Abstract
Background & Aims
Triggering receptor expressed on myeloid cells 2 (TREM2)-expressing macrophages and systemic levels of soluble TREM2 (sTREM2) appear critical in the development of chronic liver disease (CLD) and seem relevant in its detection. The aim of this study was to examine sTREM2 as a marker for early CLD and its potential to predict posthepatectomy liver failure (PHLF) in patients undergoing partial hepatectomy.
Methods
sTREM2 was assessed in the plasma of 108 patients undergoing liver resection. Blood was drawn prior to surgery (preop) and on the first and fifth postoperative day.
Results
Preop sTREM2 levels were similar across different indications for resection (p = 0.091). Higher preop sTREM2 levels were associated with advanced hepatic fibrosis (p = 0.030) and PHLF (p = 0.007). Fibrosis-4 index (FIB-4) (p = 0.619) and model for end-stage liver disease (MELD) (p = 0.590) did not show a difference between patients grouped by their CLD. Comparing the AUC from receiver-operating characteristic analysis, sTREM2 (AUC = 0.708) outperformed FIB-4 (AUC = 0.529), MELD (AUC = 0.587), Child-Pugh grading (AUC = 0.570) and LiMAx (liver maximum capacity test) (AUC = 0.516) in predicting PHLF. Similarly, in uni- and multivariate analysis, only sTREM2 proved predictive for PHLF (p = 0.023). High-risk (p = 0.003) and low-risk (p = 0.011) cut-offs for systemic sTREM2 levels could identify patients at risk for adverse outcomes after surgery. Finally, high sTREM2 was associated with decreased overall survival after liver surgery (p <0.001).
Conclusions
Circulating sTREM2 shows sensitivity for early-stage, asymptomatic liver disease, irrespective of the underlying indication for liver surgery. Assessment of CLD via sTREM2 monitoring could improve early detection of CLD and improve outcomes after liver surgery.
Impact and implications:
Soluble TREM2 (sTREM2) has previously been shown to correlate with the degree of chronic liver disease. We found that even in patients undergoing liver resection, who generally do not suffer from end-stage liver disease, sTREM2 reflects liver fibrosis status and predicts postoperative development of liver dysfunction. This is especially relevant for liver surgeons and patients, as postoperative liver dysfunction is the main reason for postoperative mortality. Our findings are also important for hepatologists, as early detection of liver fibrosis and cirrhosis is paramount for overall patient survival and we can show that even in a cohort with a median model for end-stage liver disease score of 6, sTREM2 is able to distinguish patients based on their liver fibrosis status.
Keywords: Soluble TREM2, Liver surgery, Liver regeneration, Liver fibrosis, Cirrhosis
Graphical abstract
Highlights:
-
•
Soluble TREM2 levels are similar across different indications for liver surgery.
-
•
Soluble TREM2 is elevated in patients with asymptomatic chronic liver disease.
-
•
Soluble TREM2 predicts posthepatectomy liver failure after liver surgery.
-
•
Soluble TREM2 outperforms other chronic liver disease markers for the prediction of adverse outcomes after liver surgery.
Introduction
The incidence of chronic liver disease (CLD), which is associated with the development of fibrosis and subsequent cirrhosis, has increased worldwide.1 There are multiple factors contributing to fibrosis development, with the most common cause of CLD being metabolic dysfunction-associated liver disease (MASLD) progressing to metabolic dysfunction-associated steatohepatitis (MASH).2 Alongside MASLD resulting from high caloric intake and a sedentary lifestyle, the global increase in alcohol consumption leading to alcohol-related liver disease is equally contributing to the rise in CLD.3 In general, development of CLD is a complex process and multifactorial, with patients possibly exhibiting a combination of MASLD and alcohol-related liver disease, recently coined MetALD.4 Even with a possible pharmacological treatment for MASH and non-cirrhotic fibrosis on the horizon, anti-fibrotic therapies are still extremely limited and predominantly centered around lifestyle changes.5 Progression of CLD to cirrhosis can ultimately lead to the development of hepatocellular carcinoma (HCC). HCC development is associated with poor short-term overall survival (OS), mainly due to most patients not qualifying for curative treatment at the time of diagnosis.6 Hence, detection of early-stage CLD prior to development of HCC or decompensation of liver function is vital.
A multitude of non-invasive tests including the fibrosis-4 (FIB-4) index have been assessed for their predictive potential for underlying liver fibrosis and liver function.7 In patients with end-stage liver disease, possibly requiring transplantation, liver function is categorized using classification systems like the Child-Pugh score or the model for end-stage liver disease (MELD) score.8,9 However, while patients awaiting liver transplantation usually present with decompensated liver function, patients undergoing liver resection, mostly due to oncological indications, will generally present with no or early-stage CLD.10,11 In the rare instance where patients with end-stage CLD and HCC are eligible for curative resection they still have compensated liver function, hence the predictive potential of MELD and the Child-Pugh score is limited for outcomes after liver surgery. This limitation was recently highlighted in the E-AHPBA-ESSO-ESSR consensus guidelines for preoperative (preop) liver function assessment.12 Prediction of posthepatectomy liver failure (PHLF) is especially relevant, as PHLF is the main cause for short-term mortality after liver surgery and no curative treatments are available.13,14 CLD, especially high-grade fibrosis and cirrhosis is associated with increased risk of PHLF development or postoperative (postop) mortality.15 However, non-invasive tests for detection of underlying fibrosis like the FIB-4 index do not show significant predictive potential for postop liver function.16
Recently, triggering receptor expressed on myeloid cells 2 (TREM2)-expressing macrophages were identified as a novel class of immune cells involved in a variety of different metabolic processes and their presence in the liver during CLD (including MASH) was established. TREM2+ macrophages, which are mainly derived from monocytes recruited from circulation where they already acquire increased TREM2 expression, localize to sites of hepatic injury, inflammation and fibrosis to exert protective functions against collagen deposition.17 Further, TREM2 influences macrophage plasticity and drives bone marrow-derived macrophages to adopt an anti-inflammatory, anti-fibrotic phenotype. TREM2 macrophages are also involved in the regulation of metabolic homeostasis and lipid handling, and subsequently TREM2-deficiency leads to exacerbation of steatohepatitis.17,18 TREM2 is present in its membrane-bound form on the surface of macrophages, while cleavage by ADAM10/17 results in the release of its soluble form. Data in human MASH as well as murine models for CLD have demonstrated that systemic soluble TREM2 (sTREM2) levels show high sensitivity for increasing degree of CLD. In comparison to non-invasive markers for CLD and liver injury, sTREM2 in circulation shows similar or improved predictive potential for the presence of fibrosis.17
In this study we examined the potential of sTREM2 as a marker for early-stage fibrosis with compensated liver function and for the development of PHLF in patients undergoing liver surgery. Additionally, we characterize sTREM2 levels in different tumor types and show its correlation with classical markers for liver function and injury. Finally, we examine the clinical utility of preop sTREM2-based prediction of PHLF and postop mortality for patients undergoing liver surgery.
Material and methods
Study cohort
A total of 108 patients underwent liver resection between 11/2013 and 5/2018 at the General Hospital Vienna, Clinic Landstrasse, Vienna and Clinic Favoriten, Vienna. Patient data and plasma samples were included out of a prospectively maintained biobank. Major liver resections were defined as resections of equal to or more than three liver segments.19 The study was approved by the institutional ethics committee (# 2032/2013, # 16-253-0117). Exclusion criteria included pregnancy and age below 18.
Plasma preparation
Blood was drawn preop and on the first and fifth postop day (POD1, POD5). Patient blood was then processed to plasma via a two-step centrifugation process, which has been described in previous publications.20,21
Measurement of sTREM2 concentrations
Plasma sTREM2 was measured using a human sTREM2 ELISA kit (DY1828, R&D Systems) according to manufacturer’s instructions as done previously.17
Quantification of routine blood parameters
Aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin, serum bilirubin (SB), alkaline phosphatase (AP), gamma-glutamyltransferase (GGT), prothrombin time (PT), platelet count and absolute and relative monocyte counts were measured in appropriate samples by routine laboratory blood tests.
Assessment of underlying CLD
Grade of fibrosis was recorded based on the pathologist’s assessment of the surgical specimen’s healthy liver tissue. Grade 0 represents no fibrosis, grade 1 fibrous portal expansion, grade 2 periportal fibrosis with periportal septa, grade 3 bridging fibrosis with portal fibrous septa and distorted architecture and grade 4 represents cirrhosis with nodule formation.22 For statistical analysis, patients were grouped in an early-stage fibrosis and a late-stage fibrosis group. The early-stage fibrosis group consisted of patients with no fibrosis and fibrosis grade 1 and 2. The late-stage fibrosis group consisted of patients with fibrosis grade 3 and fibrosis grade 4.
Liver maximum capacity test measurement
Liver maximum capacity test (LiMAx) was measured in the clinical routine and LiMAx values were retrospectively collected. LiMAx testing was performed following the published protocol and the manufacturer’s instructions and using the commercially available hardware (Humedics GMBH, Berlin, Germany).23
Definition of postop outcome parameters
Initial patient follow-up was 90 days. If a patient died during the initial 90-day period after surgery they were classified positive for 90-day mortality. Overall survival (OS) was calculated from the day of surgery to death or censored at last follow-up. Postop morbidity was defined using the classification put forth by Dindo et al.24 Severe postop morbidity was defined as postop morbidity grade 3 or higher. PHLF was defined and graded according to the criteria put forth by the International Study Group of Liver Surgery (ISGLS).25 PHLF was defined as elevated SB, as well as prolonged prothrombin time on POD5. If SB and PT were already deranged prior to surgery, they had to rise to levels above the preop baseline. If patients were discharged prior to POD5, due to good clinical performance, they were classified has having no PHLF.
Statistical analysis
Analysis was based on non-parametric tests to compare related or independent samples (Mann-Whitney U test, Wilcoxon signed rank test, Kruskal-Wallis test). Receiver-operating characteristic curve analysis and AUC were used to assess the predictive potential of preop parameters. To identify a cut-off for sTREM2, distinguishing our cohort into high- and low-risk groups for PHLF and postop mortality, we used Youden J statistic. To assess differences in incidence and prevalence between high- and low-risk groups, defined by a cut-off, the chi-squared test was used. To illustrate differences in OS, a log-rank test was applied, with patients lost to follow-up being censored. All analyses were performed using SPSS (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.) and GraphPad Prism (Version 10.0.0 for Macintosh, GraphPad Software, Boston, Massachusetts USA, www.graphpad.com). Figures were prepared using GraphPad Prism.
Results
sTREM2 levels are similar between different tumor types
Median preop MELD was 6, and 66% of patients underwent major liver resection. Detailed patient demographics can be found in Table 1. Except for benign tumors, hepatic resections are mainly performed for the treatment of primary or secondary malignancies of the liver. Initially, we investigated the effect of underlying tumor type on preop systemic sTREM2 levels. Hence, preop sTREM2 titers were compared between patients with colorectal cancer liver metastases, HCC, cholangiocarcinoma, benign tumors and metastases from other malignancies. Systemic sTREM2 concentrations prior to surgery were similar between all tumor types (Fig. 1A). Next, as TREM2 is already expressed by circulating monocytes we correlated preop sTREM2 with absolute and relative systemic monocyte counts measured preop and on POD1 and POD5. However, we could not see a correlative relationship between sTREM2 and circulating monocyte levels (Fig. S1).
Table 1.
Patient demographics.
| Parameters | Entire cohort (N = 108) |
|---|---|
| Median (IQR)/N (%) | |
| Age (years) | 67 (54-74) |
| Sex | |
| Female | 46 (42.6) |
| Male | 62 (57.4) |
| Tumor entity | |
| CRCLM | 48 (44.4) |
| HCC | 20 (18.5) |
| CCA | 19 (17.6) |
| Benign tumors | 13 (12) |
| Other malignancies | 8 (7.4) |
| Hepatic resection | |
| Minor | 37 (34.3) |
| Major | 71 (65.7) |
| Histology | |
| No fibrosis | 43 (39.6) |
| Fibrosis grade I | 39 (36.3) |
| Fibrosis grade II | 8 (7.7) |
| Fibrosis grade III | 11 (9.9) |
| Fibrosis grade IV | 7 (6.6) |
| Morbidity | |
| No morbidity | 47 (43) |
| Morbidity any | 61 (57) |
| I | 20 (19) |
| II | 24 (22) |
| III | 8 (7) |
| IV | 5 (5) |
| V | 4 (4) |
| Severe morbidity | |
| No severe morbidity | 92 (85) |
| Severe morbidity | 17 (15) |
| 90-day mortality | |
| No 90-day mortality | 98 (91) |
| 90-day mortality | 10 (9) |
| Posthepatectomy liver failure | |
| No PHLF | 86 (80) |
| PHLF total | 22 (20) |
| ISGLS A | 9 (8) |
| ISGLS B | 10 (9) |
| ISGLS C | 3 (3) |
| Liver function/chronic liver disease | |
| Preop sTREM2 (μg/ml) | 3.032 (1.939-4.638) |
| Preop FIB-4 | 1.8 (1-2.5) |
| Preop MELD | 6 (6-14) |
| Preop Child-Pugh | 5 (5-5) |
| Preoperative laboratory parameters | |
| SB (μmol/L) | 0.44 (0.33-0.61) |
| PT (%) | 105 (97-114) |
| AP (U/L) | 111 (75-159) |
| AST (U/L) | 31 (25-42) |
| ALT (U/L) | 31 (19-41) |
| GGT (U/L) | 69 (31-177) |
| Albumin (g/L) | 41.1 (38.3-44.7) |
| Platelets (G/L) | 241 (197-276) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; AP, alkaline phosphatase; CCA, cholangiocarcinoma; CLD, chronic liver disease; CRCLM, colorectal cancer liver metastases; FIB-4, fibrosis-4 index; GGT, gamma-glutamyltransferase; HCC, hepatocellular carcinoma; ISGLS, International Study Group of Liver Surgery; MELD, model for end-stage liver disease; PT, prothrombin time; SB, serum bilirubin; sTREM2, soluble TREM2.
Data presented as n (%) or median (IQR) as appropriate.
Fig. 1.
Systemic sTREM2 levels are associated with fibrosis stage, irrespective of tumor type.
(A) Preop sTREM2 levels in circulation of patients with different tumor types (p = 0.091, Kruskal-Wallis test). (B) Violin plots of measured preop sTREM2 levels in patients grouped according to early- and late-stage liver fibrosis (p = 0.030, Mann-Whitney U test). (C) Violin plots of FIB-4 index results of patients grouped according to early- and late-stage liver fibrosis (p = 0.619, Mann-Whitney U test). (D) Scatter plot for the correlation of preop sTREM2 levels and preop FIB-4 index (R = 0.226, p = 0.135, Spearman correlation). (E) Violin plots of MELD scores of patients grouped according to early- and late-stage liver fibrosis (p = 0.590, Mann-Whitney U test). (F) Scatter plot for the correlation of preop sTREM2 and preop MELD (R = 0.399, p <0.001, Spearman correlation). Levels of significance: ∗p <0.05. (A, B, C, E) Dotted lines represent quartiles and bold lines inside the violin plots represent median levels. CRCLM, colorectal cancer liver metastases; CCA, cholangiocarcinoma; HCC, hepatocellular carcinoma; MELD, model for end-stage liver disease; sTREM2, soluble TREM2.
Late-stage fibrosis is associated with higher levels of circulating sTREM2
To investigate whether sTREM2 concentrations were associated with different degrees of underlying liver disease in a cohort of patients undergoing liver surgery, we compared sTREM2 between early- and late-stage fibrosis. Interestingly, even in a cohort of patients undergoing liver surgery, who typically do not show symptoms of CLD, levels of sTREM2 were elevated in patients with fibrosis grade 3 and 4 in comparison with patients with no underlying liver disease or fibrosis grade 1 and 2 (Fig. 1B), confirming the association between sTREM2 and fibrosis development. FIB-4 scoring was developed for the prediction of high-grade fibrosis in patients with HCV and HIV co-infection and has since been validated for other etiologies of liver fibrosis as well.7,26 In the current cohort, FIB-4 results did not show a difference between patients with early- or late-stage fibrosis (Fig. 1C). Further, examining a possible correlation of FIB-4 with sTREM2, we could not identify a connection between preop sTREM2 and FIB-4 in patients prior to liver surgery (Fig. 1D). MELD is closely associated with survival of patients with high-grade CLD.9 Clinically, MELD is primarily used for prediction of survival and allocation of organs for patients awaiting liver transplantation.27 In addition to FIB-4, MELD was not associated with late-stage fibrosis. There was no difference between patients when grouped according to their degree of chronic underlying liver disease (Fig. 1E). In contrast, sTREM2 levels showed a positive correlation with MELD score (Fig. 1F). The Child-Pugh scoring system was developed to classify the severity of liver disease and takes into account laboratory, as well as clinical, parameters.28 A Child-Pugh class higher than A is very rare in patients undergoing liver surgery, but the frequency of early or late-stage fibrosis did show a difference in patients grouped according to the Child-Pugh criteria (Fig. S2).
Preop sTREM2 reflects liver function across the perioperative time course
Having shown an association of sTREM2 with chronic underlying liver disease, we aimed to examine if sTREM2 levels reflected preop liver function in patients undergoing liver surgery. Therefore, we assessed correlations of sTREM2 with classical laboratory parameters associated with liver function. Preop sTREM2 showed a positive correlation with preop SB levels, which has been documented to predict postop liver function.29 (Fig. 2A). During the postop time course, preop sTREM2 concentrations continued to exhibit a positive relationship with serum SB at POD1 and POD5 (Fig. 2A). Reflecting the coagulatory aspect of liver function, we assessed if sTREM2 levels correlated with PT as well. Interestingly, preop sTREM2 was inversely associated with PT from the day prior to surgery to postop time points on POD1 and POD5 (Fig. 2B). Preop sTREM2 was also negatively correlated with preop albumin and showed a positive correlation with preop ALT and AST (Fig. 2C). A positive correlation of sTREM2 with AST was also present on POD5 (Fig. S3). Finally, preop sTREM2 showed a positive correlation with preop AP, as well as AP levels on POD1 and POD5 (Fig. 2D). Taken together, these data support the association between liver injury and systemic sTREM2 levels.
Fig. 2.
sTREM2 levels correlate with parameters of non-invasive liver function assessment.
Scatter plot for the correlation of preop sTREM2 titers and (A) preop SB (R = 0.442, p <0.001, Pearson correlation), as well as SB on POD1 (R = 0.238, p = 0.021, Pearson correlation) and POD5 (R = 0.400, p <0.001, Pearson correlation) and (B) preop PT (R = -0.288, p = 0.007, Pearson correlation), as well as PT on POD1 (R = -0.312, p = 0.002, Pearson correlation) and POD5 (R = -0.442, p <0.001, Pearson correlation). (C) Scatter plot for the correlation of preop sTREM2 levels with preop albumin (R = -0.272, p = 0.025, Pearson correlation), ALT (R = 0.274, p = 0.031, Pearson correlation) and AST (R = 0.318, p = 0.009, Pearson correlation). (D) Scatter plot for the correlation of preop sTREM2 levels and preop AP (R = 0.451, p <0.001, Pearson correlation), as well as AP on POD1 (R = 0.482, p <0.001, Pearson correlation) and POD5 (R = 0.372, p = 0.002, Pearson correlation). ALT, alanine aminotransferase; AST, aspartate aminotransferase; AP, alkaline phosphatase; CLD, chronic liver disease; GGT, gamma-glutamyltransferase; POD, postoperative day; PT, prothrombin time; SB, serum bilirubin.
High sTREM2 in circulation is associated with PHLF and postop mortality and morbidity
Given the increased risk for PHLF, as well as postop morbidity and mortality in patients with underlying CLD, we investigated whether elevated preop sTREM2 levels associate with postop complications in patients undergoing liver surgery. Importantly, circulating sTREM2 was higher in patients who developed PHLF in comparison to patients with functional postop liver regeneration (Fig. 3A). Patients with postop morbidity also exhibited increased sTREM2 levels compared to patients with an uneventful postop course (Fig. S4). In patients with severe morbidity, i.e. those experiencing postop complications requiring intervention, elevated sTREM2 levels were observed compared to patients with no or minor complications (Fig. 3B). Furthermore, preop sTREM2 levels were higher in patients who died within 90 days postop compared to those with an OS greater than 90 days (Fig. 3C). These data suggest that sTREM2 levels might have predictive power for postop liver function and survival.
Fig. 3.
High systemic sTREM2 levels are associated with adverse outcomes after liver surgery.
Violin plots of sTREM2 concentrations in patients grouped according to development of (A) PHLF (p = 0.007, Mann-Whitney U test), (B) severe postop morbidity (p <001., Mann-Whitney U test), (C) 90-day mortality (p = 0.002, Mann-Whitney U test). Levels of significance: ∗p <0.05, ∗∗p <0.005. Dotted lines represent quartiles and bold lines inside the violin plots represent median levels. PHLF, posthepatectomy liver failure; sTREM2, soluble TREM2.
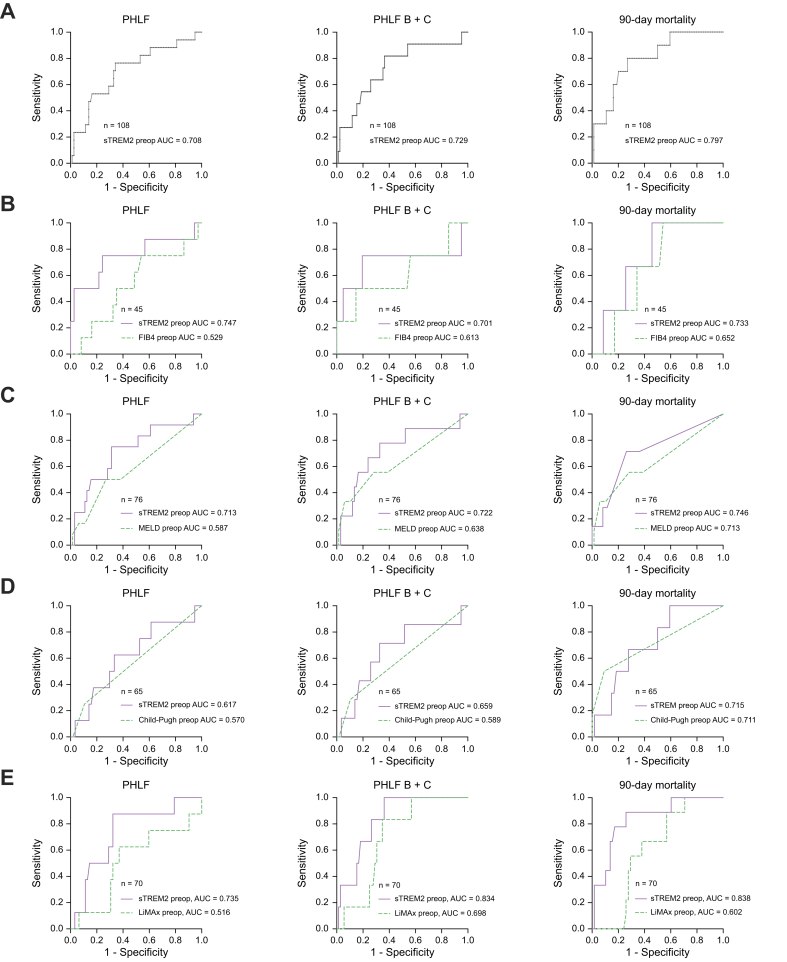
Systemic sTREM2 outperforms classical tests for underlying CLD in outcome prediction after liver surgery
Having shown an association of preop sTREM2 with classical markers of liver function and development of PHLF, we aimed to assess if preop sTREM2 could predict adverse outcomes after liver surgery, namely PHLF, PHLF B+C and 90-day mortality, using the AUC. Preop sTREM2 showed predictive potential for PHLF, PHLF B+C and 90-day mortality (Fig. 4A). Moreover, we found that preop sTREM2 outperformed FIB-4 in prediction of PHLF, PHLF B+C and 90-day mortality, as indicated by their AUCs (Fig. 4B). Similarly, we compared preop sTREM2 titers and preop MELD for their discriminatory potential for outcome after liver surgery and found that sTREM2 levels are superior to MELD in the prediction of PHLF, PHLF B+C and 90-day mortality (Fig. 4C). Finally, we evaluated the predictive performance of preop sTREM2 levels and preop Child-Pugh scores for adverse outcomes after liver surgery. In this comparison, sTREM2 was also superior for the prediction of PHLF, PHLF B+C and 90-day mortality (Fig. 4D). Additionally, we compared the predictive potential of sTREM2 with that of an established preoperative liver function test, namely LiMAx. LiMAx is primarily used in Europe and has previously shown an association with postoperative liver function.23 After comparing AUCs, sTREM2 outperformed LiMAx in its predictive potential for PHLF, PHLF B+C and 90-day mortality (Fig. 4E).
Fig. 4.
sTREM2 outperforms established markers for chronic liver disease in prediction of PHLF and postop mortality.
(A) Receiver-operating characteristic curve analysis and calculation of the AUC of systemic preop sTREM2 levels for the assessment of the predictive potential for PHLF (AUC = 0.708), PHLF B+C (AUC = 0.729) and 90-day mortality (AUC = 0.797). Receiver-operating characteristic curve analysis and calculation of the AUC of systemic preop sTREM2 levels and (B) preop FIB-4 index (PHLF: sTREM2, AUC = 0.747; FIB-4, AUC = 0.529) (PHLF B+C: sTREM2, AUC = 0.701; FIB-4, AUC = 0.613) (90-day mortality: sTREM2, AUC = 0.733; FIB-4, AUC = 0.652), (C) preop MELD score (PHLF: sTREM2, AUC = 0.713; MELD, AUC = 0.587) (PHLF B+C: sTREM2, AUC = 0.722; MELD, AUC = 0.638) (90-day mortality: sTREM2, AUC = 0.746; MELD, AUC = 0.713), (D) preop Child-Pugh score (PHLF: sTREM2, AUC = 0.617; Child-Pugh, AUC = 0.570) (PHLF B+C: sTREM2, AUC = 0.659; Child-Pugh, AUC = 0.589) (90-day mortality: sTREM2, AUC = 0.715; Child-Pugh, AUC = 0.711) and (E) LiMAx (PHLF: sTREM2, AUC = 0.747; LiMAx, AUC = 0.516) (PHLF B+C: sTREM2, AUC = 0.701; LiMAx, AUC = 0.698) (90-day mortality: sTREM2, AUC = 0.733; LiMAx, AUC = 0.602) for the comparison of the predictive potential for PHLF, PHLF B+C and 90-day mortality. n = number of patients available for comparison of the AUC. FIB-4, fibrosis-4 index; LiMAx, liver maximum capacity test; MELD, model for end-stage liver disease; PHLF, posthepatectomy liver failure; sTREM2, soluble TREM2.
sTREM2 remains independent of other metrics for severity of CLD in a multivariable model for the prediction of PHLF
To evaluate if systemic sTREM2 levels remained independent of other metrics predictive for underlying CLD, we included preop sTREM2, as well as MELD, FIB-4 index and Child-Pugh score in a multivariable logistic regression model. In univariate analysis only, sTREM2 was predictive for PHLF (Table S1). Next, we included sTREM2, FIB-4, MELD and Child-Pugh class in a multivariable model. To improve our model and account for the incidence of PHLF in our patient cohort, we proceeded with forward variable exclusion and found that preop sTREM2 remained the only predictive variable for PHLF in the model (Table S1). This reflects that sTREM2 is an independent predictor of PHLF with superior predictive potential when compared to classical markers of CLD.
High- and low-risk sTREM2 cut-offs accurately identify patients at risk for PHLF and 90-day mortality
To assess the clinical utility for the prediction of outcome after liver surgery via preop sTREM2 levels, we assessed two cut-offs for systemic sTREM2 for their positive and negative predictive values. To identify which patients could safely be resected, we tested a low-risk cut-off of ≥3.452 μg/ml for preop sTREM2. 93% of patients below the cut-off did not develop PHLF, while 37% of patients above the cut-off suffered from dysfunctional postop liver regeneration (Table 2). The low-risk cut-off was also tested for prediction of PHLF B+C. 96% of patients below the cut-off showed no signs of postop liver failure, while 26% of patients above the cut-off developed PHLF B+C (Supplemental Table 1). When assessing risk for 90-day mortality, the low-risk cut-off correctly identified 96% of patients below the cut-off, who survived the 90-day interval after surgery. 21% of patients above the cut-off died in the 90-day postop period (Table 2). Next, we tested a high-risk cut-off of ≥7.584 for preop sTREM2 designed to accurately identify patients at risk for adverse outcomes after surgery. 67% of patients above the high-risk cut-off developed PHLF, while 86% of patients below the cut-off did not develop PHLF (Table 2). Next, we tested the high-risk cut-off for the prediction of PHLF B+C, which is directly associated with short-term mortality after liver surgery.14 50% of patients above the high-risk cut-off suffered from PHLF B+C and 91% of patients below the cut-off had functional postop liver regeneration (Table 2). 50% of patients above the cut-off suffered from short-term postop mortality, while 91% of patients survived the 90-day postop period (Table 2). Overall, these data demonstrate the potential of sTREM2 to identify patients at risk for postoperative liver failure and subsequent mortality after liver surgery. Especially preop risk assessment via a low- and high-risk sTREM2 cut-off shows clinical relevance illustrated by its positive and negative predictive value for PHLF development.
Table 2.
Low- and high-risk sTREM2 cut-offs for the prediction of outcome after liver surgery.
| PHLF | PHLF B+C | 90-day mortality | |
|---|---|---|---|
| Preop sTREM2 ≥3.452 (μg/ml) cut-off | |||
| Sens. | 80% | 85% | 80% |
| Spec. | 66% | 63% | 62% |
| PPV | 37% | 26% | 21% |
| NPV | 93% | 96% | 96% |
| p value | <0.001 | 0.001 | 0.011 |
| Preop sTREM2 ≥7.584 (μg/ml) cut-off | |||
| Sens. | 24% | 27% | 30% |
| Spec. | 98% | 97% | 96% |
| PPV | 67% | 50% | 50% |
| NPV | 86% | 91% | 91% |
| p value | 0.001 | 0.002 | 0.003 |
NPV, negative predictive value; PHLF, posthepatectomy liver failure; PPV, positive predictive value; Sens, sensitivity; Spec, specificity; sTREM2, soluble TREM2.
Bold formatting highlight the outcome parameters.
High sTREM2 is associated with decreased OS after liver surgery
Next, we evaluated if high preop sTREM2 levels are associated with decreased long-term OS. Patients in the high-risk group, grouped according to our low-risk sTREM2 cut-off of ≥3.452 μg/ml, had shorter OS compared to patients in the low-risk group below the cut-off (Fig. 5). While patients in the high-risk group had a median OS of 19.8 months patients in the low-risk group did not reach median OS (Fig. 5).
Fig. 5.
High sTREM2 is associated with decreased OS after liver surgery.
Long-term OS in patients grouped according to a low-risk sTREM2 cut-off of 3.452 μg/ml (Preop sTREM2 <3.452 μg/ml: No. of events = 9, did not reach median OS; Preop sTREM2 ≥3.452 μg/ml: No. of events = 20, median OS = 19.8 months; p <0.001, Kaplan-Meier survival analysis, log-rank test). OS, overall survival; sTREM2, soluble TREM2.
sTREM2 levels decrease after liver surgery
Finally, we examined if sTREM2 levels changed across the perioperative time course. Interestingly, sTREM2 levels decreased from preop to POD1, while an elevation was noted when comparing sTREM2 on POD1 to POD5. Yet, sTREM2 levels at POD5 were still lower than concentrations of preop sTREM2 (Fig. 6A). Although we detected that preop sTREM2 is higher in patients who develop PHLF, the decrease of sTREM2 from preop to POD1 was independent of PHLF. While there was no difference between patients with and without PHLF on POD1, only in patients with normal postop liver function did sTREM2 increase from POD1 to POD5. There was no difference in sTREM2 on POD5 between patients based on the development of PHLF. However, patients with dysfunctional postop liver regeneration had lower sTREM2 levels when comparing preop sTREM2 to sTREM2 on POD5. In contrast, systemic sTREM2 on POD5 reached preop levels in patients with physiological postop liver regeneration (Fig. 6B). As sTREM2 is associated with liver fibrosis, we evaluated if there was a dynamic of perioperative sTREM2 depending on the degree of underlying liver disease. Irrespective of fibrosis stage, sTREM2 decreased from preop to POD1 in all patients. Interestingly, while sTREM2 was comparable between patients with early and late-stage liver fibrosis between POD1 and POD5, only in patients with late-stage fibrosis did sTREM2 not return to preop levels by POD5 (Fig. 6C).
Fig. 6.
Plasma sTREM2 dynamics across the perioperative time course are influenced by PHLF development and fibrosis stage.
(A) Perioperative dynamics of plasma sTREM2 levels (Preop to POD1, p <0.001, Wilcoxon test; POD1 to POD5, p = 0.015, Wilcoxon test; Preop to POD5, p = 0.011, Wilcoxon test). (B) Perioperative changes in plasma sTREM2 levels in patients grouped according to PHLF development (Preop to POD1 no PHLF, p <0.001, Wilcoxon test; Preop to POD1 PHLF, p <0.001, Wilcoxon test) (POD1 to POD5 no PHLF, p = 0.008, Wilcoxon test; POD1 to POD5 PHLF, p = 0.807, Wilcoxon test) (Preop to POD5 no PHLF, p = 0.197, Wilcoxon test; Preop to POD5 PHLF, p = 0.002, Wilcoxon test). (C) Perioperative sTREM2 dynamics in patients grouped according to fibrosis stage (Preop to POD1 no PHLF, p <0.001, Wilcoxon test; Preop to POD1 PHLF, p <0.001, Wilcoxon test) (POD1 to POD5 no PHLF, p = 0.080, Wilcoxon test; POD1 to POD5 PHLF, p = 0.575, Wilcoxon test) (Preop to POD5 no PHLF, p = 0.067, Wilcoxon test; Preop to POD5 PHLF, p = 0.017, Wilcoxon test). Levels of significance: ∗p <0.05, ∗∗p <0.005. Dotted lines represent quartiles and bold lines inside the violin plots represent median levels. PHLF, posthepatectomy liver failure; POD, postoperative day; sTREM2, soluble TREM2.
Discussion
Development of CLD leads to an increased risk of mortality due to an irreversible loss of liver function over time, as well as due to the elevated risk of HCC development.2,3 Development of symptomatic disease during progression of CLD often comes at a late stage when treatment options are limited.6,30 Here, we describe that sTREM2 levels in circulation are associated with the presence and progression of liver fibrosis and cirrhosis even in patients undergoing liver surgery. Although these patients still exhibit well compensated liver function, sTREM2 levels were able to distinguish between early- and late-stage liver fibrosis, irrespective of tumor type. Additionally, preop sTREM2 correlated with classical markers of liver function and injury across the perioperative time course and were associated with adverse outcomes after liver surgery. Finally, sTREM2 levels show significant predictive potential for postop liver function and using two sTREM2-based cut-offs we could accurately predict development of PHLF and postop mortality.
The role of TREM2 has been implicated in a variety of diseases.31 Particularly the involvement of TREM2 in the progression of neurodegenerative diseases like Alzheimer’s disease has been studied extensively.[32], [33], [34] In the central nervous system, TREM2 is mainly expressed on microglia/macrophages.35 Similarly, recent studies elucidating the role of TREM2 in progression of liver disease could show that hepatic TREM2 expression primarily arises from monocyte-derived macrophages and Kupffer cells.36 TREM2-expressing macrophages are found in livers of obese mice or in murine MASH, as well as during liver fibrosis with the expansion of TREM2-expressing scar-associated macrophages.18,37,38 Using spatial transcriptomics, we previously showed that TREM2+ macrophages localize to fibrotic and inflammatory areas. Their protective function is further supported since diet-induced MASH development is aggravated in TREM2-deficient mice in various murine models.17 Besides its membrane-bound form, sTREM2 is shedded via cleavage of the TREM2 ectodomain by metalloproteinases.39 Soluble forms of TREM2 have been demonstrated to improve microglia survival and exert a neuroprotective effect during Alzheimer’s disease.40,41 While specific functions of sTREM2 during MASH remain to be identified, sTREM2 levels correlate with the degree and progression of CLD and present a sensitive marker for underlying CLD.17,42 It remains to be seen whether sTREM2 levels during CLD are associated with elevated TREM2 cleavage.
TREM2 expression is upregulated in HCC tissue and TREM2 deficiency is associated with HCC progression in mice.43 In this study, we included a variety of different tumor types ranging from primary malignancies of the liver to metastatic and even benign disease. However, we could not identify a difference in sTREM2 levels depending on tumor type. This implies that sTREM2 levels reflect underlying CLD and liver function rather than being associated with tumor biology. Supporting this hypothesis, sTREM2 was elevated in patients with late-stage fibrosis. Additionally, sTREM2 was associated with development of PHLF or insufficient postop liver regeneration. Hepatic TREM2+ macrophage populations increase with the severity of CLD.17 Consistent with this, given that late-stage CLD poses a significant risk for liver surgery,[44], [45], [46] our data supports an association between sTREM2 levels and CLD severity. Elevated sTREM2 levels indicated inhibited liver function and a concomitantly increased risk for postop liver failure, as well as short- and long-term mortality following liver surgery.
Liver function and hepatic injury can be assessed via various non-invasive laboratory tests. Circulatory SB, PT and albumin (as classical markers of liver function), ALT and AST (as markers of hepatocyte demise), or AP (a classical cholestasis marker) together reflect a universal picture of liver health. Here we found that preop sTREM2 correlated with multiple parameters associated with liver function and injury. In line, TREM2 and sTREM2 have been found to be expressed during the development of various liver diseases ranging from cholestasis to MASH or cirrhosis.17,47 Moreover, preop sTREM2 correlated with the postop progression of SB and PT. Both SB and PT on POD5 are utilized in the most commonly used definition for PHLF. The definition of PHLF by the ISGLS defines PHLF via elevated SB and prolonged PT on POD5.25 Hence, sTREM2 levels could potentially enhance the definition of PHLF by enabling earlier detection of the onset of liver failure.
Interestingly, when examining perioperative sTREM2 levels, all patients saw a postop decrease of sTREM2, which might be related to reduced liver mass, and subsequently a proportional decrease of hepatic TREM2+ macrophage turnover reflected in measured sTREM2. In line with these findings, sTREM2 levels did not correlate with circulating monocytes during the perioperative time course, suggesting that sTREM2 levels were actually associated with hepatic TREM2+ macrophage content. Also, only patients who developed PHLF or patients with late-stage liver fibrosis failed to reach preop sTREM2 levels. Since TREM2+ macrophages exert protective effects during CLD,17 hepatic recruitment of circulating TREM2 macrophages could be vital for postop liver regeneration. However, we want to point out that we can only make assumptions about the association of sTREM2 with hepatic TREM2+ macrophage content in our patient cohort prior to liver surgery. Yet, our previous publication has shown that TREM2+ macrophages localize to sites of injury, inflammation and fibrosis in the liver, which would support our hypothesis and is in line with our findings.17 Future studies should evaluate the dynamics of TREM2+ macrophages and their function in postop liver regeneration. Moreover, given the rather small sample size presented and the lack of an external validation cohort, which might result in missing confounders in our analysis, our study would benefit from additional assessments of sTREM2 levels in more patient cohorts. It is important to note, however, that sTREM2 is not currently evaluated in clinical practice. Therefore, along with the uniqueness of our prospectively collected biorepository, validation using an independent cohort is challenging. Hence, we hope that our study will encourage further validation of sTREM2 as a biomarker for outcome after liver surgery. Furthermore, our current setting does not provide mechanistic insights as to why post-surgery outcome is related to the levels of sTREM2 in the circulation. More research is required to address this.
Patients undergoing liver surgery, while possibly presenting with late-stage CLD, will usually not suffer from symptoms associated with end-stage fulminant cirrhosis like portal hypertension, recurrent ascites or jaundice.48 This is reflected in the low median MELD and Child-Pugh scores in our patient cohort. Neither MELD nor FIB-4 were associated with fibrosis stage in this patient cohort. While Child-Pugh scoring could show a significant difference in patients with early- and late-stage fibrosis depending on Child-Pugh score, only one patient was classified with Child-Pugh grade B. This raises the question of the clinical utility of established markers for severity of CLD for risk assessment prior to liver surgery. Preop sTREM2 outperformed all evaluated parameters in the predictive potential for PHLF prediction. Strikingly, sTREM2 even outperformed an established preoperative liver function test like the LiMAx test in its predictive potential for PHLF, PHLF B+C and 90-day mortality. Additionally, when comparing published positive and negative predictive values for development of PHLF of the LiMAx test with the high- and low-risk sTREM2 cut-offs, sTREM2 outperforms LiMAx testing in identifying patients at risk for PHLF.49 Our data suggests that sTREM2 is highly sensitive for early fibrosis development prior to development of symptomatic disease. Several non-invasive tests currently in use for assessment of CLD have been developed in patient cohorts with advanced or end-stage liver disease. These tests often suffer from limited sensitivity in early-stage liver disease.50 Here, we create awareness that systemic sTREM2 could improve early detection of CLD and fibrosis in patients with asymptomatic disease. CLD is often not reflected in liver function tests or risk prediction models established for routine preop risk stratification prior to liver surgery. Integration of sTREM2 to risk assessment prior to liver surgery could improve patient outcome.
In this study, we present data on systemic sTREM2 levels in patients undergoing liver surgery. Systemic sTREM2 titers exhibited sensitivity to differences in underlying CLD even among patients with intact liver function, in a patient cohort where established tests and gradings used for detecting fibrosis and liver injury failed to differentiate. Irrespective of tumor type, sTREM2 showed an association with late-stage liver fibrosis and correlated with a broad assessment of liver function. With our proposed cut-offs, we showed that preop liver function assessment could be enhanced via the inclusion of sTREM2 into risk stratification prior to liver surgery. Taken together, our study highlights the potential of systemic sTREM2 levels as a sensitive biomarker for a variety of clinical scenarios in the context of CLD and liver surgery.
Abbreviations
ALT, alanine aminotransferase; AST, aspartate aminotransferase; AP, alkaline phosphatase; CLD, chronic liver disease; FIB-4, fibrosis-4 index; GGT, gamma-glutamyltransferase; HCC, hepatocellular carcinoma; ISGLS, International Study Group of Liver Surgery; LiMAx, liver maximum capacity test; MASH, metabolic dysfunction-associated steatohepatitis; MASLD, metabolic dysfunction-associated liver disease; MELD, model for end-stage liver disease; OS, overall survival; PHLF, posthepatectomy liver failure; POD, postoperative day; PT, prothrombin time; SB, serum bilirubin; sTREM2, soluble TREM2; TREM2, triggering receptor expressed on myeloid cells 2.
Financial support
None of the authors received funding related to the writing of this manuscript.
Authors’ contributions
J.S. collected and analyzed the data, prepared the figures and wrote the manuscript; D.R., C.H., T.P.B. performed the studies and critically revised the manuscript; G.O., B.R., P.S., J.P., M.A., A.E.K., M.A., A.S., J. J.W. collected and analyzed the data and critically revised the manuscript; T.G. provided resources and critically revised the manuscript; P.S., conceived the study, analyzed and interpreted the data, provided resources and wrote the manuscript; T.H. conceived the study, designed and performed the studies, analyzed and interpreted the data, provided resources and wrote the manuscript.
Data availability statement
The datasets generated and/or analyzed during this study are not publicly available due to sensitive patient data but are available from the corresponding author on reasonable request.
Disclosure of generative AI in scientific writing
No generative AI or AI-assisted technologies were used in the writing of this manuscript.
Conflict of interest
None of the authors have any type of conflict of interest to declare in regards to the writing of this manuscript.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
This study was in part funded by the NIH (R01DK122813) received by P.S. and by the SFU MED Research Promotion Fund (FFF 12/22, FFF 12/23) received by J.S. and T.G. We want to thank the people working at the departments of general surgery at the General Hospital Vienna and Medical University of Vienna, Clinic Landstrasse and Clinic Favoriten during the duration of this study. The Graphical abstract was created with BioRender.com.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2024.101226.
Contributor Information
Patrick Starlinger, Email: starlinger.patrick@mayo.edu.
Tim Hendrikx, Email: tim.hendrikx@meduniwien.ac.at.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Moon A.M., Singal A.G., Tapper E.B. Contemporary epidemiology of chronic liver disease and cirrhosis. Clin Gastroenterol Hepatol. 2020;18(12):2650–2666. doi: 10.1016/j.cgh.2019.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Younossi Z., Tacke F., Arrese M., et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2019;69(6):2672–2682. doi: 10.1002/hep.30251. [DOI] [PubMed] [Google Scholar]
- 3.Huang D.Q., Mathurin P., Cortez-Pinto H., et al. Global epidemiology of alcohol-associated cirrhosis and HCC: trends, projections and risk factors. Nat Rev Gastroenterol Hepatol. 2023;20(1):37–49. doi: 10.1038/s41575-022-00688-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Israelsen M., Torp N., Johansen S., et al. MetALD: new opportunities to understand the role of alcohol in steatotic liver disease. Lancet Gastroenterol Hepatol. 2023;8(10):866–868. doi: 10.1016/S2468-1253(23)00206-6. [DOI] [PubMed] [Google Scholar]
- 5.Harrison S.A., Bedossa P., Guy C.D., et al. A phase 3, randomized, controlled trial of resmetirom in NASH with liver fibrosis. N Engl J Med. 2024;390(6):497–509. doi: 10.1056/NEJMoa2309000. [DOI] [PubMed] [Google Scholar]
- 6.Goutté N., Sogni P., Bendersky N., et al. Geographical variations in incidence, management and survival of hepatocellular carcinoma in a Western country. J Hepatol. 2017;66(3):537–544. doi: 10.1016/j.jhep.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 7.Vallet-Pichard A., Mallet V., Nalpas B., et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46(1):32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 8.Tsoris A., Marlar C.A. StatPearls Publishing LLC.; 2024. Use of the Child pugh score in liver disease. StatPearls. Treasure island (FL): StatPearls publishing copyright © 2024. [PubMed] [Google Scholar]
- 9.Kamath P.S., Wiesner R.H., Malinchoc M., et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33(2):464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 10.Györi G.P., Pereyra D., Rumpf B., et al. The von Willebrand Factor Facilitates Model for End-Stage Liver Disease-Independent Risk Stratification on the Waiting List for Liver Transplantation. Hepatology. 2020;72(2):584–594. doi: 10.1002/hep.31047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santol J., Pereyra D., Haegele S., et al. The ratio of activin A and follistatin-like 3 is associated with posthepatectomy liver failure and morbidity in patients undergoing liver resection. Gastro Hep Adv. 2023;2(5):642–651. doi: 10.1016/j.gastha.2023.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Primavesi F., Maglione M., Cipriani F., et al. E-AHPBA-ESSO-ESSR Innsbruck consensus guidelines for preoperative liver function assessment before hepatectomy. Br J Surg. 2023;110(10):1331–1347. doi: 10.1093/bjs/znad233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilg S., Sandström P., Rizell M., et al. The impact of post-hepatectomy liver failure on mortality: a population-based study. Scand J Gastroenterol. 2018;53(10–11):1335–1339. doi: 10.1080/00365521.2018.1501604. [DOI] [PubMed] [Google Scholar]
- 14.Baumgartner R., Gilg S., Björnsson B., et al. Impact of post-hepatectomy liver failure on morbidity and short- and long-term survival after major hepatectomy. BJS Open. 2022;6(4) doi: 10.1093/bjsopen/zrac097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sparrelid E., Olthof P.B., Dasari B.V.M., et al. Current evidence on posthepatectomy liver failure: comprehensive review. BJS Open. 2022;6(6) doi: 10.1093/bjsopen/zrac142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santol J., Kim S., Gregory L.A., et al. An APRI+ALBI based multivariable model as preoperative predictor for posthepatectomy liver failure. Ann Surg. 2023 doi: 10.1097/SLA.0000000000006127. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hendrikx T., Porsch F., Kiss M.G., et al. Soluble TREM2 levels reflect the recruitment and expansion of TREM2(+) macrophages that localize to fibrotic areas and limit NASH. J Hepatol. 2022;77(5):1373–1385. doi: 10.1016/j.jhep.2022.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Jaitin D.A., Adlung L., Thaiss C.A., et al. Lipid-associated macrophages control metabolic homeostasis in a Trem2-dependent manner. Cell. 2019;178(3):686–698.e14. doi: 10.1016/j.cell.2019.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strasberg S., Belghiti J., Clavien P.-A., et al. The Brisbane 2000 terminology of liver anatomy and resections. Hpb. 2000;2(3):333–339. [Google Scholar]
- 20.Mussbacher M., Schrottmaier W.C., Salzmann M., et al. Optimized plasma preparation is essential to monitor platelet-stored molecules in humans. PLoS One. 2017;12(12) doi: 10.1371/journal.pone.0188921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mussbacher M., Krammer T.L., Heber S., et al. Impact of anticoagulation and sample processing on the quantification of human blood-derived microRNA signatures. Cells. 2020;9(8) doi: 10.3390/cells9081915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Batts K.P., Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol. 1995;19(12):1409–1417. doi: 10.1097/00000478-199512000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Stockmann M., Lock J.F., Riecke B., et al. Prediction of postoperative outcome after hepatectomy with a new bedside test for maximal liver function capacity. Ann Surg. 2009;250(1):119–125. doi: 10.1097/SLA.0b013e3181ad85b5. [DOI] [PubMed] [Google Scholar]
- 24.Dindo D., Demartines N., Clavien P.-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rahbari N.N., Garden O.J., Padbury R., et al. Posthepatectomy liver failure: a definition and grading by the international study group of liver surgery (ISGLS) Surgery. 2011;149(5):713–724. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Sterling R.K., Lissen E., Clumeck N., et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 27.Freeman R.B., Jr., Wiesner R.H., Harper A., et al. The new liver allocation system: moving toward evidence-based transplantation policy. Liver Transpl. 2002;8(9):851–858. doi: 10.1053/jlts.2002.35927. [DOI] [PubMed] [Google Scholar]
- 28.Pugh R.N.H., Murray-Lyon I.M., Dawson J.L., et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 2005;60(8):646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 29.Balzan S., Belghiti J., Farges O., et al. The "50-50 criteria" on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242(6):824–828. doi: 10.1097/01.sla.0000189131.90876.9e. discussion 828-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dam Fialla A., Schaffalitzky de Muckadell O.B., Touborg Lassen A. Incidence, etiology and mortality of cirrhosis: a population-based cohort study. Scand J Gastroenterol. 2012;47(6):702–709. doi: 10.3109/00365521.2012.661759. [DOI] [PubMed] [Google Scholar]
- 31.Kober D.L., Brett T.J. TREM2-Ligand interactions in health and disease. J Mol Biol. 2017;429(11):1607–1629. doi: 10.1016/j.jmb.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guerreiro R., Wojtas A., Bras J., et al. TREM2 variants in Alzheimer's disease. N Engl J Med. 2013;368(2):117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jonsson T., Stefansson H., Steinberg S., et al. Variant of TREM2 associated with the risk of Alzheimer's disease. N Engl J Med. 2013;368(2):107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y., Cella M., Mallinson K., et al. TREM2 lipid sensing sustains the microglial response in an Alzheimer's disease model. Cell. 2015;160(6):1061–1071. doi: 10.1016/j.cell.2015.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colonna M., Wang Y. TREM2 variants: new keys to decipher Alzheimer disease pathogenesis. Nat Rev Neurosci. 2016;17(4):201–207. doi: 10.1038/nrn.2016.7. [DOI] [PubMed] [Google Scholar]
- 36.Wen Y., Lambrecht J., Ju C., et al. Hepatic macrophages in liver homeostasis and diseases-diversity, plasticity and therapeutic opportunities. Cell Mol Immunol. 2021;18(1):45–56. doi: 10.1038/s41423-020-00558-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiong X., Kuang H., Ansari S., et al. Landscape of intercellular crosstalk in healthy and NASH liver revealed by single-cell secretome gene analysis. Mol Cel. 2019;75(3):644–660.e5. doi: 10.1016/j.molcel.2019.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramachandran P., Dobie R., Wilson-Kanamori J.R., et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature. 2019;575(7783):512–518. doi: 10.1038/s41586-019-1631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wunderlich P., Glebov K., Kemmerling N., et al. Sequential proteolytic processing of the triggering receptor expressed on myeloid cells-2 (TREM2) protein by ectodomain shedding and γ-secretase-dependent intramembranous cleavage. J Biol Chem. 2013;288(46):33027–33036. doi: 10.1074/jbc.M113.517540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhong L., Chen X.F., Wang T., et al. Soluble TREM2 induces inflammatory responses and enhances microglial survival. J Exp Med. 2017;214(3):597–607. doi: 10.1084/jem.20160844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhong L., Xu Y., Zhuo R., et al. Soluble TREM2 ameliorates pathological phenotypes by modulating microglial functions in an Alzheimer's disease model. Nat Commun. 2019;10(1):1365. doi: 10.1038/s41467-019-09118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kothari V., Savard C., Tang J., et al. sTREM2 is a plasma biomarker for human NASH and promotes hepatocyte lipid accumulation. Hepatol Commun. 2023;7(11) doi: 10.1097/HC9.0000000000000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Esparza-Baquer A., Labiano I., Sharif O., et al. TREM-2 defends the liver against hepatocellular carcinoma through multifactorial protective mechanisms. Gut. 2021;70(7):1345–1361. doi: 10.1136/gutjnl-2019-319227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bruix J., Castells A., Bosch J., et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology. 1996;111(4):1018–1022. doi: 10.1016/s0016-5085(96)70070-7. [DOI] [PubMed] [Google Scholar]
- 45.Reddy S.K., Marsh J.W., Varley P.R., et al. Underlying steatohepatitis, but not simple hepatic steatosis, increases morbidity after liver resection: a case-control study. Hepatology. 2012;56(6):2221–2230. doi: 10.1002/hep.25935. [DOI] [PubMed] [Google Scholar]
- 46.Capussotti L., Muratore A., Amisano M., et al. Liver resection for hepatocellular carcinoma on cirrhosis: analysis of mortality, morbidity and survival--a European single center experience. Eur J Surg Oncol. 2005;31(9):986–993. doi: 10.1016/j.ejso.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 47.Labiano I., Agirre-Lizaso A., Olaizola P., et al. TREM-2 plays a protective role in cholestasis by acting as a negative regulator of inflammation. J Hepatol. 2022;77(4):991–1004. doi: 10.1016/j.jhep.2022.05.044. [DOI] [PubMed] [Google Scholar]
- 48.Zaydfudim V.M., Turrentine F.E., Smolkin M.E., et al. The impact of cirrhosis and MELD score on postoperative morbidity and mortality among patients selected for liver resection. Am J Surg. 2020;220(3):682–686. doi: 10.1016/j.amjsurg.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santol J., Ammann M., Reese T., et al. Comparison of the LiMAx test vs. the APRI+ALBI score for clinical utility in preoperative risk assessment in patients undergoing liver surgery - a European multicenter study. Eur J Surg Oncol. 2024;50(4) doi: 10.1016/j.ejso.2024.108048. [DOI] [PubMed] [Google Scholar]
- 50.Patel K., Sebastiani G. Limitations of non-invasive tests for assessment of liver fibrosis. JHEP Rep. 2020;2(2) doi: 10.1016/j.jhepr.2020.100067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during this study are not publicly available due to sensitive patient data but are available from the corresponding author on reasonable request.