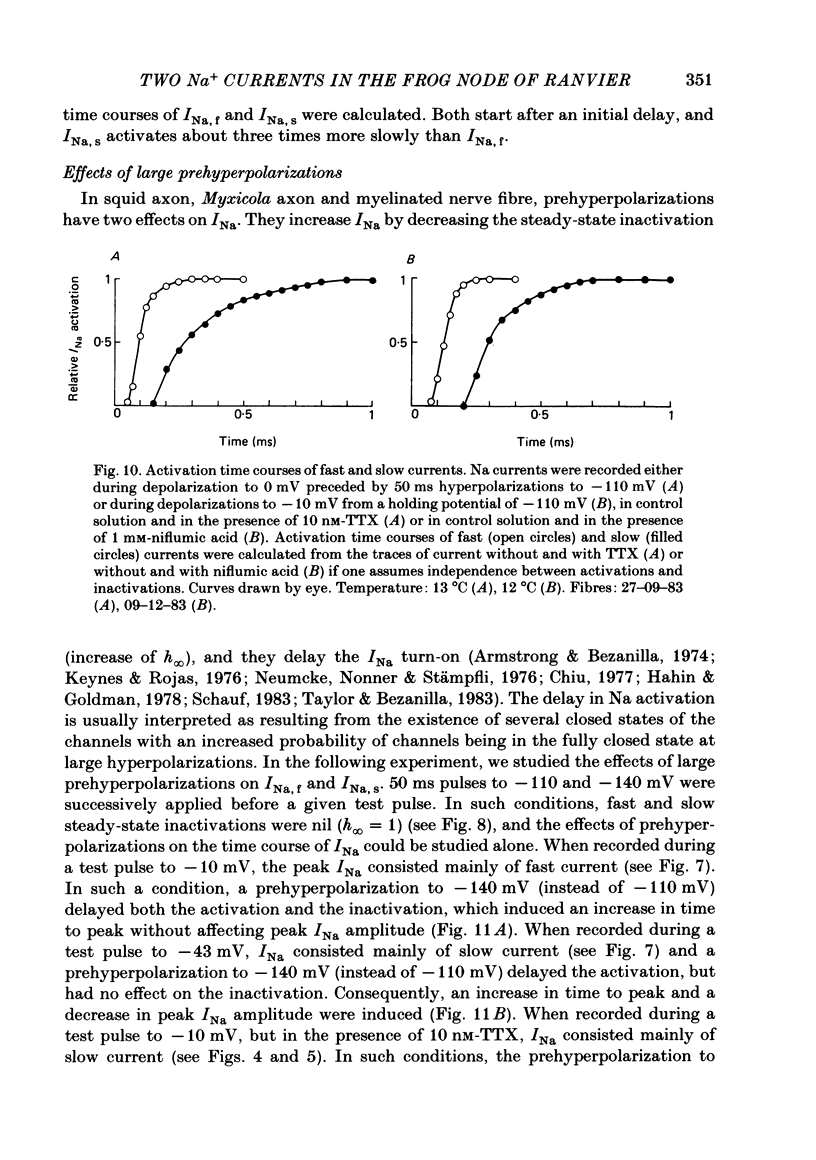
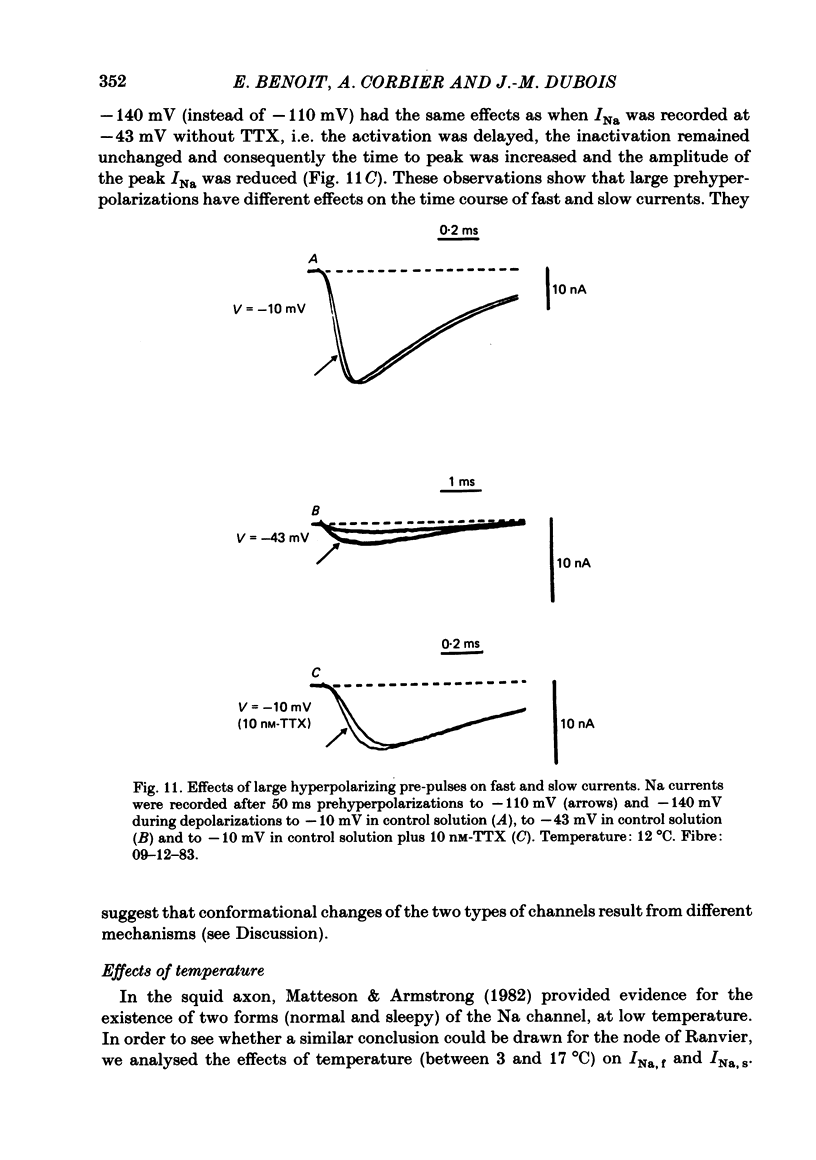
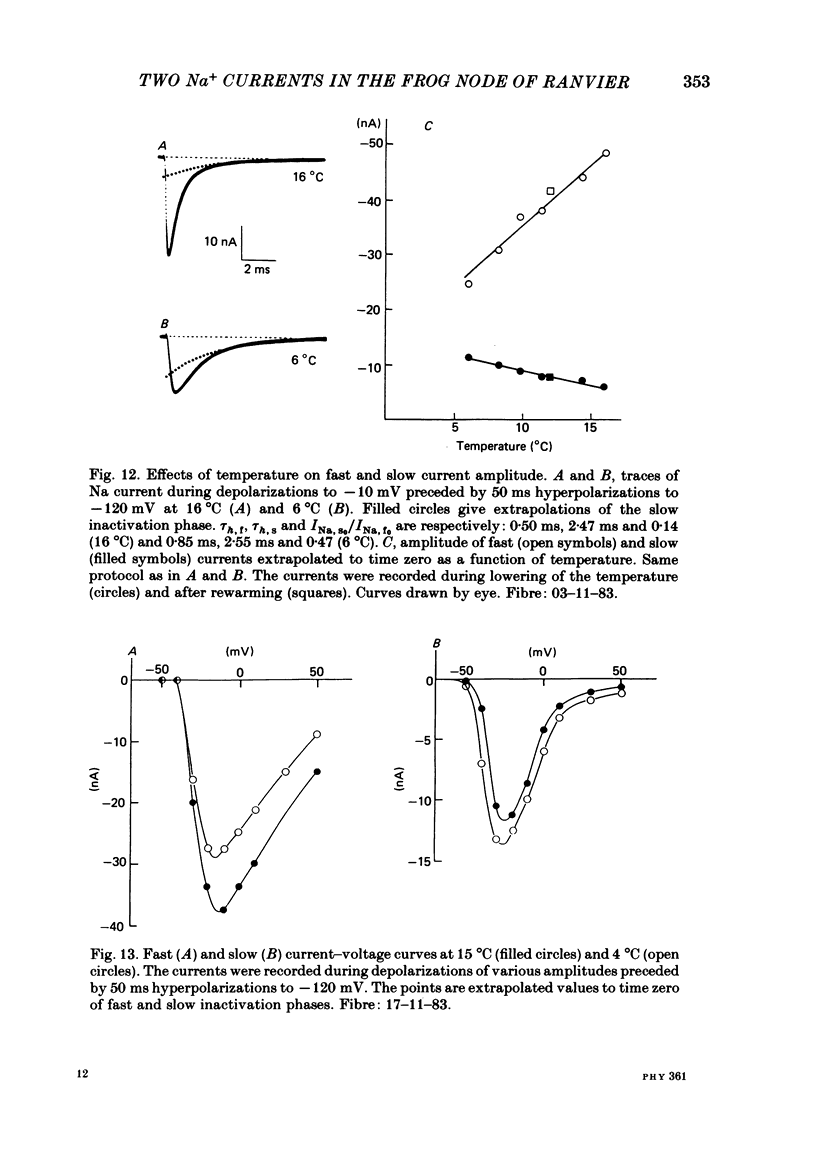
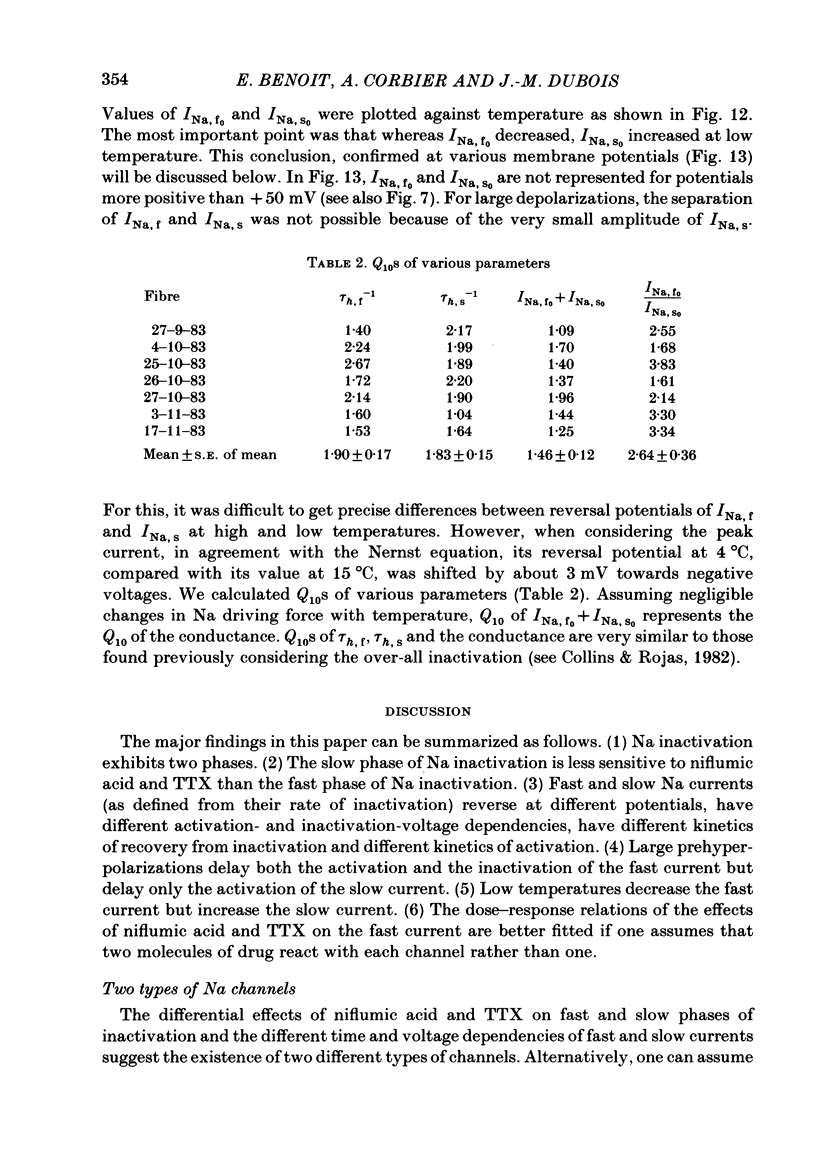
Abstract
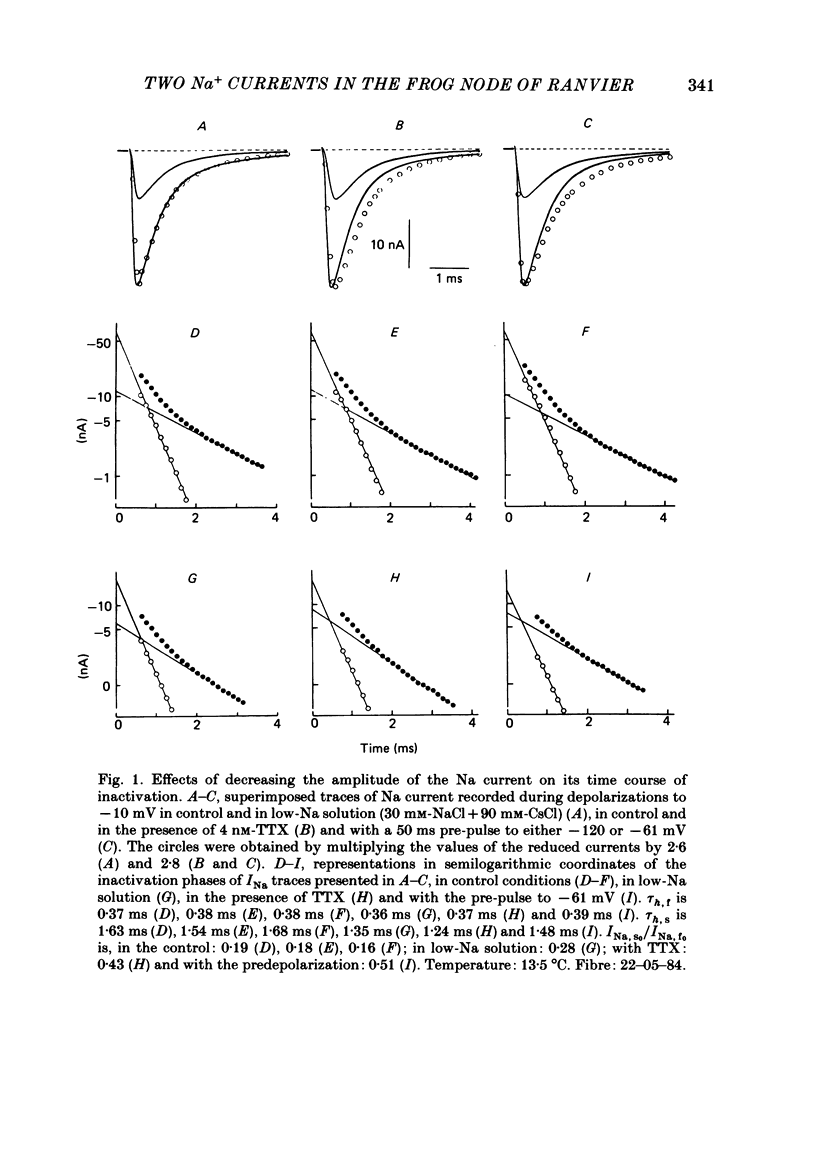
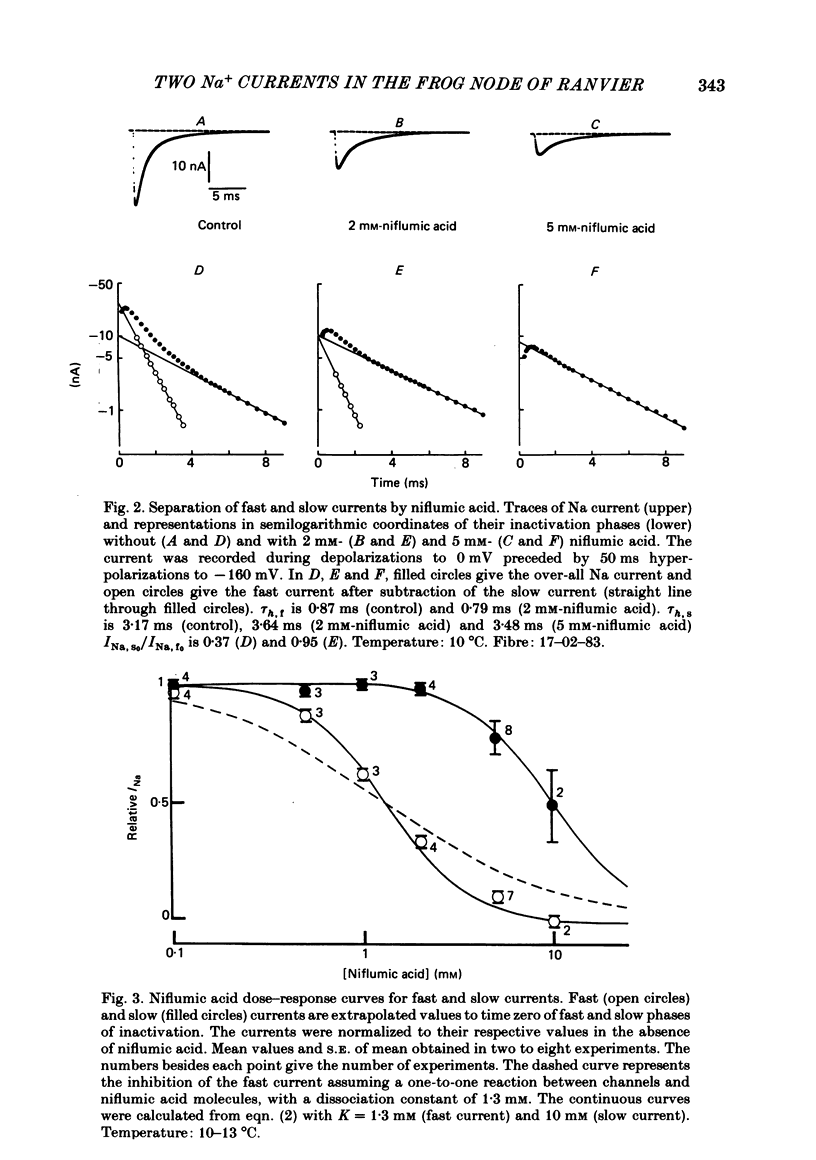
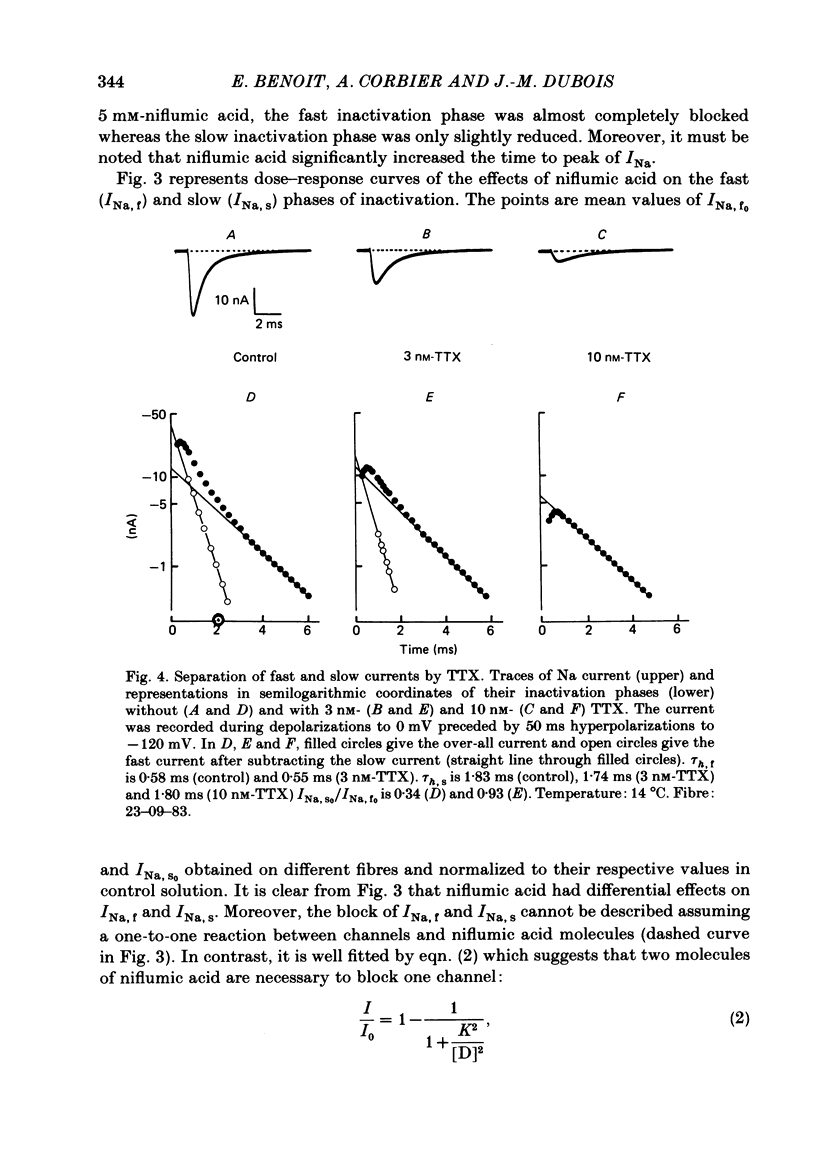
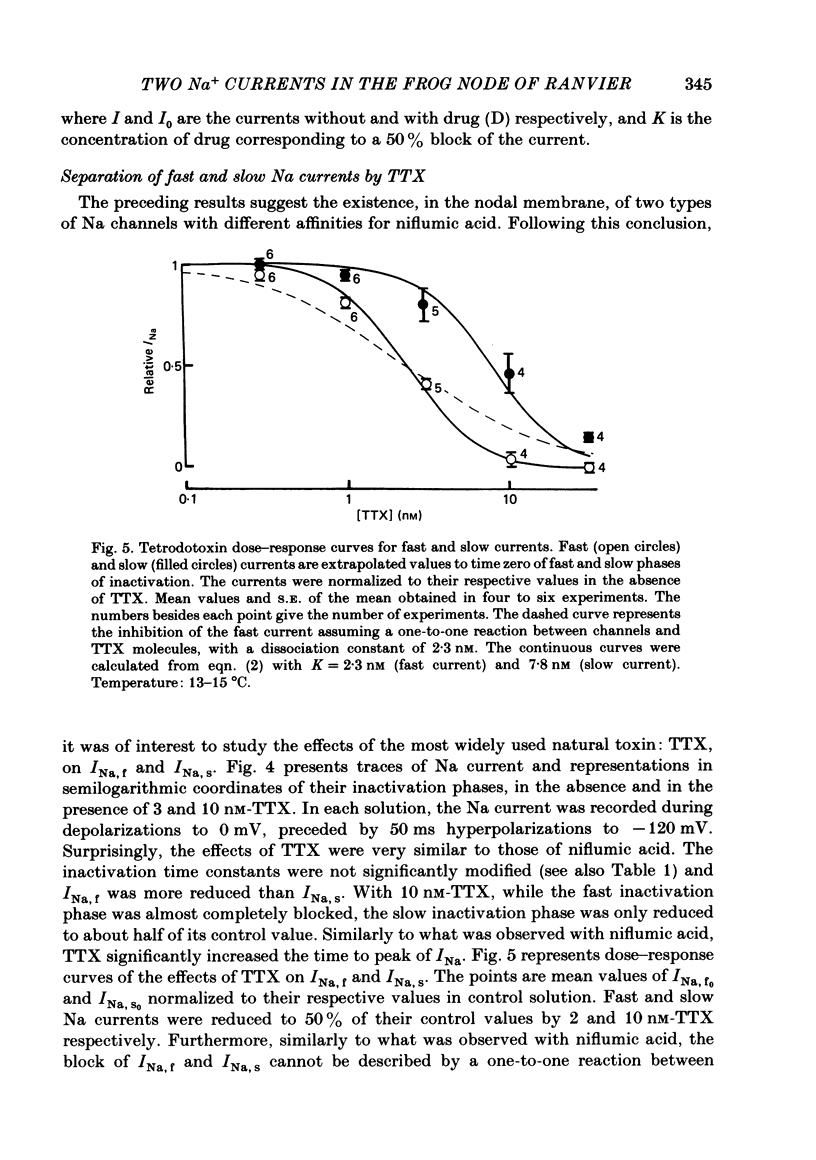
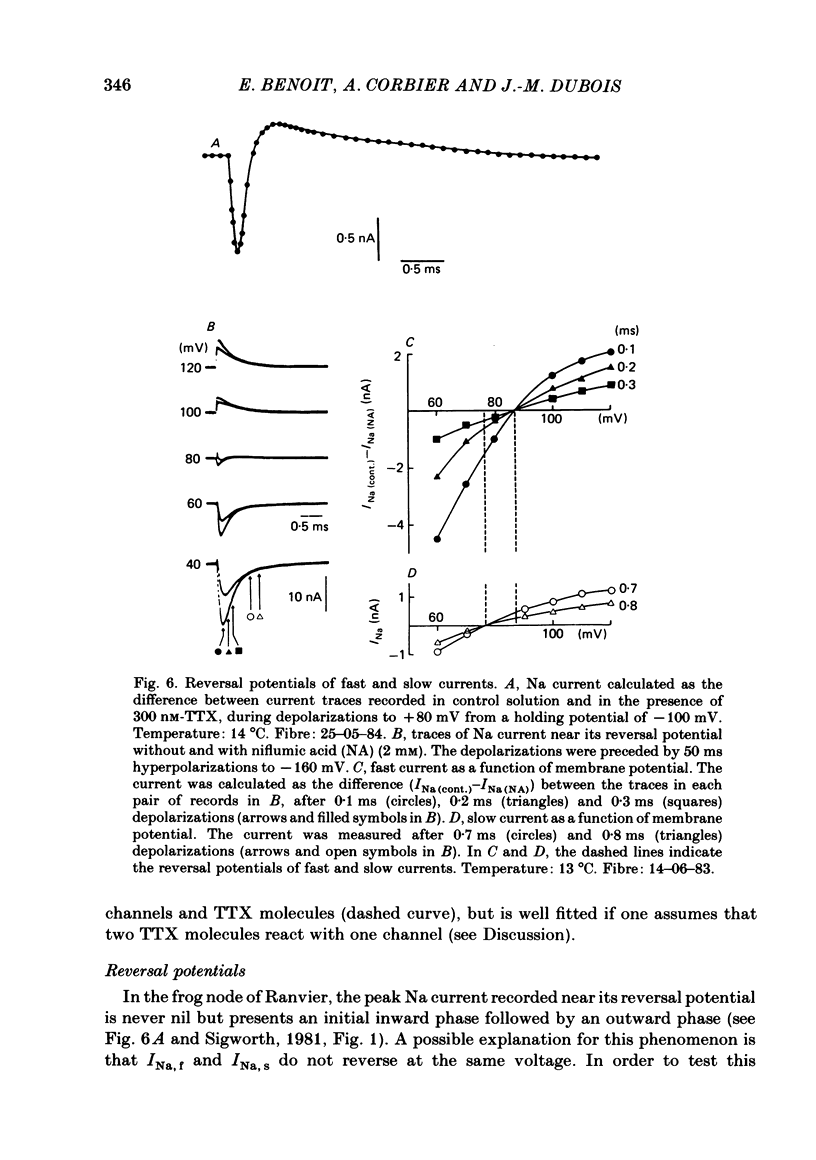
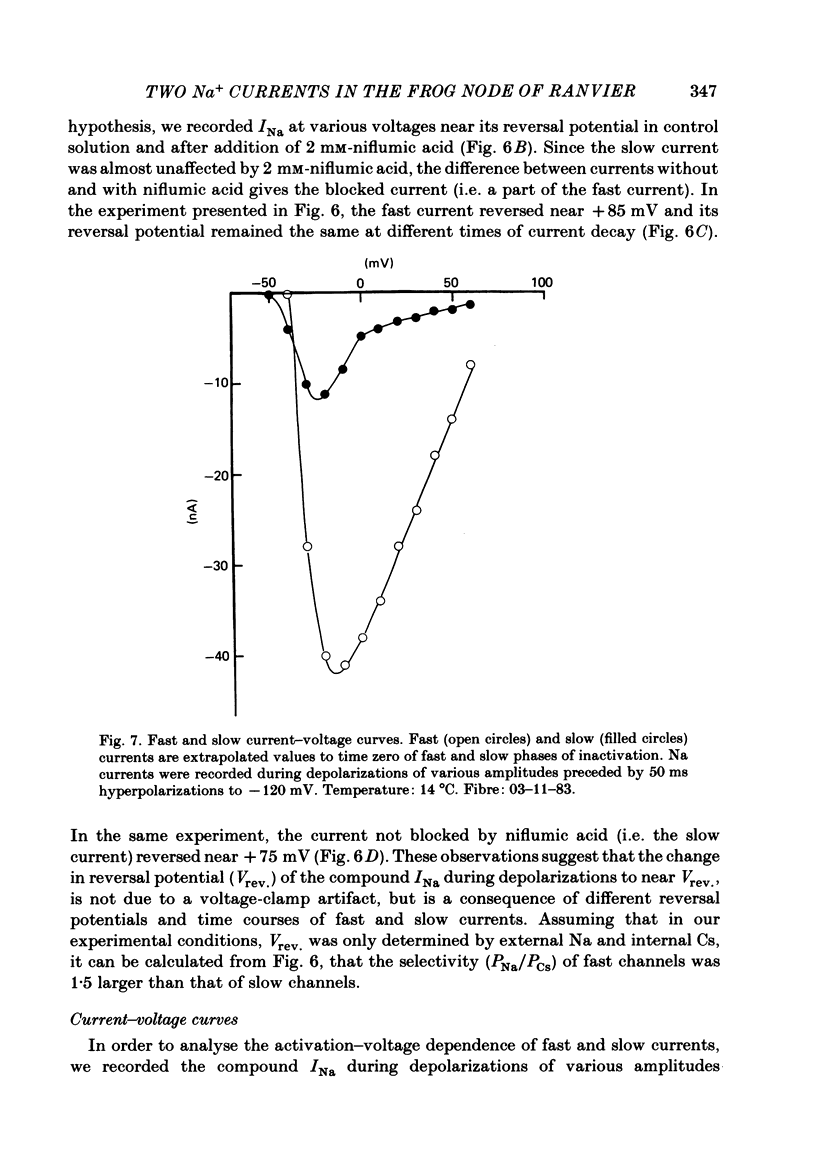
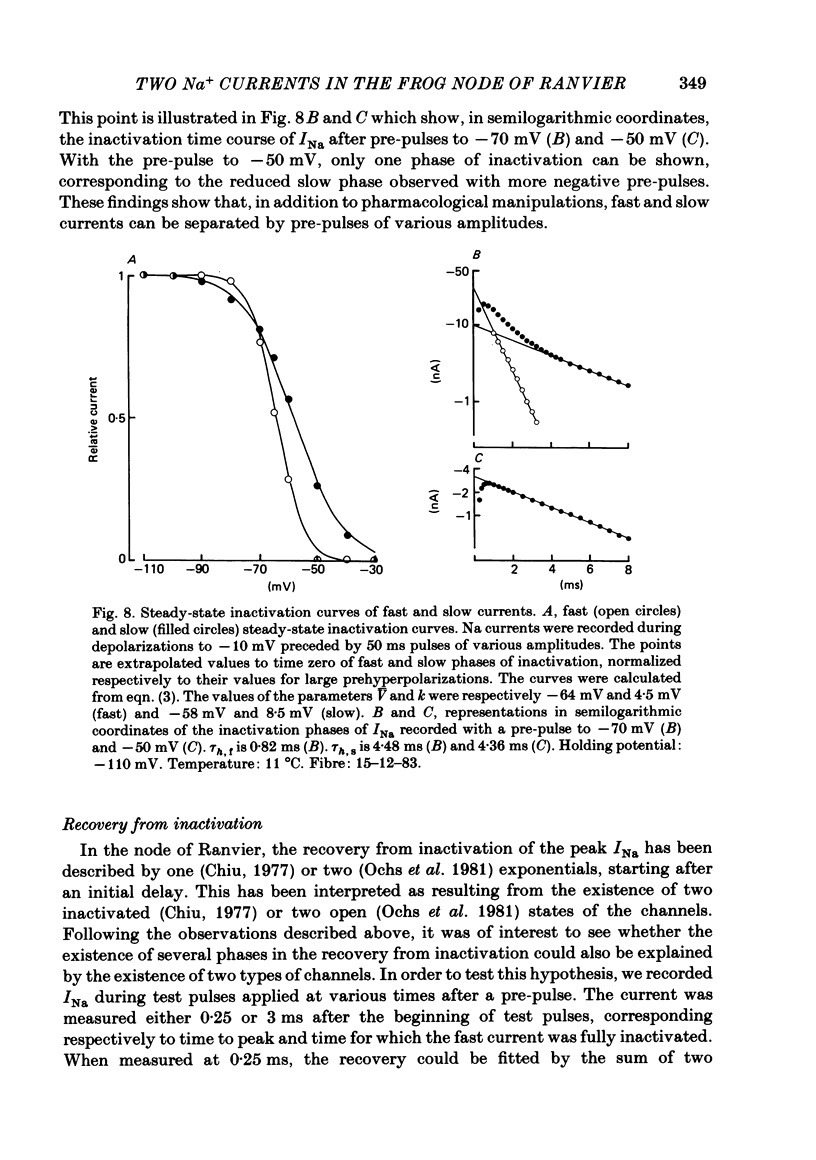
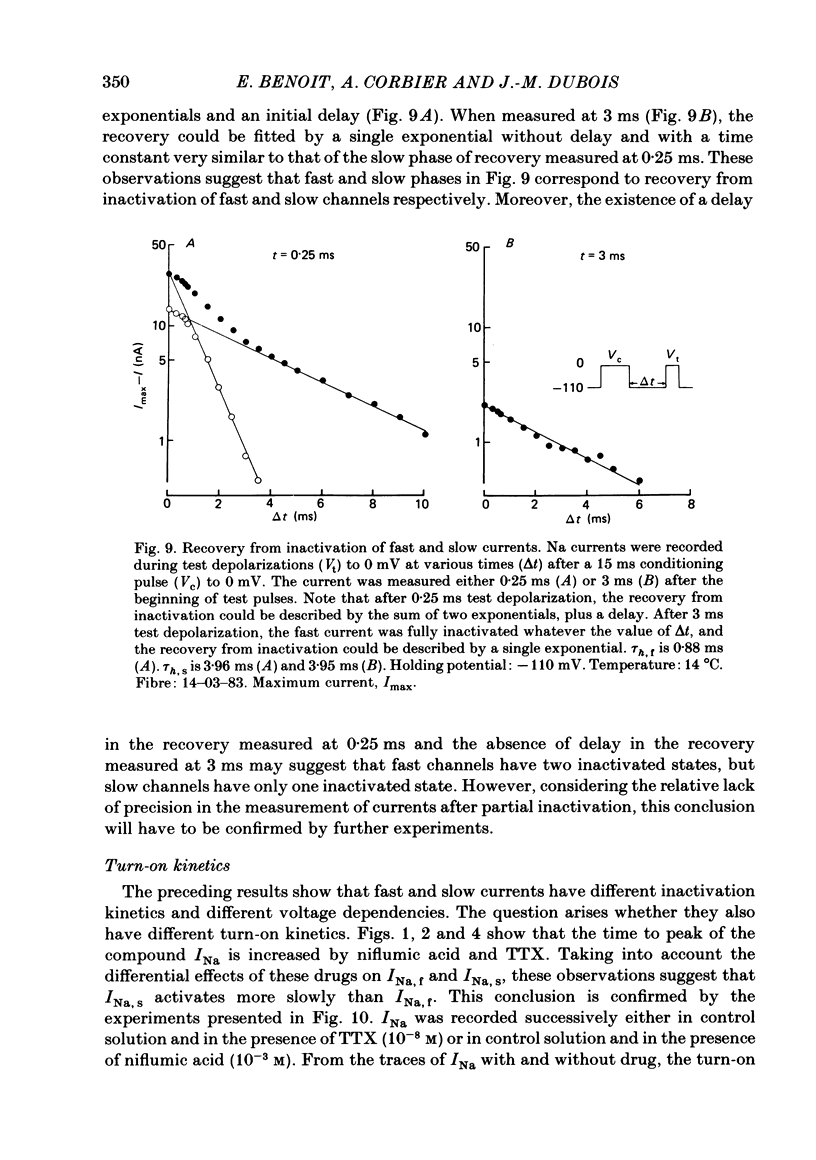
Na current (INa) was monitored in isolated voltage-clamped frog nodes of Ranvier in order to analyse the pharmacological and kinetic properties of fast and slow phases of inactivation. Niflumic acid (0.1-10 mM) and tetrodotoxin (0.3-30 nM) did not alter fast and slow inactivation time courses but preferentially reduced the amplitude of the fast phase of inactivation. The block of both phases of inactivation by niflumic acid and tetrodotoxin was well described if one assumed that more than one molecule of drug reacted with one channel. Fast and slow currents, corresponding respectively to fast and slow phases of inactivation, reversed at different potentials, had different threshold voltages of activation and the slopes of their steady-state inactivation curves were different. The recovery from inactivation of the compound INa could be described by the sum of two exponentials (plus a delay) corresponding respectively to fast and slow currents. When calculated from INa recorded without and with niflumic acid or tetrodotoxin, the slow current activated about three times more slowly than the fast current. Large prehyperpolarizations delayed both the activation and the inactivation of the fast current but only the activation of the slow current. Lowering the temperature decreased the fast current but increased the slow current. We conclude that the inactivatable Na current of the nodal membrane is made up of two components (INa,f and INa,s) corresponding to two different and interconvertible forms of the Na channel.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angelides K. J., Nutter T. J. Molecular and cellular mapping of the voltage-dependent na channel. Biophys J. 1984 Jan;45(1):31–34. doi: 10.1016/S0006-3495(84)84096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F. Charge movement associated with the opening and closing of the activation gates of the Na channels. J Gen Physiol. 1974 May;63(5):533–552. doi: 10.1085/jgp.63.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Croop R. S. Simulation of Na channel inactivation by thiazine dyes. J Gen Physiol. 1982 Nov;80(5):641–662. doi: 10.1085/jgp.80.5.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Gilly W. F. Fast and slow steps in the activation of sodium channels. J Gen Physiol. 1979 Dec;74(6):691–711. doi: 10.1085/jgp.74.6.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud C., Kado R. T., Marcher K. Sodium channels induced by depolarization of the Xenopus laevis oocyte. Proc Natl Acad Sci U S A. 1982 May;79(10):3188–3192. doi: 10.1073/pnas.79.10.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brismar T., Rydqvist B. Effect of the nonionic detergent triton X-100 on sodium permeability of the myelinated nerve fibre of Xenopus laevis. Acta Physiol Scand. 1978 Apr;102(4):425–433. doi: 10.1111/j.1748-1716.1978.tb06090.x. [DOI] [PubMed] [Google Scholar]
- Brûlé G., Haudecoeur G., Jdâaa H., Guilbault P. Inhibition par une substance amphiphile, l'acide niflumique, de la rectification dans le sens entrant de la fibre musculaire de Crustacé. Arch Int Physiol Biochim. 1983 Nov;91(4):269–277. doi: 10.3109/13813458309067974. [DOI] [PubMed] [Google Scholar]
- Chiu S. Y. Inactivation of sodium channels: second order kinetics in myelinated nerve. J Physiol. 1977 Dec;273(3):573–596. doi: 10.1113/jphysiol.1977.sp012111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins C. A., Rojas E. Temperature dependence of the sodium channel gating kinetics in the node of Ranvier. Q J Exp Physiol. 1982 Jan;67(1):41–55. doi: 10.1113/expphysiol.1982.sp002623. [DOI] [PubMed] [Google Scholar]
- Cousin J. L., Motais R. Inhibition of anion permeability by amphiphilic compounds in human red cell: evidence for an interaction of niflumic acid with the band 3 protein. J Membr Biol. 1979 Apr 20;46(2):125–153. doi: 10.1007/BF01961377. [DOI] [PubMed] [Google Scholar]
- Drouin H., Neumcke B. Specific and unspecific charges at the sodium channels of the nerve membrane. Pflugers Arch. 1974;351(3):207–229. doi: 10.1007/BF00586919. [DOI] [PubMed] [Google Scholar]
- Dubois J. M., Bergman C. Late sodium current in the node of Ranvier. Pflugers Arch. 1975;357(1-2):145–148. doi: 10.1007/BF00584552. [DOI] [PubMed] [Google Scholar]
- Dubois J. M., Coulombe A. Current-dependent inactivation induced by sodium depletion in normal and batrachotoxin-treated frog node of Ranvier. J Gen Physiol. 1984 Jul;84(1):25–48. doi: 10.1085/jgp.84.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J. M. Potassium currents in the frog node of Ranvier. Prog Biophys Mol Biol. 1983;42(1):1–20. doi: 10.1016/0079-6107(83)90002-0. [DOI] [PubMed] [Google Scholar]
- Eick R. T., Yeh J., Matsuki N. Two types of voltage dependent na channels suggested by differential sensitivity of single channels to tetrodotoxin. Biophys J. 1984 Jan;45(1):70–73. doi: 10.1016/S0006-3495(84)84113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. M. Ultra-slow inactivation of the ionic currents through the membrane of myelinated nerve. Biochim Biophys Acta. 1976 Mar 5;426(2):232–244. doi: 10.1016/0005-2736(76)90334-5. [DOI] [PubMed] [Google Scholar]
- French R. J., Horn R. Sodium channel gating: models, mimics, and modifiers. Annu Rev Biophys Bioeng. 1983;12:319–356. doi: 10.1146/annurev.bb.12.060183.001535. [DOI] [PubMed] [Google Scholar]
- Gilly W. F., Armstrong C. M. Threshold channels--a novel type of sodium channel in squid giant axon. 1984 May 31-Jun 6Nature. 309(5967):448–450. doi: 10.1038/309448a0. [DOI] [PubMed] [Google Scholar]
- Gundersen C. B., Miledi R., Parker I. Voltage-operated channels induced by foreign messenger RNA in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1983 Nov 22;220(1218):131–140. doi: 10.1098/rspb.1983.0092. [DOI] [PubMed] [Google Scholar]
- Hahin R., Goldman L. Initial conditions and the kinetics of the sodium conductance in Myxicola giant axons. I. effects on the time-course of the sodium conductance. J Gen Physiol. 1978 Dec;72(6):863–877. doi: 10.1085/jgp.72.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Rojas E. The temporal and steady-state relationships between activation of the sodium conductance and movement of the gating particles in the squid giant axon. J Physiol. 1976 Feb;255(1):157–189. doi: 10.1113/jphysiol.1976.sp011274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniffki K. D., Siemen D., Vogel W. Development of sodium permeability inactivation in nodal membranes. J Physiol. 1981;313:37–48. doi: 10.1113/jphysiol.1981.sp013649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980 Aug;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombet A., Frelin C., Renaud J. F., Lazdunski M. Na+ channels with binding sites of high and low affinity for tetrodotoxin in different excitable and non-excitable cells. Eur J Biochem. 1982 May;124(1):199–203. doi: 10.1111/j.1432-1033.1982.tb05925.x. [DOI] [PubMed] [Google Scholar]
- Matteson D. R., Armstrong C. M. Evidence for a population of sleepy sodium channels in squid axon at low temperature. J Gen Physiol. 1982 May;79(5):739–758. doi: 10.1085/jgp.79.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy K., Kiss T., Hof D. Single Na channels in mouse neuroblastoma cell membrane. Indications for two open states. Pflugers Arch. 1983 Dec;399(4):302–308. doi: 10.1007/BF00652757. [DOI] [PubMed] [Google Scholar]
- Neumcke B., Nonner W., Stämpfli R. Asymmetrical displacement current and its relation with the activation of sodium current in the membrane of frog myelinated nerve. Pflugers Arch. 1976 Jun 22;363(3):193–203. doi: 10.1007/BF00594601. [DOI] [PubMed] [Google Scholar]
- Neumcke B., Stämpfli R. Alteration of the conductance of Na+ channels in the nodal membrane of frog nerve by holding potential and tetrodotoxin. Biochim Biophys Acta. 1983 Jan 5;727(1):177–184. doi: 10.1016/0005-2736(83)90382-6. [DOI] [PubMed] [Google Scholar]
- Neumcke B., Stämpfli R. Sodium currents and sodium-current fluctuations in rat myelinated nerve fibres. J Physiol. 1982 Aug;329:163–184. doi: 10.1113/jphysiol.1982.sp014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonner W. A new voltage clamp method for Ranvier nodes. Pflugers Arch. 1969;309(2):176–192. doi: 10.1007/BF00586967. [DOI] [PubMed] [Google Scholar]
- Nonner W. Relations between the inactivation of sodium channels and the immobilization of gating charge in frog myelinated nerve. J Physiol. 1980 Feb;299:573–603. doi: 10.1113/jphysiol.1980.sp013143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs G., Bromm B., Schwarz J. R. A three-state model for inactivation of sodium permeability. Biochim Biophys Acta. 1981 Jul 20;645(2):243–252. doi: 10.1016/0005-2736(81)90195-4. [DOI] [PubMed] [Google Scholar]
- Ritchie J. M., Rogart R. B. The binding of saxitoxin and tetrodotoxin to excitable tissue. Rev Physiol Biochem Pharmacol. 1977;79:1–50. doi: 10.1007/BFb0037088. [DOI] [PubMed] [Google Scholar]
- Schauf C. L. Insensitivity of activation delays in potassium and sodium channels to heavy water in Myxicola giant axons. J Physiol. 1983 Apr;337:173–182. doi: 10.1113/jphysiol.1983.sp014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz J. R., Bromm B., Spielmann R. P., Weytjens J. L. Development of Na inactivation in motor and sensory myelinated nerve fibres of Rana esculenta. Pflugers Arch. 1983 Jul;398(2):126–129. doi: 10.1007/BF00581059. [DOI] [PubMed] [Google Scholar]
- Schwarz W. Temperature experiments on nerve and muscle membranes of frogs. Indications for a phase transition. Pflugers Arch. 1979 Oct;382(1):27–34. doi: 10.1007/BF00585900. [DOI] [PubMed] [Google Scholar]
- Sevcik C. Binding of tetrodotoxin to squid nerve fibers. Two kinds of receptors? J Gen Physiol. 1976 Jul;68(1):95–103. doi: 10.1085/jgp.68.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth F. J. Covariance of nonstationary sodium current fluctuations at the node of Ranvier. Biophys J. 1981 Apr;34(1):111–133. doi: 10.1016/S0006-3495(81)84840-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth F. J. The variance of sodium current fluctuations at the node of Ranvier. J Physiol. 1980 Oct;307:97–129. doi: 10.1113/jphysiol.1980.sp013426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. E., Bezanilla F. Sodium and gating current time shifts resulting from changes in initial conditions. J Gen Physiol. 1983 Jun;81(6):773–784. doi: 10.1085/jgp.81.6.773. [DOI] [PMC free article] [PubMed] [Google Scholar]



