Abstract
We investigated the dynamics of sarcomere length change during imposed stretches and releases of unstimulated single fibres of frog skeletal muscle. Three independent methods were used: an on-line method in which sarcomere length is computed from the striation pattern; laser diffraction; and a segment length tracking device. During steady ramp releases and stretches, both sarcomere and segment length changes occurred in stepwise fashion; i.e. periods of pause were interspersed between periods of rapid shortening. The above result indicates that activation of the fibre is not required to elicit stepwise length changes. Increasing the ramp velocity caused the steps to increase in size and the pauses to decrease in duration. Ramp releases and stretches were imposed at each of several initial sarcomere lengths up to 4.0 microns. Stepwise length changes were observed at all lengths, and their size was independent of initial sarcomere length. The observation of stepwise length changes beyond overlap indicates that the underlying mechanism probably does not lie in synchronous action of cross-bridges; an alternative hypothesis is advanced.
Full text
PDF


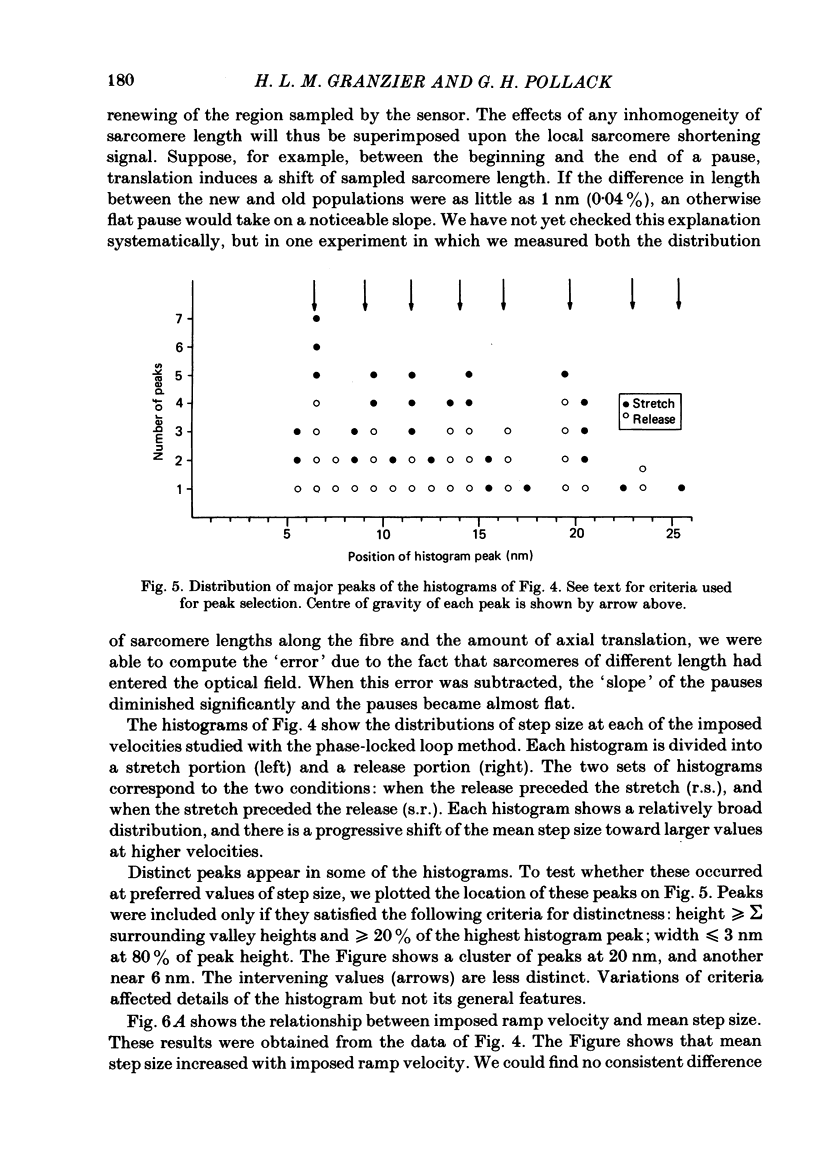
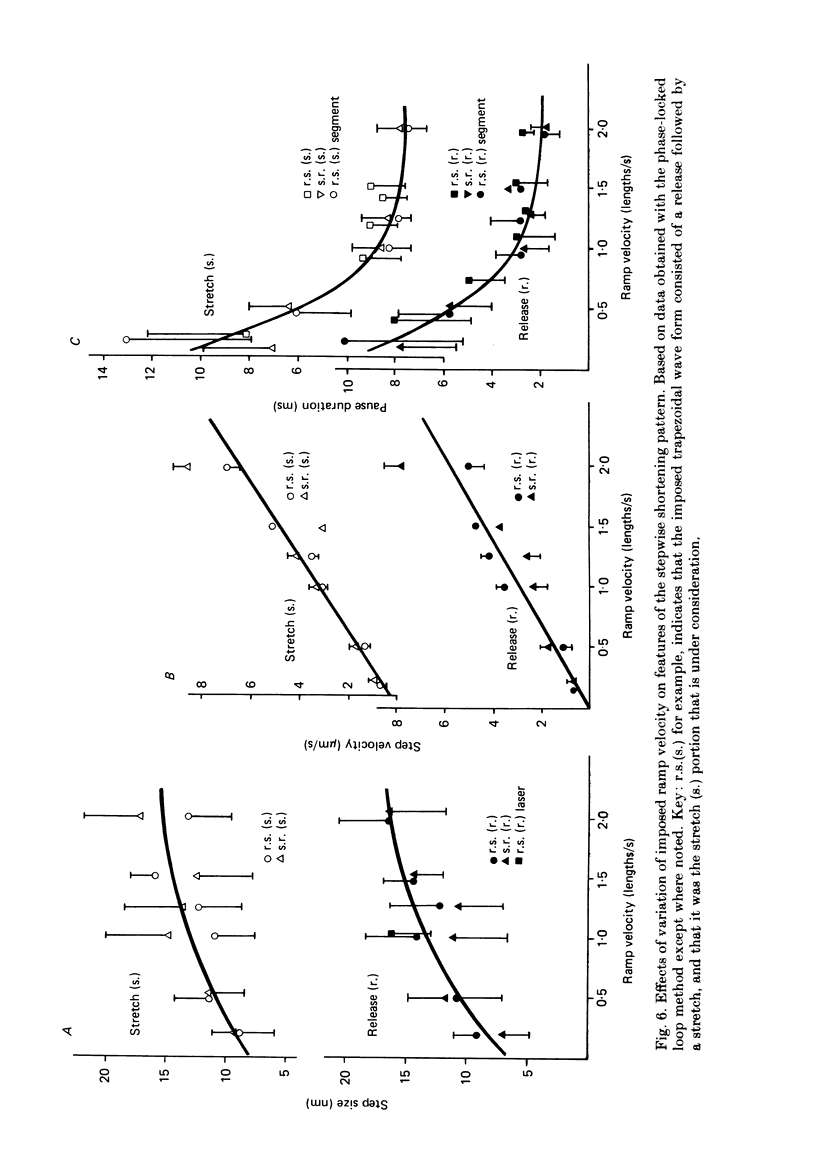
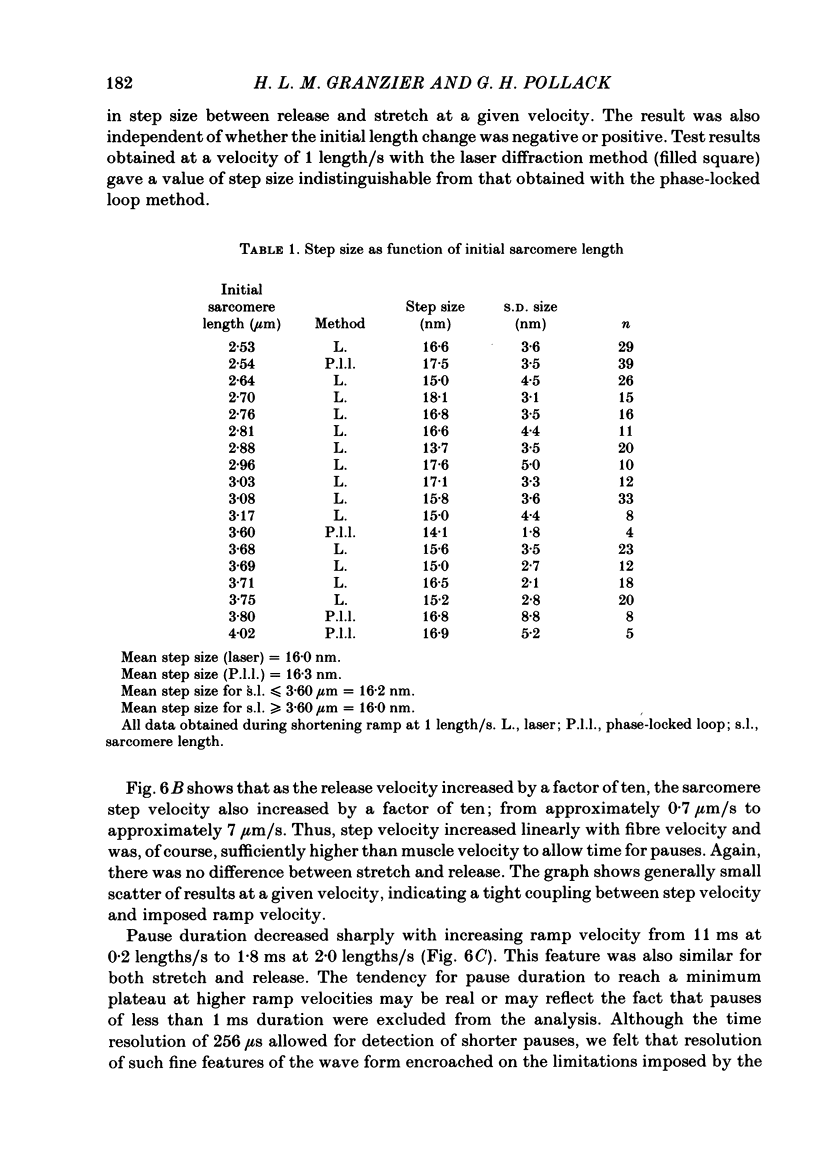






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altringham J. D., Bottinelli R., Lacktis J. W. Is stepwise sarcomere shortening an artefact? Nature. 1984 Feb 16;307(5952):653–655. doi: 10.1038/307653a0. [DOI] [PubMed] [Google Scholar]
- Delay M. J., Ishide N., Jacobson R. C., Pollack G. H., Tirosh R. Stepwise sarcomere shortening: analysis by high-speed cinemicrography. Science. 1981 Sep 25;213(4515):1523–1525. doi: 10.1126/science.7280674. [DOI] [PubMed] [Google Scholar]
- Edman K. A., Elzinga G., Noble M. I. Residual force enhancement after stretch of contracting frog single muscle fibers. J Gen Physiol. 1982 Nov;80(5):769–784. doi: 10.1085/jgp.80.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F., PEACHEY L. D. The maximum length for contraction in vertebrate straiated muscle. J Physiol. 1961 Apr;156:150–165. doi: 10.1113/jphysiol.1961.sp006665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D. K. Tension due to interaction between the sliding filaments in resting striated muscle. The effect of stimulation. J Physiol. 1968 Dec;199(3):637–684. doi: 10.1113/jphysiol.1968.sp008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwazumi T., Pollack G. H. On-line measurement of sarcomere length from diffraction patterns in muscle. IEEE Trans Biomed Eng. 1979 Feb;26(2):86–93. doi: 10.1109/tbme.1979.326514. [DOI] [PubMed] [Google Scholar]
- Jacobson R. C., Tirosh R., Delay M. J., Pollack G. H. Quantized nature of sarcomere shortening steps. J Muscle Res Cell Motil. 1983 Oct;4(5):529–542. doi: 10.1007/BF00712113. [DOI] [PubMed] [Google Scholar]
- Locker R. H., Leet N. G. Histology of highly-stretched beef muscle. I. The fine structure of grossly stretched single fibers. J Ultrastruct Res. 1975 Jul;52(1):64–75. doi: 10.1016/s0022-5320(75)80022-0. [DOI] [PubMed] [Google Scholar]
- Magid A., Ting-Beall H. P., Carvell M., Kontis T., Lucaveche C. Connecting filaments, core filaments, and side-struts: a proposal to add three new load-bearing structures to the sliding filament model. Adv Exp Med Biol. 1984;170:307–328. doi: 10.1007/978-1-4684-4703-3_26. [DOI] [PubMed] [Google Scholar]
- Myers J., Tirosh R., Jacobson R. C., Pollack G. H. Phase-locked loop measurement of sarcomere length with high time resolution. IEEE Trans Biomed Eng. 1982 Jun;29(6):463–466. doi: 10.1109/TBME.1982.324975. [DOI] [PubMed] [Google Scholar]
- Pollack G. H. Is stepwise sarcomere shortening an artefact? A response. Nature. 1984 Jun 21;309(5970):712–714. doi: 10.1038/309712a0. [DOI] [PubMed] [Google Scholar]
- Pollack G. H., Iwazumi T., ter Keurs H. E., Shibata E. F. Sarcomere shortening in striated muscle occurs in stepwise fashion. Nature. 1977 Aug 25;268(5622):757–759. doi: 10.1038/268757a0. [DOI] [PubMed] [Google Scholar]
- Pollack G. H. The cross-bridge theory. Physiol Rev. 1983 Jul;63(3):1049–1113. doi: 10.1152/physrev.1983.63.3.1049. [DOI] [PubMed] [Google Scholar]
- Pollack G. H., Tirosh R., Brozovich F. V., Lacktis J. W., Jacobson R. C., Tameyasu T. Stepwise shortening: evidence and implications. Adv Exp Med Biol. 1984;170:765–786. doi: 10.1007/978-1-4684-4703-3_74. [DOI] [PubMed] [Google Scholar]
- Rüdel R., Zite-Ferenczy F. Do laser diffraction studies on striated muscle indicate stepwise sarcomere shortening? Nature. 1979 Apr 5;278(5704):573–575. doi: 10.1038/278573a0. [DOI] [PubMed] [Google Scholar]
- Wang K. Cytoskeletal matrix in striated muscle: the role of titin, nebulin and intermediate filaments. Adv Exp Med Biol. 1984;170:285–305. doi: 10.1007/978-1-4684-4703-3_25. [DOI] [PubMed] [Google Scholar]
- Wang K., Ramirez-Mitchell R., Palter D. Titin is an extraordinarily long, flexible, and slender myofibrillar protein. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3685–3689. doi: 10.1073/pnas.81.12.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]


