half of the Na replaced by K significantly reduced subsequent K-stimulated Ca uptake, pre-incubation in non-depolarizing solution, with one-half of the Na replaced by choline, had no significant effect on subsequent K-stimulated Ca uptake. In solutions containing a high (0.5-2 mM) concentration of Ca, the rate of K-stimulated Ca uptake measured after 0-0.12 s was 40 times greater than that measured after 5-10 s. High Ca accelerated the rate at which K-stimulated Ca uptake declined with prolonged depolarization. The effect was mimicked by high (10 mM) concentrations of Sr, but not of Ba. The accelerated rate of decline observed with high Ca could be either a direct effect of Ca on the Ca channels or, more probably, an indirect effect of Ca loading on the nerve terminals. The apparent efficacy of several Ca-channel blockers (Ni, La and verapamil) in reducing K-stimulated Ca uptake was enhanced when the synaptosomes were depolarized in the presence of inhibitory agents for brief (less than 1 s) intervals before K-stimulated Ca uptake was measured.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft F. M., Stanfield P. R. Calcium dependence of the inactivation of calcium currents in skeletal muscle fibers of an insect. Science. 1981 Jul 10;213(4504):224–226. doi: 10.1126/science.213.4504.224. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P. Effects of potassium, veratridine, and scorpion venom on calcium accumulation and transmitter release by nerve terminals in vitro. J Physiol. 1975 Jun;247(3):617–655. doi: 10.1113/jphysiol.1975.sp010950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Goldring J. M. Membrane potentials in pinched-off presynaptic nerve ternimals monitored with a fluorescent probe: evidence that synaptosomes have potassium diffusion potentials. J Physiol. 1975 Jun;247(3):589–615. doi: 10.1113/jphysiol.1975.sp010949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Oborn C. J. The influence of sodium on calcium fluxes in pinched-off nerve terminals in vitro. J Physiol. 1975 Jun;247(3):657–686. doi: 10.1113/jphysiol.1975.sp010951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm P., Eckert R., Tillotson D. Calcium-mediated inactivation of calcium current in Paramecium. J Physiol. 1980 Sep;306:193–203. doi: 10.1113/jphysiol.1980.sp013391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota G., Nicola Siri L., Stefani E. Calcium-channel gating in frog skeletal muscle membrane: effect of temperature. J Physiol. 1983 May;338:395–412. doi: 10.1113/jphysiol.1983.sp014679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau P., Blaustein M. P. Initial release of [3H]dopamine from rat striatal synaptosomes: correlation with calcium entry. J Neurosci. 1983 Apr;3(4):703–713. doi: 10.1523/JNEUROSCI.03-04-00703.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Tillotson D. L. Calcium-mediated inactivation of the calcium conductance in caesium-loaded giant neurones of Aplysia californica. J Physiol. 1981 May;314:265–280. doi: 10.1113/jphysiol.1981.sp013706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein A. Specific pharmacology of calcium in myocardium, cardiac pacemakers, and vascular smooth muscle. Annu Rev Pharmacol Toxicol. 1977;17:149–166. doi: 10.1146/annurev.pa.17.040177.001053. [DOI] [PubMed] [Google Scholar]
- Fox A. P. Voltage-dependent inactivation of a calcium channel. Proc Natl Acad Sci U S A. 1981 Feb;78(2):953–956. doi: 10.1073/pnas.78.2.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotgil'f N. M., Magazanik L. G. Vliianie blokatorov kal'tsievykh kanalov (verapamil, D-600 i iony margantsa) na osvobozhdenie mediatora iz dvigatel'nykh nervnykh okonchanii v myshtse liagushki. Neirofiziologiia. 1977;9(4):415–422. [PubMed] [Google Scholar]
- Haeusler G. Differential effect of verapamil on excitation-contraction coupling in smooth muscle and on excitation-secretion coupling in adrenergic nerve terminals. J Pharmacol Exp Ther. 1972 Mar;180(3):672–682. [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hajós F. An improved method for the preparation of synaptosomal fractions in high purity. Brain Res. 1975 Aug 15;93(3):485–489. doi: 10.1016/0006-8993(75)90186-9. [DOI] [PubMed] [Google Scholar]
- Jansson S. E., Gripenberg J., Härkönen M., Korpijoki P. Methodological studies on the uptake of radiocalcium by nerve endings isolated from rat brain. Life Sci. 1977 Apr 15;20(8):1431–1440. doi: 10.1016/0024-3205(77)90372-1. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. Calcium-dependence of catecholamine release from bovine adrenal medullary cells after exposure to intense electric fields. J Membr Biol. 1982;68(2):107–140. doi: 10.1007/BF01872259. [DOI] [PubMed] [Google Scholar]
- Krueger B. K., Ratzlaff R. W., Strichartz G. R., Blaustein M. P. Saxitoxin binding to synaptosomes, membranes, and solubilized binding sites from rat brain. J Membr Biol. 1979 Nov 30;50(3-4):287–310. doi: 10.1007/BF01868894. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. Mechanism of calcium channel blockade by verapamil, D600, diltiazem and nitrendipine in single dialysed heart cells. Nature. 1983 Apr 28;302(5911):790–794. doi: 10.1038/302790a0. [DOI] [PubMed] [Google Scholar]
- Nachshen D. A., Blaustein M. P. Influx of calcium, strontium, and barium in presynaptic nerve endings. J Gen Physiol. 1982 Jun;79(6):1065–1087. doi: 10.1085/jgp.79.6.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachshen D. A., Blaustein M. P. Some properties of potassium-stimulated calcium influx in presynaptic nerve endings. J Gen Physiol. 1980 Dec;76(6):709–728. doi: 10.1085/jgp.76.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachshen D. A., Blaustein M. P. The effects of some organic "calcium antagonists" on calcium influx in presynaptic nerve terminals. Mol Pharmacol. 1979 Sep;16(2):576–586. [PubMed] [Google Scholar]
- Nachshen D. A. Selectivity of the Ca binding site in synaptosome Ca channels. Inhibition of Ca influx by multivalent metal cations. J Gen Physiol. 1984 Jun;83(6):941–967. doi: 10.1085/jgp.83.6.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. W. Calcium channels in excitable cell membranes. Annu Rev Physiol. 1983;45:341–358. doi: 10.1146/annurev.ph.45.030183.002013. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Kloot W., Kita H. The effects of the "calcium-antagonist" verapamil on muscle action potentials in the frog and crayfish and on neuromuscular transmission in the crayfish. Comp Biochem Physiol C. 1975 Jan 1;50(1):121–125. [PubMed] [Google Scholar]
- Verjovski-Almeida S., Kurzmack M., Inesi G. Partial reactions in the catalytic and transport cycle of sarcoplasmic reticulum ATPase. Biochemistry. 1978 Nov 14;17(23):5006–5013. doi: 10.1021/bi00616a023. [DOI] [PubMed] [Google Scholar]


