Abstract
Background & Aims
Hepatocellular carcinoma (HCC) frequently undergoes regional chromosomal amplification, resulting in elevated gene expression levels. We aimed to elucidate the role of these poorly understood genetic changes by using CRISPR activation (CRISPRa) screening in mouse livers to identify which genes within these amplified loci are cancer driver genes.
Methods
We used data from The Cancer Genome Atlas to identify that frequently copy number-amplified and up-regulated genes all reside on human chromosomes 1q and 8q. We generated CRISPRa screening transposons that contain oncogenic Myc to drive tumor formation. We conducted CRISPRa screens in vivo in the liver to identify tumor driver genes. We extensively validated the findings in separate mice and performed RNA sequencing analysis to explore mechanisms driving tumorigenesis.
Results
We targeted genes that frequently undergo amplification in human HCC using an in vivo CRISPRa screening system in mice, which induced extensive liver tumorigenesis. Human chromosome 1q genes Zbtb7b, Vps72, Gba1, and Mrpl9 emerged as drivers of liver tumorigenesis. In human HCC there is a trend in correlation between levels of MRPL9, VPS72, or GBA1 and poor survival. In validation assays, activation of Vps72, Gba1, or Mrpl9 resulted in extensive liver tumorigenesis and decreased survival in mice. RNA sequencing revealed different mechanisms driving HCC, with Mrpl9 activation altering genes functionally related to mitochondrial function, Vps72 levels altering phospholipid metabolism, and Gba1 activation enhancing endosomal-lysosomal activity, all leading to promotion of cellular proliferation. Analysis of human tumor tissues with high levels of MRPL9, VPS72, or GBA1 revealed congruent results, indicating conserved mechanisms driving HCC.
Conclusions
This study reveals chromosome 1q genes Vps72, Gba1, and Mrpl9 as drivers of HCC. Future efforts to prevent or treat HCC can focus on these new driver genes.
Keywords: Genetic Screen, Liver Cancer, Somatic Amplification, Carcinogenesis, dCas9
Summary.
VPS72, GBA1, and MRPL9 are chromosome 1q genes that undergo amplification in human hepatocellular carcinoma and are found in this study to be cancer driver genes, suggesting their potential as targets for liver cancer prevention and treatment.
Liver cancer represents a significant health challenge, ranking as the third leading cause of cancer-related deaths globally with approximately 900,000 new cases annually.1 Hepatocellular carcinoma (HCC), constituting about 90% of liver cancer cases,2 emerges from chronic liver injury, inflammation, and cirrhosis, accumulating genetic and epigenetic changes that culminate in highly heterogenous tumors. Current therapeutic approaches offer only modest improvement in outcomes, and there is no reliable way to determine which patients are likely to achieve tumor responses.3 There is a need to identify more effective and personalized targets for HCC treatment.
Although numerous genes have been identified as prognostic markers in HCC, the functional annotation of genes lags. It remains challenging to distinguish the true drivers and suppressors in tumors from mere passengers with no substantive impact on HCC. Genetic screening techniques, particularly CRISPR-based methods, can systematically ascertain specific gene functions such as the promotion of cellular proliferation. But these techniques have not been applied in vivo in HCC research.1
CRISPR-based systems offer a versatile approach, requiring only the modification of the guide RNA (gRNA) coding sequence to specifically target a gene.4, 5, 6 Activation screens using CRISPRa provide unique insights compared with loss-of-function or interference screens, because the up-regulation or absence of genes can lead to distinct phenotypes.1,7,8 With CRISPRa, gRNA(s) tether catalytically dead Cas9 (dCas9) to the promoter region of each target gene, together with a transcriptional activator (TA), which boosts the expression of the gene. To date, CRISPRa screens have not been applied to study HCC.
We hypothesized that CRISPRa screening in a mouse tumor model will identify genes that directly promote liver cancer. We established a sensitive and accurate CRISPRa platform to screen the function of genes that are amplified and up-regulated in human HCCs. The results revealed VPS72, GBA1, and MRPL9 as drivers of liver cancer. Our innovative approach advances our understanding of HCC pathogenesis and identifies potential targets for HCC therapy.
Results
Identification of Genes Amplified and Enriched in Hepatocellular Carcinomas
We first identified the genes that undergo amplification and up-regulation in expression level in human HCC by mining The Cancer Genome Atlas (TCGA) liver cancer dataset TCGA-LIHC. Interestingly, the top hits were all genes located in 2 clusters on chromosome arms 1q and 8q (Figure 1). A cutoff for genes amplified in ≥10% and up-regulated in ≥40% of human HCCs yielded a set of 57 genes, of which 51 are conserved in mice. Of these, 35 reside on human chromosome 1q and 16 on 8q. The well-established HCC oncogene MYC is found at the locus on chromosome 8q.9, 10, 11, 12 However, no other definite HCC driver gene is located on chromosomes 1 or 8. In fact, only 2 (PRCC and SETDB1) out of the 51 genes were annotated as potential drivers of cancer in the COSMIC cancer gene consensus database.13 Therefore, we hypothesized that we could determine which genes are key drivers of HCC using an in vivo CRISPR activation system.
Figure 1.
Genome-wide copy number amplification and mRNA up-regulation in human HCC. (A) Chart of the percentage of patients with copy-number amplification of the tumor tissue genomic DNA at the given locus. (B) Chart of the percentage of patients with up-regulated gene expression by RNA-seq (Z-score >1.5). Data source is the TCGA-LIHC dataset of human HCCs.
In Vivo CRISPRa Screening in a MYC-Driven Model
Overexpression of MYC in vivo drives oncogenesis in the liver with a long latency, which is greatly accelerated with the co-expression of other oncogenes or the inhibition of tumor suppressors.14, 15, 16 In other words, MYC expression provides a basal tumor formation activity that can be greatly enhanced by the dysregulation of a second gene. To identify which of the genes that are amplified and enriched in human HCC can function as tumor driver genes, we engineered an in vivo CRISPRa screen that combines MYC expression with gRNA expression. We designated the CRISPRa screening plasmid as Myc-TA-gRNA. We made a control plasmid that did not express MYC to identify the tumorigenesis activity of CRISPRa gene targeting alone, if any, which was designated TA-gRNA (Figure 2A). We then generated a screening library containing 5 gRNAs targeted to each of the 51 amplified and up-regulated genes from human HCC, plus 3 known driver genes (Vegfa, Ccnd1, Tert) as positive controls, for a total of 270 gene-targeting gRNAs, along with 20 non-targeting gRNAs (sg-NTs) as negative controls. The Myc-TA-gRNA or TA-gRNA plasmid libraries were hydrodynamic tail vein–injected into dCas9+ and wild-type (WT) control mice (Figure 2A). At post-injection week 5.5 and 8–10, respectively, the Myc-TA-gRNA– and TA-gRNA–injected mice were euthanized. The tumor burden (Figure 2B and C), along with liver-to-body weight ratio (Figure 2D), were markedly greater in dCas9+ mice injected with the Myc-TA-gRNA library as compared with WT mice or TA-gRNA–injected mice, indicating that the combination of exogenous MYC overexpression and CRISPRa targeting of genes from the library can accelerate tumor formation. Immunohistochemical (IHC) staining of tumor tissues showed a high proportion of Ki67-positive tumor cells, indicating aggressive tumor cell proliferation (Figure 2E).
Figure 2.
In vivo CRISPRa screen in a MYC-driven model. (A) Design of the CRISPRa tumorigenesis screen. Plasmid libraries Myc-TA-gRNA or TA-gRNA were hydrodynamically injected into dCas9+ or WT control mice. Tumorigenesis proceeded for up to 10 weeks. The enriched gRNA sequences correspond to genetic drivers of tumorigenesis. (B) (Left) Liver images from dCas9+ or WT mice injected with Myc-TA-gRNA (+Myc) or TA-gRNA (NoMyc) libraries for the tumorigenesis screen. (Right) Gross quantification of tumors on the anterior surface of mouse livers. Statistical analysis performed with one-way ANOVA with Tukey multiple comparisons test. ∗∗∗∗P < .0001. Data are shown as mean ± standard deviation (SD). (C) Representative (top) liver images and (bottom) hematoxylin-eosin (H&E) sections from WT mice and dCas9+ mice hydrodynamically injected with Myc-TA-gRNA. Scale bars represent (top) 10 mm and (bottom) 2 mm. (D) Liver-to-body weight ratios from dCas9+ or WT mice injected with Myc-TA-gRNA or TA-gRNA libraries. Statistical analysis performed with one-way ANOVA with Tukey multiple comparisons test (∗∗∗∗P < .0001). Data are shown as mean ± SD. (E) Panel of liver sections from the +Myc;dCas9+ mice stained with H&E, MYC, and Ki67. Scale bars represent 650 μm.
In Vivo CRISPRa Screening to Identify Drivers of Liver Cancer
First, we assessed the performance of CRISPRa screening during liver tumorigenesis. There was low sequencing error, because most reads mapped correctly to expected sequences (Figure 3A). The input libraries and the dCas9+ and WT samples clustered separately by principal component analysis (Figure 3B). The number of different gRNAs recovered was highest in input libraries, followed by bulk liver tissue samples, and lowest for tumor tissue samples (Figure 3C), with an inverse pattern in gRNA reads evenness score, which reflects a drop in gRNA diversity in the tumors (Figure 3D). A mean of 10 unique gRNAs were identified in each tumor (Figure 3E), and the proportion of gRNAs was consistent with clonality (Figure 4).
Figure 3.
Analysis of in vivo CRISPRa screening during liver tumorigenesis. (A) Percent of mapped and unmapped reads across groups. Statistical analysis performed with two-way ANOVA with Šídák's multiple comparisons test. (B) Principal component analysis plot of dCas9+ and WT samples and input libraries. (C) The number of gRNAs present per sample out of the total 290 gRNAs across conditions. (D) Gini index (measure of evenness) across all conditions. (E) The number of gRNAs present per tumor out of the total 290 gRNAs. Statistical analyses performed with one-way ANOVA with Tukey multiple comparisons test. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001. Data are shown as mean ± SD. BL, bulk liver tissue; T, individual tumors.
Figure 4.
Proportion of gRNAs per tumor. Pie charts representing the proportion of each gRNA detected in individual tumors from +Myc;dCas9+ livers.
Next, to identify the genes that are driving HCC, we determined the gRNAs that were enriched in tumor tissues. We analyzed the gRNAs recovered either from bulk liver tissues or from individual tumors from dCas9+ mice hydrodynamically injected with the Myc-TA-gRNA library (+Myc;dCas9+) (Figure 5A). The putative cancer driver genes were the ones with gRNAs significantly enriched during the screen (Figure 5B). We identified positive control driver genes Vegfa and Ccnd1 among top genes with enriched gRNAs, validating our CRISPRa screening approach. Multiple gRNAs targeting chromosome 1q genes Zbtb7b, Gba1, Vps72, and Mrpl9 were significantly enriched in +Myc;dCas9+ bulk liver tissue compared with the input library (Figure 5C, Supplementary Tables 2 and 4). In addition, gRNAs targeting these genes were among the highest ranked gRNAs within individual tumors (Figure 5D).
Figure 5.
In vivo CRISPRa screen to identify drivers of liver cancer. (A) Schematic representation of library preparation for next-generation sequencing. Genomic DNA was extracted from individual tumors or bulk liver tissue of Myc-TA-gRNA dCas9+ and WT conditions, gRNA region was amplified and indexed, and samples were pooled for next-generation sequencing. (B) MAGeCK global analysis of screen showing top positively selected gRNAs by gene in the +Myc;dCas9+ bulk liver tissues as compared with the input library. (C) The -Log10(positive P values) for gRNAs ranked by genes alphabetically in the +Myc;dCas9+ bulk liver tissues, normalized to the input library. (D) Proportion of all tumors containing each indicated gRNA (n = 23 tumors from n = 4 dCas9+ mice injected with Myc-TA-gRNA library).
In Vivo CRISPRa Screen in Liver Hyperproliferation
Most HCCs occur in patients with chronic liver injury and cirrhosis. Pure oncogene-driven HCC models do not include liver injury, which may provide an additional stimulus for hepatocyte proliferation.5,17,18 Therefore, we performed a second, independent CRISPRa screen in the Fah-/- model of liver injury and repopulation. This model has been shown to develop HCC in 6–8 weeks after hydrodynamic injection of mice with plasmids containing the Fah gene plus oncogenes.18,19 We hydrodynamically injected Fah–/–;dCas9+, or Fah–/– control mice with Fah-Myc-TA-gRNA plasmids that contained the same set of gRNAs targeting the 51 genes amplified and enriched in human HCC (Figure 6A) and allowed mice to repopulate their livers until they displayed signs of illness by body condition score at 11–21 days after injection (Figure 6B). No differences in liver-to-body weight ratio or liver morphology were detected between Fah–/– control (WT) and Fah–/–;dCas9+ (dCas9+) mice (Figure 6C). Next, to identify which genes enhance MYC-driven hyperproliferation, we examined the changes in gRNA prevalence in the bulk liver tissue. Quality control analysis showed high correlation between replicates (Figure 6D), high quality sequencing data (Figure 6E), high gRNA coverage (Figure 6F), and gRNA evenness (Figure 6G). Like with the tumorigenesis screen, Zbtb7b, Vps72, Gba1, and Mrpl9 were top scoring genes with enriched gRNAs (Figure 6I and J, Supplementary Tables 3 and 4). To summarize, 2 independent CRISPR screening assays, one with tumorigenesis driven by MYC and the other with hyperproliferation with MYC and FAH in a liver injury and repopulation context, identified the same putative 4 tumor driver genes: Zbtb7b, Vps72, Gba1, and Mrpl9.
Figure 6.
CRISPRa screen in liver injury and hyperproliferation model identifies driver genes. (A) Design of the CRISPRa hyperproliferation screen. The gRNA library was cloned into plasmids containing Fah, Myc, and TA sequences. The Fah-Myc-TA-gRNA plasmid library was hydrodynamically injected into Fah–/–;dCas9+ (dCas9+) or Fah–/– control mice, followed by proliferation and repopulation for 11 days. Genomic DNA was extracted from bulk liver tissue, and gRNA sequences in the livers were determined by next-generation sequencing. (B) Body weights measured during the screen graphed as percent of the weight at the time of HTVI. Dashed line marks 80% of body weight. (C) (Left) Liver images from WT or dCas9+ mice injected with Fah-Myc-TA-gRNA library for the hyperproliferation screen. (Right) Liver-to-body weight ratios measured after euthanasia of mice injected with Fah-Myc-TA-gRNA library. Statistical analysis performed with unpaired two-sided Student t test. Data are shown as mean ± SD. NS, not significant. (D) Principal component analysis plot of WT and dCas9+ samples from mice injected with the Fah-Myc-TA-gRNA plasmid or input library. (E) Percent of mapped and unmapped reads across groups. Statistical analysis performed with two-way ANOVA with Šídák's multiple comparisons test. (F) The number of gRNAs present per sample out of the total 290 gRNAs across all conditions. (G) Gini index representing gRNA reads evenness across all conditions. Statistical analyses performed with one-way ANOVA with Tukey multiple comparisons test. ∗P < .05, ∗∗∗P < .001, ∗∗∗∗P < .0001. Data are shown as mean ± SD. (H) MAGeCK global analysis of screen showing top positively selected gRNAs by gene in the bulk liver of dCas9+ mice injected with the Fah-Myc-TA-gRNA library, as compared with the input library. (I) The -Log10(positive P values) for gRNAs ranked by genes alphabetically in the Fah-Myc;dCas9+ bulk liver tissues, normalized to the input library. (J) Comparison of gRNA Log2FC between tumorigenesis and hyperproliferation screens.
Putative Drivers Identified in CRISPRa Screens Are Promising Targets in Liver Cancer
The putative drivers from the screen, ZBTB7B, VPS72, GBA1, and MRPL9, are all enriched in human HCCs as compared with normal liver tissue (Figure 7A). Furthermore, high levels of expression of VPS72, GBA1, or MRPL9, but not ZBTB7B, correlate with poor survival in patients with HCC (Figure 7B).
Figure 7.
Candidate HCC driver genes are up-regulated in HCC tissue. (A) Gene expression of candidate genes in human HCCs from TCGA-LIHC as compared with normal tissues. Statistical analysis was performed with one-way ANOVA. (B) Overall survival of HCC patients in TCGA-LIHC with a median group cutoff of gene expression. The log-rank test is used for calculating P value. (C) Validation of CRISPRa activity by gRNA in cell lines. The relative expression of the genes is shown after transducing T226 dCas9-expressing hepatoma cells with CRISPRa lentivirus expressing gRNAs or a sg-NT. (D) (Left) HCC cell proliferation after transduction with CRISPRa plasmids containing Zbtb7b-targeting, Vps72-targeting, Gba1-targeting, Mrpl9-targeting, or sg-NT control gRNA. (Right) Area under the curve calculated for each condition. The P value is calculated by Wilcoxon rank-sum test (∗P < .05; ∗∗P < .01). (E) (Left) HCC cell proliferation after transduction with transgene plasmids containing Zbtb7b, Vps72, Gba1, Mrpl9, or Gfp coding sequence as control. (Right) Area under the curve calculated for each condition. Proliferation index is the luminescence normalized to Gfp control. Data are shown as mean ± SD. TE, transgene expression. The P value is calculated by Wilcoxon rank-sum test (∗P < .05; ∗∗P < .01).
We sought to validate the CRISPRa activity of gRNAs targeting each of the top ranked genes from the screen. In T226, a mouse HCC cell line expressing dCas9,5 gRNAs activated target gene expression in the 1.3- to 2-fold range (Figure 7C). To determine whether the observed expression increases are sufficient to induce proliferation in vitro, we performed CRISPRa and exogenous overexpression of Zbtb7b, Vps72, Gba1, or Mrpl9 in T226 cells. Both CRISPRa (Figure 7D) and transgene expression (Figure 7E) of all 4 genes increased proliferation of HCC cells, demonstrating that the observed expression levels were sufficient to drive proliferation in vitro.
Activation of Vps72, Gba1, or Mrpl9 Drives Liver Tumorigenesis
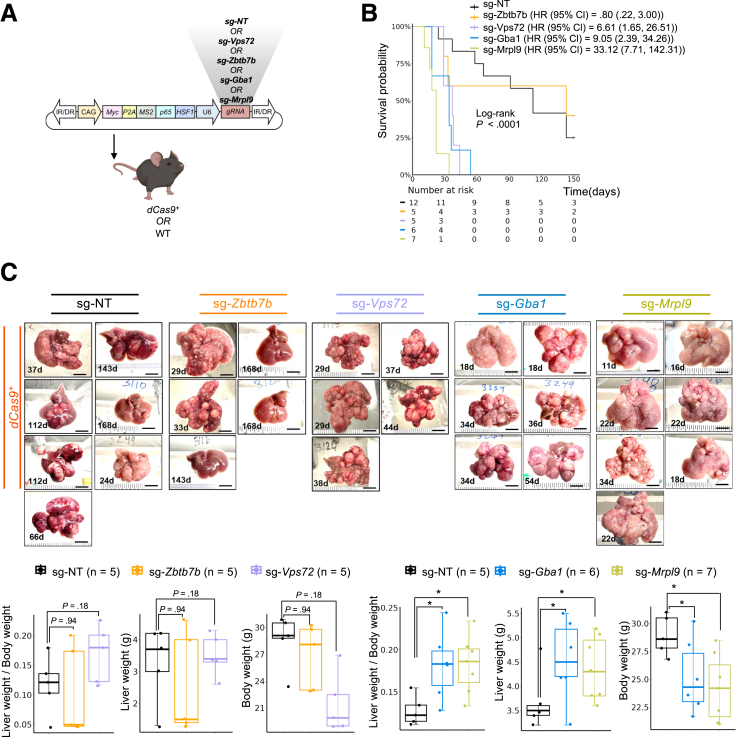
To validate whether the activation of Zbtb7b, Vps72, Gba1, or Mrpl9 can promote MYC-driven tumorigenesis in vivo, we injected dCas9+ or WT control mice with plasmids containing (1) Myc coding sequence, (2) the TA sequences, and (3) a single gRNA targeting Zbtb7b, Vps72, Gba1, Mrpl9, or sg-NT control (Figure 8A). Intriguingly, CRISPRa of Vps72, Gba1, and Mrpl9, but not Zbtb7b, led to significantly decreased survival (Figure 8B), along with markedly increased tumor burden (Figure 8C), compared with NT controls, which resulted in few tumors in dCas9+ or WT mice. Strikingly, the earliest mice to reach the humane end point were the dCas9+ mice with activation of Mrpl9, with livers showing an expansive amount of cell proliferation (Figure 8C). In contrast, CRISPRa experiments activating Zbtb7b showed no increase in MYC-driven tumorigenesis above baseline (Figures 8 and 9).
Figure 8.
Activation of Vps72, Gba1, or Mrpl9 in combination with MYC drives aggressive proliferation in mouse livers. (A) Design of an in vivo CRISPRa validation experiment. dCas9+ mice were injected with Myc-TA-gRNA plasmids expressing Zbtb7b-, Vps72-, Gba1-, Mrpl9-targeting, or NT gRNA. (B) Kaplan-Meier survival plots of dCas9+ mice injected with Myc-TA-gRNA plasmids. Statistical analysis performed with log-rank test and Cox PH model. (C) Representative liver images from dCas9+ mice injected with Myc-TA-gRNA plasmids targeting Zbtb7b, Vps72, Gba, Mrpl9, or NT control. Scale bar represents 10 mm, and bottom left text represents survival days after injection. The lower panel shows mouse body weight, liver weight, and liver weight/body weight from each group. Kruskal-Wallis with Dunn multiple comparisons test was used to calculate statistics (∗P < .05, ∗∗P < .01).
Figure 9.
Activation of Zbtb7b does not drive tumorigenesis in single gRNA assay. (A) Kaplan-Meier survival plot of dCas9+ mice injected with Myc-TA-gRNA plasmid containing Zbtb7b-targeting or NT control gRNA. Statistical analysis performed with log-rank test. (B) Liver images from dCas9+ or WT mice injected with Myc-TA-gRNA plasmids. Scale bar represents 10 mm, and bottom left text represents survival days after injection.
We repeated the validation assay, followed by euthanizing all mice at 20 days after the hydrodynamic injection. Similar to the prior result, there was significantly increased tumor burden with Gba1 and Mrpl9 activation, a trend toward increased tumor burden for Vps72, and no significantly increased tumor burden for Zbtb7b as compared with the NT control (Figure 10A). The average liver tissue area involved by tumor was increased with activation of Mrpl9, Vps72, or Gba1, but not with Zbtb7b (Figure 10B). The tumor tissue stained positive for Ki-67 in 70%–90% of tumor cells in all 4 activation groups, which was higher than in the control groups, which was 50%–70% (Figure 10C). Overall, the rate of cell division, tumor burden, and mortality was greatest for Mrpl9 activation, followed by Gba1 and Vps72. The mortality and tumor burden with Zbtb7b was similar to controls, although it had a high Ki-67 index, possibly related to fewer tumors overall than with activation of the other genes.
Figure 10.
Activation of Vps72, Gba1, or Mrpl9 in combination with MYC drives larger tumor and increased intratumoral KI-67 proliferation index in mouse livers. (A) Representative liver images from dCas9+ or WT mice injected with Myc-TA-gRNA plasmids. All livers were harvested after 20 days post-injection for direct comparison. Scale bar represents 10 mm. The lower panel shows mouse body weight, liver weight, and liver weight/body weight from each group. Kruskal-Wallis with Dunn multiple comparisons test was used to calculate statistics (∗P < .05, ∗∗P < .01). (B) H&E staining for tumor area quantification demonstrated an extensive tumor growth in the group of Vps72, Gba1, or Mrpl9 activation, but not Zbtb7b, as compared with the NT group. Scale bar represents 2 mm for top panel, 100 μm for bottom panel. (C) Immunohistochemistry staining of KI-67 showed an increased intratumoral cell proliferation rate when these 4 genes are activated as compared with NT (∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001). Scale bar represents 2 mm for top panel, 100 μm for bottom panel.
Mrpl9 Enhances Mitochondrial Function, Vps72 Promotes Phospholipid Metabolism, and Gba1 Affects Endosome/Lysosome Activity to Drive Tumorigenesis
We queried the mechanisms by which Vps72, Gba1, and Mrpl9 promote MYC-driven tumorigenesis by performing RNA sequencing (RNA-seq) of liver tumors. Principal component analysis showed separate clustering of the NT controls and the CRISPRa samples (Figure 11A). Unsupervised clustering showed biological replicates generally clustered together, indicating similar expression profile (Figure 11B). Differential expression analysis showed hundreds of differentially expressed genes (DEGs) in gene-targeting groups compared with controls (Figure 11C). Each target gene was significantly up-regulated according to the CRISPRa condition in the tumors (Figure 11D).
Figure 11.
Quality control analyses of RNA sequencing. (A) Principal component analysis of samples represented by DESeq2 normalized counts of expressed genes identified across samples. (B) Heatmap representing all commonly expressed genes across samples. Samples were unsupervised clustered by hierarchical clustering method ‘ward.D2’. (C) Volcano plots of all the expressed genes (mean > 0 in at least 1 group) representing the Log2FC and -Log10(P value). Blue dots: genes with Log2FC <-1 and P value < .05. Red dots: genes with Log2FC >1 and P value < .05. (D) DESeq2 normalized reads normalized to sg-NT controls from RNA-seq of the candidate genes in liver tumors from dCas9+ mice injected with Myc-TA-gRNA plasmids. Asterisks represent FDR adjusted P values from differential expression analyses as follows: ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001. Data are shown as mean ± SD.
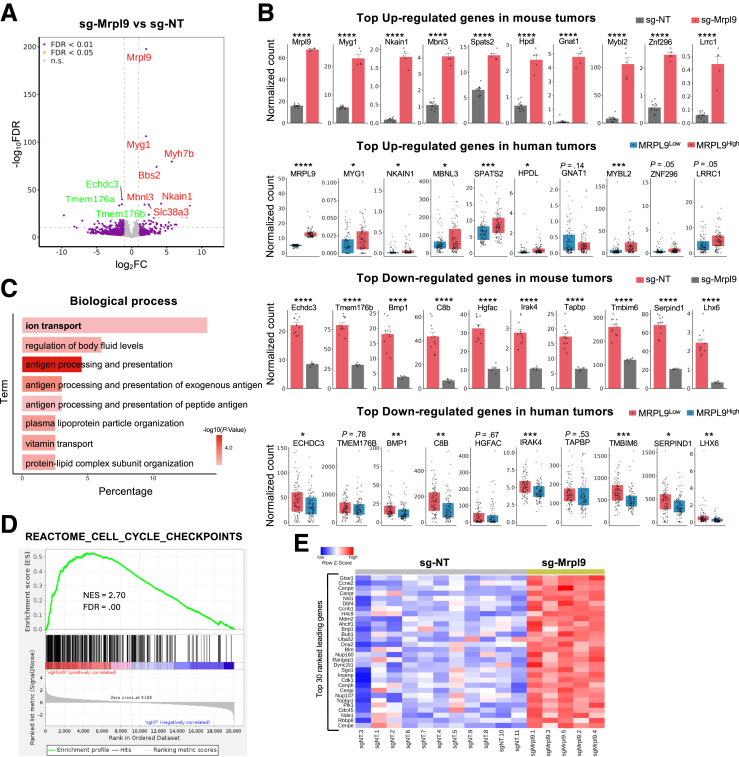
MRPL9, mitochondrial ribosomal protein L9, encodes a component of the 39S subunit of the mitochondrial ribosome. Differential gene expression analysis of tumors with Mrpl9 activation showed mitochondria-related gene Myg1 was the most significantly up-regulated gene (Figure 12A and B). By analyzing the human liver cancer samples extracted from TCGA, we found that the human homolog MYG1 was also significantly up-regulated in human liver cancers with high expression of MRPL9 (Figure 12B). Similarly, we found another mitochondria-related gene Echdc3 was the most down-regulated gene, and the human homolog ECHDC3 was down-regulated in human tumors highly expressing MRPL9 (Figure 12A and B). Other genes among the top 10 altered genes included regulators of ion transport activity: Nkain1, which encodes Na+/K+ transporting ATPase interacting 1, and Slc38a3, which encodes solute carrier family 38, member 3 (Figure 12A). Gene ontology (GO) analysis revealed 14% of the top 100 up-regulated or down-regulated genes are involved in ion transport, indicating a close connection between abnormal ion transport activity and mitochondrial dysfunction (Figure 12C). Human NKAIN1 was also up-regulated in human liver cancers with higher expression of MRPL9. Furthermore, additional top-ranked genes included apoptosis-related genes Mybl2 and Tmbim6, which changed in the same direction in human cancer samples (Figure 12B). The remarkably short survival of mice with Mrpl9 activation prompted us to examine cell division–related genes in those tumors. Although cell cycle–related genes were not among the top 10 DEGs, we observed many of them were ranked between 100–1000, indicating a secondary change. Indeed, gene set enrichment analysis (GSEA) revealed the cell cycle checkpoint was among the top enriched gene sets, with typical genes such as Cdk1, Ccnb1, Ccne2, and Mdm2 among the top genes that were up-regulated (Figure 12D and E). This is consistent with the observed fast tumor growth and high Ki-67 proliferation index (Figure 10).
Figure 12.
Mrpl9 activation on tumors enhances mitochondria function. (A) Volcano plot showing DEGs in sg-Mrpl9 tumors (n = 5) compared with sg-NT controls (n = 11). The top 10 genes either up-regulated (red text) or down-regulated (green text) are shown on the plot. (B) Bar plots showing the up-regulation (the first row) or down-regulation (the third row) of top changed genes in sg-Mrpl9 tumors compared with sg-NT; box plots (the second and fourth rows) showing the expression changes of top genes in human liver cancers extracted from TCGA-LIHC cohort by comparing the top quartile samples (MRPL9high) with the bottom quartile samples (MRPL9low) based on gene expression rank. The genes were selected from top 30 up-regulated or top 20 down-regulated genes for the comparison between mouse and human samples. For mouse RNA-seq, DESeq2 adjusted P values are shown. For TCGA-LIHC RNA-seq, P values using Student t test and corrected for multiple testing by the BH method are shown (∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001). (C) GO showed top 8 enriched signatures for biological process using DAVID Functional Annotation Analysis on DEGs when comparing sg-Mrpl9 with sg-NT. The top 100 up-regulated and 100 down-regulated genes were selected, respectively. The P value was calculated by Fisher exact test. (D) GSEA showing enrichment of REACTOME_CELL_CYCLE_CHECKPOINTS for sg-Mrpl9. The ES, NES, and FDR are calculated according to the software’s recommended algorithms. (E) Heatmap of the top 30 genes from the differential gene expression analysis comparing sg-Mrpl9 vs sg-NT.
VPS72, vacuolar protein sorting 72 homologue, is a component of the histone acetyltransferase complex TRRAP/TIP60 and the chromatin remodeling SRCAP-containing complex. RNA-seq analysis showed a dominant enrichment of genes involved in the regulation of phosphatidylinositol 4,5-bisphosphate (PIP2) activity upon Vps72 activation, such as the PIP2-interacting protein Twf2,20 the enzyme Pip5k1a, which catalyzes phosphatidylinositol 4-phosphate (PI4P) to form PIP2,21 Prelid2, which contains PRELI/MSF1 motif known for regulating phospholipids transport,22 and Fig4, a lipid phosphatase that reverses the reaction generating phosphatidylinositol 3,5-bisphosphate (PI(3,5)P2) from phosphatidylinositol 3-phosphate (PI3P) (Figure 13A and B). All of these genes were up-regulated in both mouse and human tumors highly expressing VPS72 (Figure 13B). Consistently, our GO analysis revealed ∼23% genes were enriched in phosphorus metabolic process, and lipid metabolic process is the most significantly enriched signature (Figure 13C). GSEA on the whole expression profile revealed the mitotic prometaphase was among the top enriched gene sets, with many genes related to the centrosome, microtubule, and centromere, indicating promotion of cell division as a causative factor for the tumor growth (Figure 13D and E).
Figure 13.
Vps72 activation on tumors promotes phospholipid metabolism. (A) Volcano plot showing DEGs in sg-Vps72 (n = 4) tumors compared with sg-NT controls (n = 11). The top 10 genes either up-regulated (red text) or down-regulated (green text) are shown on the plot. (B) Bar plots showing the up-regulation (the first row) or down-regulation (the third row) of top changed genes in sg-Vps72 tumors compared with sg-NT; box plots (the second and fourth rows) showing the expression changes of top genes in human liver cancers extracted from TCGA-LIHC cohort by comparing the top quartile (VPS72high) with the bottom quartile (VPS72low) of VPS72 expression. The genes were selected from top 30 up-regulated or down-regulated gene list for the comparison. For mouse RNA-seq, DESeq2 adjusted P values are shown. For TCGA-LIHC RNA-seq, P values using Student t test and corrected for multiple testing by the BH method are shown (∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001). (C) GO showed top 8 enriched signatures for biological process when comparing sg-Vps72 with sgNT. The top 100 up-regulated and 100 down-regulated genes were selected, respectively. The P value was calculated by Fisher exact test. (D) GSEA showing enrichment of REACTOME_MITOTIC_PROMETAPHASE for sg-Vps72. The ES, NES, and FDR are calculated according to the software’s recommended algorithms. (E) Heatmap of the top 30 genes from the differential gene expression analysis comparing sg-Vps72 vs sg-NT.
GBA1 encodes lysosomal glucosylceramidase, which breaks down glucosylceramide into glucose and ceramide.23 Ceramide plays an important role in the endosome by triggering budding of exosome vesicles into multivesicular endosomes.24 Indeed, RNA-seq analysis of Gba1-activated tumors indicated a key role in endosome regulation. The most significantly changed gene was Ocrl, a phosphatase that hydrolyzes the 5′ phosphate of PIP2 (Figure 14A). It controls endosome trafficking via PIP2-dependent regulation of endosomal actin.25 Human liver tumors with high expression of GBA1 showed nominally increased expression of OCRL, although it did not reach significance (P = .16, Figure 14B). PIP2-mediated regulation of endosomal F-actin and microtubules is important for endosomal dynamics.26 Among the top 50 genes up-regulated with activation of GBA1, we found other F-actin and microtubule related genes including Enah, encoding an actin regulator,27 Map2, encoding a microtubule-stabilizing protein,28 and Brsk1, a microtubule-associated protein phosphorylase.29 All of these genes had a trend of increased expression in human liver cancers with high expression of GBA1 (Figure 14B). Furthermore, GO analysis revealed proteolysis is the most significantly enriched signature, which is in line the function of the endosome/lysosome (Figure 14C). These results indicate a key role of endosomal-lysosomal system and associated trafficking in driving the tumor progression when Gba1 was activated. Similar to Vps72 activation, GSEA revealed an enrichment of the mitotic prometaphase among the top enriched gene sets, with genes related to the centrosome, microtubule, and centromere, indicating GBA1 promotes cell division to drive tumor growth (Figure 14D and E).
Figure 14.
Gba1 activation affects endosomal-lysosomal activity. (A) Volcano plot showing significantly changed genes in sg-Gba1 tumors (n = 5) compared with sg-NT control (n = 11). The top 10 genes either up-regulated (red text) or down-regulated (green text) are shown on the plot. (B) Bar plots showing the up-regulation (the first row) or down-regulation (the third row) of top changed genes in sg-Gba1 tumors compared with sg-NT; box plots (the second and fourth rows) showing the expression changes of top genes in human liver cancers extracted from TCGA-LIHC cohort by comparing the top quartile (GBA1high) with the bottom quartile (GBA1low) of GBA1 expression. The genes were selected from top 50 up-regulated or down-regulated gene list for the comparison between mouse and human samples, respectively. For mouse RNA-seq, DESeq2 adjusted P values are shown. For TCGA-LIHC RNA-seq, P values using Student t test and corrected for multiple testing by the BH method are shown (∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001). (C) GO showing the top 8 enriched signatures for biological process when comparing sg-Gba1 with sg-NT and including the top 100 up-regulated and 100 down-regulated genes. The P value was calculated by Fisher exact test. (D) GSEA showing enrichment of REACTOME_MITOTIC_PROMETAPHASE for sg-Gba1. The ES, NES, and FDR are calculated according to the software’s recommended algorithms. (E) Heatmap of the top 30 genes extracted from sg-Gba1 vs sg-NT.
Discussion
We successfully conducted the first CRISPRa screen to directly annotate gene function in liver cancer. From a pool of 51 genes exhibiting copy number-expansion and up-regulation in HCC, we identified 3 drivers of liver tumorigenesis, VPS72, GBA1, and MRPL9, all of which are associated with poor prognosis in HCC. Overall, our CRISPRa screening platform can ascertain the driver genes that contribute to tumorigenesis.
In patients with HCC, there are several recurrent focal genomic amplifications that occur in chromosomes 1q and 8q (Figure 1).30 Candidate genes ZBTB7B (1q21.3), VPS72 (1q21.3), GBA1 (1q22), and MRPL9 (1q21.3) are all located on 1q, whereas MYC (8q24.21) is located on 8q, which undergoes one of the earliest amplifications in HCC.31 Interestingly, our screen did not identify any additional putative drivers on chromosome 8q, suggesting that MYC might be the sole driver of HCC at that locus. Here, we propose VPS72, GBA1, and MRPL9 as the main drivers of HCC located on the 1q gain peak. Indeed, the dramatically accelerated mortality seen in mice with MRPL9 expression could indicate that this gene may be a main driver of tumorigenesis or progression in the amplified region on chromosome 1.
As a component of the mitochondrial ribosome, MRPL9 supports mitochondrial translation. Indeed, our RNA-seq result revealed the most significantly up-regulated gene Myg1 and down-regulated gene Echdc3 are both mitochondrial-related genes. Myg1 exonuclease was reported as a coordinator of nucleo-mitochondrial crosstalk through RNA processing. In the nucleolus, MYG1 functions in ribosome maturation and promotes translation. In the mitochondrial matrix, MYG1 cleaves nuclear encoded mitochondrial transcripts and promotes their turnover.32 Mitochondria plays a central role in controlling energy metabolism,33 and the liver is the central organ in lipogenesis, gluconeogenesis, and cholesterol metabolism.34 In livers, the overactivated mitochondrial functionality induced by Mrpl9 activation could lead to aberrations of metabolism to favor energy use by cancer cells. The most significant down-regulated gene Echdc3, encodes Enoyl-CoA hydratase domain-containing protein 3, which is located in mitochondria, was reported to play a role in insulin sensitivity and Akt phosphorylation in adipocytes. However, the role in cancer cells is not clear yet.35 Future investigations can focus on elucidating more detailed mechanisms and can connect the changes in mitochondrial gene expression to the changes in cell cycle checkpoints that occur in cells to drive tumor formation.
Vps72 activation revealed a significant enrichment of genes involved in the regulation of phospholipid, particularly PIP2 metabolism, exemplified by the top 10 DEGs Twf2, Pip5k1a, and Prelid2, implicating a PI3K/AKT-dependent mechanism driving HCC, which aligns with a previous study.36 However, another recent article used an AKT/β-catenin mouse model to investigate the effect of VPS72 in driving liver tumorigenesis and suggested regulation of a non-AKT dependent signaling pathway.37 The inconsistency could be caused by a different context of tumor microenvironment using different mouse models. Our study does not clearly favor one mechanistic hypothesis over another. Compared with the previous studies, our approach used unbiased screens to assign driver functions to multiple genes across 2 genomic regions that are amplified in human HCC rather than a candidate gene approach.
GBA1 produces ceramide, and importantly, ceramide plays an important role in the endosomal-lysosomal system.24 Our RNA-seq result showed altered expression of endosome-associated genes in GBA1-high tumors, which suggests GBA1 may drive tumorigenesis through ceramide production. Notably, Ocrl, a gene encoding a phosphatase that catalyzes the hydrolysis of the 5′ phosphate of PIP2, was reported to deplete PIP238 but also promotes early endosome trafficking.25 A possible unifying mechanism could be the removal of accumulated PIP2, increasing its turnover, promotes endosome-associated actin reorganization and recycling.25,39 Future research should investigate whether Vps72 and Gba1 cooperate in the same phospholipid and endosome pathway or work in parallel.
Interestingly, the majority of cell cycle or mitosis-related genes are ranked from 100–1000 among the DEGs, indicating a secondary change attributable to the activation of Mrpl9, Vps72, or Gba1. Mrpl9 activation led to transcriptional regulation of cell cycle checkpoint genes, whereas Vps72 and Gba1 activation up-regulated genes associated with mitosis: the centrosome, microtubules, and centromeres. Considering the close physical proximity on chromosome 1q, amplification of all of these genes could work cooperatively to drive tumorigenesis. Future work should examine how these genes interact with each other, and whether targeting any one of them can block tumor progression in HCCs that undergo amplification of the entire region.
Despite identifying Zbtb7b as a putative driver in the 2 CRISPRa screens and its activity in a cell line, its activation alone did not drive tumorigenesis when tested alone in vivo (Figures 8 and 9). One possibility is that Zbtb7b is a weak driver on its own but may synergize with other driver genes. In our screen, tumor clones contained an average of 10 unique gRNAs (Figure 4), and synergistic interactions between Zbtb7b and other target genes could drive tumorigenesis. Another possible explanation could be that the activation of ZBTB7B confers a competitive advantage to MYC in cell-cell competition. Cells with higher MYC expression have been described to outcompete or eliminate neighboring cells with lower MYC expression.40 Similarly, MYC-high hepatocytes with ZBTB7B activation may outcompete MYC-high cells lacking ZBTB7B activation, which would not show any signal if all the surrounding clones also express ZBTB7B, which was the case in the validation experiments. Future experiments could explore whether synergy or competition are mechanisms driving tumor formation when ZBTB7B is activated.
Overall, our results demonstrate CRISPRa screening can identify bona fide, novel tumor driver genes relevant to human HCC.
Materials and Methods
Study Approval
All animals received humane care in compliance with the Mayo Clinic Institutional Animal Care and Use Committee (protocol #A00006617-22) or the University of Pennsylvania Institutional Animal Care and Use Committee (protocol #806401).
Mice
Fah-/- mice were obtained with permission from the Oregon Health & Science University. dCas9+ mice were previously deposited to the Mutant Mouse Regional Resource to facilitate distribution to the research community. The dCas9+ mice were crossed with Fah–/– mice to obtain Fah–/–;dCas9+ mice.4 Fah-/- and Fah-/-;dCas9+ mice were maintained on 7.5 μg/mL nitisinone (Sigma-Aldrich) until hydrodynamic tail vein injection with plasmids at 6–15 weeks of age for the Fah-Myc CRISPRa screen. dCas9+ and WT mice [STOCK Gt(ROSA)26Sortm1(CAG-cas9∗,-BFP)Khk/Mmjax, RRID:MMRRC_043926-JAX and B6(Cg)-Tyrc-2J/J RRID: IMSR_JAX:000058, respectively] were obtained from the Mutant Mouse Resource and Research Center at The Jackson Laboratory (Bar Harbor, ME). These strains were used for downstream validation studies because of easier detection of tail vein for hydrodynamic injections and referred to as mixed background.
Cell Lines
T226 cells were derived from a MYC-TGFα induced mouse HCC5 and cultured with advanced modified Eagle medium (Thermo #12492013), 5% fetal bovine serum, 1% P/S, Glutamax 1X. The human embryonic kidney cell line 293T/17 (ATCC #CRL-11268) was cultured with Dulbecco modified Eagle medium high glucose (ATCC #30-2002), 10% fetal bovine serum, and 1% P/S.
Plasmids
For the TA components, we modified pKT2/Fah-TA//SB, described previously,4 where the original TA was the scFv-VP64 fusion by replacing the scFv-VP64 TA with the MS2-P65-HSF1 (MPH) fusion protein, which was polymerase chain reaction amplified from the plasmid lenti MS2-P65-HSF1_Hygro (Addgene #6142641) using primers MPH-EcoRI_F and MPH-BsiWI_R (Table 1). The MPH fragment was inserted at the EcoRI and BsiWI restriction sites. Stop codons were added to the 3′ end of the MPH fusion by polymerase chain reaction amplifying the fragment using the primers CAG-F and HSF1_stop_R (Table 1). The gRNA scaffold was replaced with an optimized gRNA scaffold containing MS2 stem loops (gRNA 2.0) as described by Liao et al42 in pKT2/Fah-MPH-gRNA//SB. To construct Myc-TA-gRNA plasmids, we amplified Myc cDNA (primers Myc+EcoRI_F and Myc+P2A_R, Table 1), inserted the P2A sequence downstream of Myc (primers Myc+EcoRI_F and EcoRI+P2A_R2, Table 1), and flanked the Myc-P2A sequence with EcoRI sites. The pKT2-Fah-MPH-gRNA2.0//SB digested vector and insert (Myc-P2A) were ligated to synthesize pKT2-Fah-Myc-MPH-gRNA2.0//SB. For the hyperproliferation screen plasmid, the piggyBac (PB) transposon backbone and Fah-Myc-MPH insert were ligated to generate the pPBT-Fah-Myc-MPH-gRNA2.0 plasmid referred to as Fah-Myc-TA-gRNA. For the tumorigenesis screen plasmids, Fah was removed from pPBT-Fah-MPH-gRNA2.0 and pPBT-Fah-Myc-MPH-gRNA2.0 plasmids by digestion and re-ligation to generate pPBT-MPH-gRNA2.0 (TA-gRNA) and pPBT-Myc-MPH-gRNA2.0 (Myc-TA-gRNA) plasmids. Myc was removed from the pPBT-Fah-Myc-MPH-gRNA2.0 plasmid by digestion and re-ligation for the NoMyc control plasmid. For the Myc-Gfp-sgNT plasmid, the U6-gRNA2.0 insert was ligated with pPBT-Myc-Gfp vector, and NT control gRNA oligo was inserted.
Table 1.
List of Primers for Plasmid Constructs
| Primer | Sequence (5′-3′) |
|---|---|
| MPH-EcoRI_F | AATTGAATTCGCCACCATGGCTTCAAACTTTACTC |
| MPH-BsiWI_R | AATTCGTACGGGAGACAGTGGGGTCCTTGG |
| CAG-F | CTCTGCTAACCATGTTCATGC |
| HSF1_stop_R | AATTTCGTACGTCAGGAGACAGTGGGGTCCTTGGC |
| Myc+EcoRI_F | ggcaaaGAATTCatgcccctcaacgtgaactt |
| Myc+P2A_R | gcttcagcagggagaagttggtggctgcaccagagtttcgaagctgttcg |
| Myc+EcoRI_F | ggcaaaGAATTCatgcccctcaacgtgaactt |
| EcoRI+P2A_R2 | tggtggcGAATTCggggccggggttctcctccacgtcgccggcctgcttcagcag |
| AdaBioID-a-f | TCGATCCGGGAGTATACA |
| AdaBioIDa-R2 | GTGTCGAACTCTTGTAGG |
For in vitro and in vivo CRISPRa validation studies, the lenti CRISPRa plasmid (pXPR-502, Addgene #96923) was ligated with annealed gRNA oligos (Table 2) using T7 DNA ligase (NEB #M0318) by Golden Gate reaction. For in vitro transgene expression studies, the MPH-gRNA2.0 sequences were removed from pPBT-Myc-MPH-gRNA2.0 plasmid. Gfp and mouse Vps72 and Zbtb7b coding sequences followed by the rabbit beta globin polyA sequence were cloned into the pPBT-Myc vector via 5′ and 3′ HindIII by recombination by Azenta Life Sciences. For in vivo CRISPRa studies, annealed gRNA oligos (Table 2) were ligated with pPBT-Myc-MPH-gRNA2.0 plasmid.
Table 2.
List of gRNA Oligos for in Vitro and in Vivo Validation
| Target gene | gRNA | Sense | In vitro oligo sequence | In vivo oligo sequence |
|---|---|---|---|---|
| Non-targeting | sg-NT | Fwd | caccgGCTCCTGGGGCAGTCACCCA | accGCTCCTGGGGCAGTCACCCA |
| Rev | aaacTGGGTGACTGCCCCAGGAGCc | aacTGGGTGACTGCCCCAGGAGC | ||
| Vps72 | sg1-Vps72 | Fwd | caccgGAACCCTGGTACTGTGCCCT | accGAACCCTGGTACTGTGCCCT |
| Rev | aaacAGGGCACAGTACCAGGGTTCc | aacAGGGCACAGTACCAGGGTTC | ||
| sg2-Vps72 | Fwd | caccgGCACTCATAGGCAGCGCCTA | accGCACTCATAGGCAGCGCCTA | |
| Rev | aaacTAGGCGCTGCCTATGAGTGCc | aacTAGGCGCTGCCTATGAGTGC | ||
| sg3-Vps72 | Fwd | caccgGCGCCTAGGGCACAGTACCA | accGCGCCTAGGGCACAGTACCA | |
| Rev | aaacTGGTACTGTGCCCTAGGCGCc | aacTGGTACTGTGCCCTAGGCGC | ||
| sg4-Vps72 | Fwd | caccgGGGCTGCTGTGGCCACTCAT | accGGGCTGCTGTGGCCACTCAT | |
| Rev | aaacATGAGTGGCCACAGCAGCCCc | aacATGAGTGGCCACAGCAGCCC | ||
| sg5-Vps72 | Fwd | caccgGTTCACCTTAAGTCTCTTTG | accGTTCACCTTAAGTCTCTTTG | |
| Rev | aaacCAAAGAGACTTAAGGTGAACc | aacCAAAGAGACTTAAGGTGAAC | ||
| Zbtb7b | sg1-Zbtb7b | Fwd | caccgGACAGTGTCCGCAGTGGCCA | accGACAGTGTCCGCAGTGGCCA |
| Rev | aaacTGGCCACTGCGGACACTGTCc | aacTGGCCACTGCGGACACTGTC | ||
| sg2-Zbtb7b | Fwd | caccgGAGGAGGCAAAGGGTGGCGG | accGAGGAGGCAAAGGGTGGCGG | |
| Rev | aaacCCGCCACCCTTTGCCTCCTCc | aacCCGCCACCCTTTGCCTCCTC | ||
| sg3-Zbtb7b | Fwd | caccgGAGGGCTGGACGGGGGTCGG | accGAGGGCTGGACGGGGGTCGG | |
| Rev | aaacCCGACCCCCGTCCAGCCCTCc | aacCCGACCCCCGTCCAGCCCTC | ||
| sg4-Zbtb7b | Fwd | caccgGTGACAGAGGGTGTTGCTAA | accGTGACAGAGGGTGTTGCTAA | |
| Rev | aaacTTAGCAACACCCTCTGTCACc | aacTTAGCAACACCCTCTGTCAC | ||
| sg5-Zbtb7b | Fwd | caccgGTGGAAGCCAGGTGAATGTA | accGTGGAAGCCAGGTGAATGTA | |
| Rev | aaacTACATTCACCTGGCTTCCACc | aacTACATTCACCTGGCTTCCAC | ||
| Gba | sg1-Gba | Fwd | caccgGGAATTTTGGGGAGGCCTAA | accGGAATTTTGGGGAGGCCTAA |
| Rev | aaacTTAGGCCTCCCCAAAATTCCc | aacTTAGGCCTCCCCAAAATTCC | ||
| sg2-Gba | Fwd | caccgGGGCCTCCCCAAAATTCACA | accGGGCCTCCCCAAAATTCACA | |
| Rev | aaacTGTGAATTTTGGGGAGGCCCc | aacTGTGAATTTTGGGGAGGCCC | ||
| sg3-Gba | Fwd | caccgGTAAGCCGGTCACGTGATGC | accGTAAGCCGGTCACGTGATGC | |
| Rev | aaacGCATCACGTGACCGGCTTACc | aacGCATCACGTGACCGGCTTAC | ||
| sg4-Gba | Fwd | caccgGTGAATACAGTGGCCCTCAC | accGTGAATACAGTGGCCCTCAC | |
| Rev | aaacGTGAGGGCCACTGTATTCACc | aacGTGAGGGCCACTGTATTCAC | ||
| sg5-Gba | Fwd | caccgGTGTATGGACAGCTGAGTAG | accGTGTATGGACAGCTGAGTAG | |
| Rev | aaacCTACTCAGCTGTCCATACACc | aacCTACTCAGCTGTCCATACAC | ||
| Mrpl9 | sg1-Mrpl9 | Fwd | caccgGCTCTCCGCTGTCCTATCGT | accGCTCTCCGCTGTCCTATCGT |
| Rev | aaacACGATAGGACAGCGGAGAGCc | aacACGATAGGACAGCGGAGAGC | ||
| sg2-Mrpl9 | Fwd | caccgGGCCTGAGTGGTATAGATAT | accGGCCTGAGTGGTATAGATAT | |
| Rev | aaacATATCTATACCACTCAGGCCc | aacATATCTATACCACTCAGGC | ||
| sg3-Mrpl9 | Fwd | caccgGGGCGTGCAAGGCGGGGTTG | accGGGCGTGCAAGGCGGGGTTG | |
| Rev | aaacCAACCCCGCCTTGCACGCCCc | aacCAACCCCGCCTTGCACGCCC | ||
| sg4-Mrpl9 | Fwd | caccgGTGCCCGCGGCGCGCCTGAG | accGTGCCCGCGGCGCGCCTGAG | |
| Rev | aaacCTCAGGCGCGCCGCGGGCACc | aacCTCAGGCGCGCCGCGGGCAC | ||
| sg5-Mrpl9 | Fwd | caccgGTTAAAAAAAGAGGCGGAGG | accGTTAAAAAAAGAGGCGGAGG | |
| Rev | aaacCCTCCGCCTCTTTTTTTAACc | aacCCTCCGCCTCTTTTTTTAAC |
Design and Cloning of gRNA Library for CRISPRa Screens
We first queried the Liver Hepatocellular Carcinoma (LIHC) dataset from TCGA to download RNA expression and copy number data using cBioportal.43,44 We calculated the frequency of copy-number amplification and mRNA up-regulation across all genes and generated the chromosome plots using the KaryoploteR package in R. We identified all 104 genes that were up-regulated in at least 40% of HCCs with a Z-score of ≥1.5. Next, we further filtered the gene list to include all genes with a copy-number amplification (defined as an amplification event with many copies44) in ≥10% of HCCs to identify 51 genes that were both amplified and up-regulated in HCC patients from the TCGA-LIHC dataset. We included Tert, Vegfa, and Ccnd1 as control genes with known roles in HCC. Then we designed a gRNA library with 5 gRNAs per gene and 20 NT control gRNAs for a 290-gRNA CRISPRa library (Supplementary Table 1). CRISPRa gRNA sequences were from Horlbeck et al.45 We designed oligonucleotides for the gRNA library as described previously.4 The CRISPRa oligo library (Agilent Technologies, Santa Clara, CA) was then amplified using primers AdaBioID-a-f and AdaBioIDa-R2 (Table 1) and purified (Qiagen Minelute #28004, Germantown, MD). The digested gRNA library was ligated with CRISPRa vectors, and the new plasmid libraries were purified from bacteria. To achieve robust gRNA coverage, we purified at least 1.2 million colonies for an estimated 300-fold coverage per gRNA. We used sanger sequencing to confirm gRNAs were cloned correctly.
In Vivo CRISPRa Screening
For the tumorigenesis screen, a total of 13 μg of piggyBac transposon (Myc-TA-gRNA or TA-gRNA) and 6.7–10 μg of piggyBac transposase plasmid for a 1:1 molar ratio was hydrodynamically tail-vein injected into dCas9+ or C57BL6 as controls. Mice were euthanized 5.5 and 8 weeks after injection of the Myc-TA-gRNA and TA-gRNA plasmids, respectively. For hyperproliferation screen, 13 μg of Fah-Myc-TA-gRNA and 6.7–10 μg of piggyBac transposase plasmid for a 1:1 molar ratio were injected into Fah-/-;dCas9+ mice or Fah-/- mice as controls. Mice were euthanized 11–14 days after injection. To remove the LoxP-stop-LoxP cassette from dCas9fl+ mice, 1 × 1011 genome copies of AAV8-Cre virus (Addgene #107787-AAV8) were added to the DNA solution at the time of hydrodynamic tail vain injections. For Fah-/- mice, nitisinone was removed after injection to induce liver injury and repopulation with hepatocytes that incorporated plasmids.18 All plasmids used for CRISPRa screen are described in the section on Plasmids.
gRNA Sequencing Analysis
At the indicated end points of the experiments, liver tissue genomic DNA was extracted and gRNA sequences were amplified, followed by indexing, as described previously.4 Libraries were sequenced using an Illumina NextSeq500 [75-base-pair single-end reads and geometry of 75 × 8 × 8 (Read 1, index 1, index 2 cycles)]. Reads were demultiplexed to generate one fastq file per sample. The Model-based Analysis of Genome-wide CRISPR/Cas9 Knockout (MAGeCK) was used to generate read count tables containing the number of gRNA reads per sample for each of the 290 gRNAs, mapping rates, gRNAs present, and gini indexes. A modified robust ranking aggregation algorithm in MAGeCK software was used to identify positively selected genes from the CRISPRa screens.46
RNA Extraction and Quantitative PCR
Total RNA was extracted using TRIzol (Thermo, cat. no. 15596026). Complementary DNA was synthesized from DNase-treated RNA (Promega, cat. no. M6101) by reverse transcription (Applied Biosystems, cat. no. 4368814). Quantitative PCR (qPCR) was performed using Taq DNA polymerase SYBR green master mix (Applied Biosystems, cat. no. A25741 or BioRad, cat. no. 172-5120) and 300 nmol/L of primers (Table 3) in a StepOne Plus or BioRad CFX Connect qPCR detection system. Each reaction was performed in triplicates. Calculations for relative mRNA expression are as follows: ΔCT was calculated by subtracting the CT of the housekeeping gene Gapdh from the CT of the target gene. Then, 2-ΔCT was calculated, and the experimental samples were normalized to the average of the control samples to obtain the relative mRNA expression.
Table 3.
Primers for qPCR Experiments of Mouse Genes
| Gene target | Sense | Forward (5′-3′) |
|---|---|---|
| Vps72 | Fwd | AACTTACGGGGGTTTCACCG |
| Rev | GCTCTTCCGCTTCTCCATCAC | |
| Gba | Fwd | GCCAGGCTCATCGGATTCTTC |
| Rev | GAGTGCTCTCGTAACGGCT | |
| Mrpl9 | Fwd | CCGCGAATTGAAGGGAGTACC |
| Rev | CCTCCACCAACTTATACACGC | |
| Zbtb7b | Fwd | CCCGAGGATGACCTGATTGG |
| Rev | CCTGCGTCCTGATGGTGAG | |
| Gapdh | Fwd | AGGTCGGTGTGAACGGATTTG |
| Rev | TGTAGACCATGTAGTTGAGGTCA |
Lentivirus Packaging and Infection
Lentivirus CRISPRa vector (Addgene #96923) encoding gene-targeting or control gRNAs were co-transfected with pCMV-VSV-G and pCMV-dR8.2 in 293T/17 cells at a ratio of 1:0.05:0.45 using lipofectamine 3000. The medium was refreshed 12–18 hours later. The supernatant, which contained virus particles, was collected 48 and 72 hours later and filtered. Lentivirus was concentrated 100-fold using Lenti-X concentrator (Takara Bio USA, San Jose, CA, #631232). Target cells were transduced in the presence of 8 μg/mL polybrene and incubated overnight. T226 cells were selected with 3 μg/mL puromycin starting 24 hours after transduction. For in vitro gRNA validation, RNA was extracted, and qPCR was performed to detect relative expression of the target genes.
Immunohistochemical Staining
Formalin-fixed paraffin embedded sections were deparaffinized and rehydrated. Antigen retrieval was performed in a citrate buffer (R-buffer A, pH 6.0) for one heating cycle (Aptum 2100-Retriever). Sections were treated with 3% hydrogen peroxide, blocked with CAS-Block (Thermo #008120), incubated with primary antibody (Table 4) overnight at 4°C, and incubated with rabbit or goat horseradish peroxidase polymer (Biocare Medical #RMR622, #GHP516, Pacheco, CA). We developed the signal with DAB substrate for peroxidases (Vector Labs #SK4100, Newark, CA) until strong signal was detected on positive control section, and same incubation time was kept across sections. Next, sections were dehydrated, mounted (Fisher #22-050-262), and sealed with coverslips (Fisher #125485E). Stained sections were imaged using a light microscope (Zeiss, Oberkochen, Germany Primo Star) or scanned with a slide scanning microscope (Motic EasyScan Pro).
Table 4.
Antibodies for IHC Experiments
| Antibody target | Target species | Host | Dilution | Manufacturer | Catalog no. |
|---|---|---|---|---|---|
| MYC | Mouse | Rabbit | 1:400 | Abcam | ab32072 |
| FAH | Mouse | Rabbit | 1:800 | Invitrogen | PA5-42049 |
| KI67 | Mouse | Rabbit | 1:100 | Abcam | ab16667 |
Measuring HCC Proliferation
For CRISPRa-mediated overexpression, T226 cells stably expressing TAs and a gRNA targeting Mrpl9, Gba1, Zbtb7b, or Vps72, or NT as control were seeded in 96-well plates at a confluency of 650–750 cells per well. For transgene-mediated overexpression, T226 cells were transfected with 0.1 μg Myc-Zbtb7b, Myc-Vps72, or Myc-Gfp control at a 3:1 (transfection reagent: DNA) ratio (Promega, Madison, WI, #E2311) or transduced with lentiviral vectors expressing Mrpl9, Gba1, or Gfp control. Proliferation was monitored for 72 hours with 6- to 12-hour time points using a luminescence detection system with 0.5-second integration time (Promega, #GM3000).
RNA-seq Analysis
Tumor RNA-seq was performed on a NovaSeq platform (150-base-pair dual-end reads). The data are deposited on the Gene Expression Omnibus database (accession #GSE250070). The mouse RNA-seq data were size factor-based normalized using DESeq2.47 Volcano plots were generated to show a generic view of DEGs, with the top 10 significantly altered genes text marked. The top 100 up-regulated and top 100 down-regulated genes were selected for GO biological process analysis using The Database for Annotation, Visualization and Integrated Discovery (DAVID). Next, the top DEGs were selected for verifying the consistency of gene expression changes between mouse and human samples.
TCGA Analysis
For gene expression analysis, the top DEGs from RNA-Seq of Mrpl9, Vps72, and Gba1 activation levels were examined in human liver cancers in TCGA-LIHC. The top 30 up-regulated and top 20 down-regulated genes were selected for Mrpl9 activation, top 30 up-regulated and down-regulated genes were selected for Vps72 activation, and top 50 up-regulated and down-regulated genes were selected for Gba1 activation because of the observed different consistencies. All 374 human liver tumors were ordered according to the decreased expression of MRPL9, VPS72, or GBA1 and then filtered into 2 groups, with one group having the gene expression greater than the upper quartile (n = 94) and another less than the lower quartile (n = 94). For survival analysis, the expression profile was downloaded along with the clinical and biospecimen data. Combined matrices were generated using in-house scripts, and Kaplan–Meier survival plots were generated for MRPL9, VPS72, GBA1, and ZBTB7B.
GSEA
GSEA was performed for the whole expression profile with all 21,274 genes prefiltered by DESeq2 for Mrpl9, Vps72, and Gba1. The 2664 mouse curated gene sets were selected for analysis. The significance of the gene signature in GSEA was determined by taking enrichment score (ES), normalized enrichment score (NES), and false discovery rate (FDR) into consideration. All the gene sets were obtained from the Molecular Signatures Database.48, 49, 50
Software
R package karyoploteR was used for generating Manhattan plots. Prism (GraphPad, San Diego, CA) was used for creating line chart and dot plots. R package ggplot2 was used for generating bar plots, box plots, volcano plots, and scatter plots. R package heatmap.2 was used for generating heatmaps. R package ggsurvplot was used for creating survival curve. gRNA screen plots were generated by MAGeCK. Python module pandas were used for generating combined matrix for survival analysis. All the analyses were performed under the conda environment with R 4.4.0 and Python 3.11.
Statistical Analysis
For gene expression analysis of the TCGA-LIHC cohort, Student t test was used to compare gene expression levels in tumors with high or low expression of Mrpl9, Vps72, or Gba1, and the P values were adjusted by the Benjamini-Hochberg method for multiple testing. Otherwise, Wilcoxon rank-sum test or unpaired Student t test was used for 2 sample comparison, and one-way analysis of variance (ANOVA) with Tukey multiple comparisons test or Kruskal–Wallis test followed by Dunn test was applied for multiple comparisons in appropriate places, as indicated in the figure legends. For survival analysis, log-rank test and Cox proportional hazards model are applied, and Fisher exact test is for contingency tables as detailed in the figure legends.
All authors had access to the study data and reviewed and approved the final manuscript. Additional detailed information is provided in the supplementary figure legends.
Acknowledgments
The authors thank Dr Zvi Cramer for technical support and Drs Adam Zahm and Vijay Shah for invaluable feedback on this manuscript.
CRediT Authorship Contributions
Alexandra M. Vázquez Salgado (Conceptualization: Supporting; Data curation: Supporting; Formal analysis: Supporting; Funding acquisition: Supporting; Investigation: Supporting; Methodology: Supporting; Validation: Supporting; Visualization: Supporting; Writing – original draft: Supporting; Writing – review & editing: Supporting)
Chunmiao Cai, PhD (Conceptualization: Supporting; Data curation: Supporting; Methodology: Supporting; Visualization: Supporting; Writing – original draft: Supporting; Writing – review & editing: Supporting)
Markcus A Lee II (Investigation: Supporting; Visualization: Supporting; Writing – review & editing: Supporting)
Dingzi Yin, PhD (Conceptualization: Supporting; Formal analysis: Supporting; Investigation: Supporting; Writing – review & editing: Supporting)
Marie-Lise Chrystostome (Methodology: Supporting; Validation: Supporting; Writing – review & editing: Supporting)
Adrienne F. Gefre (Formal analysis: Supporting; Investigation: Supporting; Writing – review & editing: Supporting)
Shirui He (Investigation: Supporting)
Julia E. Kieckhaefer, PhD (Investigation: Supporting; Writing – review & editing: Supporting)
Kirk J Wangensteen, MD/PhD (Conceptualization: Lead; Formal analysis: Lead; Funding acquisition: Lead; Investigation: Lead; Project administration: Lead; Resources: Lead; Supervision: Lead; Writing – original draft: Lead; Writing – review & editing: Lead)
Footnotes
Conflicts of interest This author discloses the following: Kirk J. Wangensteen has received research support from Calico Life Sciences and Pfizer. The remaining authors disclose no conflicts.
Funding Supported by NIH R37CA259201, NIH R01CA249929, and NIH P30DK084567.
Note: To access the supplementary material accompanying this article, go to the full text version at https://doi.org/10.1016/j.jcmgh.2025.101460.
Supplementary Material
References
- 1.Adlat S., Vazquez Salgado A.M., Lee M., et al. Emerging and potential use of CRISPR in human liver disease. Hepatology. 2023 doi: 10.1097/HEP.0000000000000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dal Bo M., De Mattia E., Baboci L., et al. New insights into the pharmacological, immunological, and CAR-T-cell approaches in the treatment of hepatocellular carcinoma. Drug Resist Updat. 2020;51 doi: 10.1016/j.drup.2020.100702. [DOI] [PubMed] [Google Scholar]
- 3.Mandlik D.S., Mandlik S.K., Choudhary H.B. Immunotherapy for hepatocellular carcinoma: current status and future perspectives. World J Gastroenterol. 2023;29:1054–1075. doi: 10.3748/wjg.v29.i6.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wangensteen K.J., Wang Y.J., Dou Z., et al. Combinatorial genetics in liver repopulation and carcinogenesis with a in vivo CRISPR activation platform. Hepatology. 2018;68:663–676. doi: 10.1002/hep.29626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saito Y., Yin D., Kubota N., et al. A therapeutically targetable TAZ-TEAD2 pathway drives the growth of hepatocellular carcinoma via ANLN and KIF23. Gastroenterology. 2023;164:1279–1292. doi: 10.1053/j.gastro.2023.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jia Y., Li L., Lin Y.H., et al. In vivo CRISPR screening identifies BAZ2 chromatin remodelers as druggable regulators of mammalian liver regeneration. Cell Stem Cell. 2022;29:372–385.e8. doi: 10.1016/j.stem.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu X., Xuan W., Li J., et al. AMPK protects against alcohol-induced liver injury through UQCRC2 to up-regulate mitophagy. Autophagy. 2021;17:3622–3643. doi: 10.1080/15548627.2021.1886829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka S., Hikita H., Tatsumi T., et al. Rubicon inhibits autophagy and accelerates hepatocyte apoptosis and lipid accumulation in nonalcoholic fatty liver disease in mice. Hepatology. 2016;64:1994–2014. doi: 10.1002/hep.28820. [DOI] [PubMed] [Google Scholar]
- 9.Sequera C., Grattarola M., Holczbauer A., et al. MYC and MET cooperatively drive hepatocellular carcinoma with distinct molecular traits and vulnerabilities. Cell Death Dis. 2022;13:994. doi: 10.1038/s41419-022-05411-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zucman-Rossi J., Villanueva A., Nault J.C., et al. Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology. 2015;149:1226–1239.e4. doi: 10.1053/j.gastro.2015.05.061. [DOI] [PubMed] [Google Scholar]
- 11.Shang N., Arteaga M., Zaidi A., et al. FAK is required for c-Met/β-catenin-driven hepatocarcinogenesis. Hepatology. 2015;61:214–226. doi: 10.1002/hep.27402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shang N., Wang H., Bank T., et al. Focal adhesion kinase and β-catenin cooperate to induce hepatocellular carcinoma. Hepatology. 2019;70:1631–1645. doi: 10.1002/hep.30707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sondka Z., Bamford S., Cole C.G., et al. The COSMIC Cancer Gene Census: describing genetic dysfunction across all human cancers. Nat Rev Cancer. 2018;18:696–705. doi: 10.1038/s41568-018-0060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molina-Sanchez P., Ruiz de Galarreta M., Yao M.A., et al. Cooperation between distinct cancer driver genes underlies intertumor heterogeneity in hepatocellular carcinoma. Gastroenterology. 2020;159:2203–2220.e14. doi: 10.1053/j.gastro.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santoni-Rugiu E., Nagy P., Jensen M.R., et al. Evolution of neoplastic development in the liver of transgenic mice co-expressing c-myc and transforming growth factor-alpha. Am J Pathol. 1996;149:407–428. [PMC free article] [PubMed] [Google Scholar]
- 16.Lee J.S., Chu I.S., Mikaelyan A., et al. Application of comparative functional genomics to identify best-fit mouse models to study human cancer. Nat Genet. 2004;36:1306–1311. doi: 10.1038/ng1481. [DOI] [PubMed] [Google Scholar]
- 17.Vazquez Salgado A.M., Preziosi M.E., Yin D., et al. In vivo screen identifies liver X receptor alpha agonism potentiates sorafenib killing of hepatocellular carcinoma. Gastro Hep Adv. 2022;1:905–908. doi: 10.1016/j.gastha.2022.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wangensteen K.J., Wilber A., Keng V.W., et al. A facile method for somatic, lifelong manipulation of multiple genes in the mouse liver. Hepatology. 2008;47:1714–1724. doi: 10.1002/hep.22195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vazquez Salgado A.M., Wangensteen K.J. Death after high-dose rAAV9 gene therapy in a patient with Duchenne’s muscular dystrophy. N Engl J Med. 2023;389:2211. doi: 10.1056/NEJMc2312288. [DOI] [PubMed] [Google Scholar]
- 20.Palmgren S., Ojala P.J., Wear M.A., et al. Interactions with PIP2, ADP-actin monomers, and capping protein regulate the activity and localization of yeast twinfilin. J Cell Biol. 2001;155:251–260. doi: 10.1083/jcb.200106157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shulga Y.V., Anderson R.A., Topham M.K., et al. Phosphatidylinositol-4-phosphate 5-kinase isoforms exhibit acyl chain selectivity for both substrate and lipid activator. J Biol Chem. 2012;287:35953–35963. doi: 10.1074/jbc.M112.370155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu Y., Zou R., Sha H., et al. Lipid metabolism-related proteins of relevant evolutionary and lymphoid interest (PRELI) domain containing family proteins in cancer. Am J Transl Res. 2020;12:6015–6026. [PMC free article] [PubMed] [Google Scholar]
- 23.Magalhaes J., Gegg M.E., Migdalska-Richards A., et al. Autophagic lysosome reformation dysfunction in glucocerebrosidase deficient cells: relevance to Parkinson disease. Hum Mol Genet. 2016;25:3432–3445. doi: 10.1093/hmg/ddw185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trajkovic K., Hsu C., Chiantia S., et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 25.Vicinanza M., Di Campli A., Polishchuk E., et al. OCRL controls trafficking through early endosomes via PtdIns4,5P(2)-dependent regulation of endosomal actin. EMBO J. 2011;30:4970–4985. doi: 10.1038/emboj.2011.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Granger E., McNee G., Allan V., et al. The role of the cytoskeleton and molecular motors in endosomal dynamics. Semin Cell Dev Biol. 2014;31:20–29. doi: 10.1016/j.semcdb.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krugmann S., Jordens I., Gevaert K., et al. Cdc42 induces filopodia by promoting the formation of an IRSp53:Mena complex. Curr Biol. 2001;11:1645–1655. doi: 10.1016/s0960-9822(01)00506-1. [DOI] [PubMed] [Google Scholar]
- 28.Dehmelt L., Halpain S. The MAP2/Tau family of microtubule-associated proteins. Genome Biol. 2005;6:204. doi: 10.1186/gb-2004-6-1-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshida H., Goedert M. Phosphorylation of microtubule-associated protein tau by AMPK-related kinases. J Neurochem. 2012;120:165–176. doi: 10.1111/j.1471-4159.2011.07523.x. [DOI] [PubMed] [Google Scholar]
- 30.Franch-Exposito S., Bassaganyas L., Vila-Casadesus M., et al. CNApp, a tool for the quantification of copy number alterations and integrative analysis revealing clinical implications. Elife. 2020;9 doi: 10.7554/eLife.50267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding X., He M., Chan A.W.H., et al. Genomic and epigenomic features of primary and recurrent hepatocellular carcinomas. Gastroenterology. 2019;157:1630–1645.e6. doi: 10.1053/j.gastro.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Grover R., Burse S.A., Shankrit S., et al. Myg1 exonuclease couples the nuclear and mitochondrial translational programs through RNA processing. Nucleic Acids Res. 2019;47:5852–5866. doi: 10.1093/nar/gkz371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cardoso A.R., Queliconi B.B., Kowaltowski A.J. Mitochondrial ion transport pathways: role in metabolic diseases. Biochim Biophys Acta. 2010;1797:832–838. doi: 10.1016/j.bbabio.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 34.Bechmann L.P., Hannivoort R.A., Gerken G., et al. The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol. 2012;56:952–964. doi: 10.1016/j.jhep.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 35.Sharma N.K., Chuang Key C.C., Civelek M., et al. Genetic regulation of enoyl-CoA hydratase domain-containing 3 in adipose tissue determines insulin sensitivity in African Americans and Europeans. Diabetes. 2019;68:1508–1522. doi: 10.2337/db18-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen T., Tu Y., Lv D., et al. Vacuolar protein sorting-associated protein 72 homolog (VPS72) binding to lysine acetyltransferase 5 (KAT5) promotes the proliferation, invasion and migration of hepatocellular carcinoma through regulating phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) signaling pathway. Bioengineered. 2022;13:9197–9210. doi: 10.1080/21655979.2022.2056692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu F., Liao Z., Qin L., et al. Targeting VPS72 inhibits ACTL6A/MYC axis activity in HCC progression. Hepatology. 2023;78:1384–1401. doi: 10.1097/HEP.0000000000000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bohdanowicz M., Balkin D.M., De Camilli P., et al. Recruitment of OCRL and Inpp5B to phagosomes by Rab5 and APPL1 depletes phosphoinositides and attenuates Akt signaling. Mol Biol Cell. 2012;23:176–187. doi: 10.1091/mbc.E11-06-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simonetti B., Cullen P.J. Actin-dependent endosomal receptor recycling. Curr Opin Cell Biol. 2019;56:22–33. doi: 10.1016/j.ceb.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 40.Gallant P. Myc, cell competition, and compensatory proliferation. Cancer Res. 2005;65:6485–6487. doi: 10.1158/0008-5472.CAN-05-1101. [DOI] [PubMed] [Google Scholar]
- 41.Konermann S., Brigham M.D., Trevino A.E., et al. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature. 2015;517:583–588. doi: 10.1038/nature14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liao H.K., Hatanaka F., Araoka T., et al. In vivo target gene activation via CRISPR/Cas9-mediated trans-epigenetic modulation. Cell. 2017;171:1495–1507.e15. doi: 10.1016/j.cell.2017.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cerami E., Gao J., Dogrusoz U., et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao J., Aksoy B.A., Dogrusoz U., et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6 doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horlbeck M.A., Gilbert L.A., Villalta J.E., et al. Compact and highly active next-generation libraries for CRISPR-mediated gene repression and activation. eLife. 2016;5 doi: 10.7554/eLife.19760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li W., Xu H., Xiao T., et al. MAGeCK enables robust identification of essential genes from genome-scale CRISPR/Cas9 knockout screens. Genome Biology. 2014;15:554. doi: 10.1186/s13059-014-0554-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liberzon A., Subramanian A., Pinchback R., et al. Molecular signatures database (MSigDB) 3.0. Bioinformatics. 2011;27:1739–1740. doi: 10.1093/bioinformatics/btr260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Subramanian A., Tamayo P., Mootha V.K., et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castanza A.S., Recla J.M., Eby D., et al. Extending support for mouse data in the Molecular Signatures Database (MSigDB) Nature Methods. 2023;20:1619–1620. doi: 10.1038/s41592-023-02014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.