Abstract
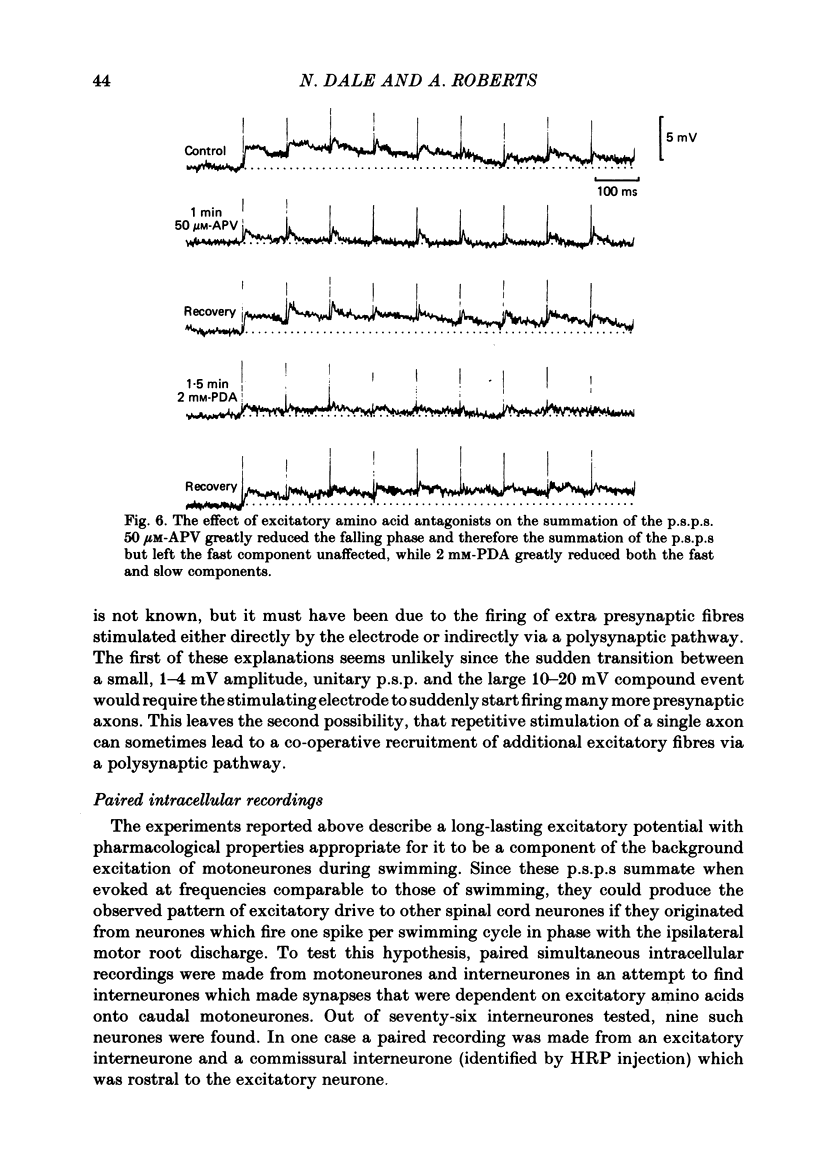
The neuronal basis of the excitation received by motoneurones during swimming in curarized Xenopus embryos has been investigated further. Extracellular stimulation of axons in the fibre tracts of the spinal cord has been used to evoke unitary excitatory post-synaptic potentials (p.s.p.s) in motoneurones. The p.s.p.s. had a rise time of 3-5 ms and a long falling phase lasting up to 200 ms. These potentials consist of two components: a 'fast' p.s.p. which is insensitive to 50 microM-(+/-)-2-amino-5-phosphonovaleric acid (APV) but is blocked by 2 mM-cis-2,3-piperidine dicarboxylic acid (PDA) and is therefore probably mediated by kainate/quisqualate receptors, and a 'slow' p.s.p. which is blocked by both APV and PDA and is therefore probably mediated by N-methyl-D-aspartate (NMDA) receptors. Paired intracellular recordings from motoneurones and interneurones have revealed a class of spinal cord interneurone which makes descending excitatory amino-acid-dependent synapses onto motoneurones and commissural interneurones. The p.s.p.s evoked by intracellular stimulation of these excitatory interneurones consist of 'fast' and 'slow' components identical in shape and pharmacological properties to those of the extracellularly evoked potentials. One neurone may, therefore, be able to release a transmitter which activates both NMDA and non-NMDA receptors on the same post-synaptic neurone generating fast and slow post-synaptic potentials. The excitatory interneurones play an important role in the generation of the swimming pattern in the curarized Xenopus embryo. Like motoneurones, they fire once per swimming cycle in phase with the ipsilateral motoneurones and receive a background excitation during swimming that is excitatory amino acid mediated. They are therefore part of the swimming rhythm generator. The temporal summation of the extracellularly evoked p.s.p.s shows that these excitatory interneurones are sufficient to generate the excitatory drive received by motoneurones during swimming.
Full text
PDF























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong-James M., Millar J. Carbon fibre microelectrodes. J Neurosci Methods. 1979 Oct;1(3):279–287. doi: 10.1016/0165-0270(79)90039-6. [DOI] [PubMed] [Google Scholar]
- Ault B., Evans R. H., Francis A. A., Oakes D. J., Watkins J. C. Selective depression of excitatory amino acid induced depolarizations by magnesium ions in isolated spinal cord preparations. J Physiol. 1980 Oct;307:413–428. doi: 10.1113/jphysiol.1980.sp013443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROCK L. G., COOMBS J. S., ECCLES J. C. The recording of potentials from motoneurones with an intracellular electrode. J Physiol. 1952 Aug;117(4):431–460. doi: 10.1113/jphysiol.1952.sp004759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. G. On the nature of the fundamental activity of the nervous centres; together with an analysis of the conditioning of rhythmic activity in progression, and a theory of the evolution of function in the nervous system. J Physiol. 1914 Mar 31;48(1):18–46. doi: 10.1113/jphysiol.1914.sp001646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS D. R., ECCLES J. C. The time courses of excitatory and inhibitory synaptic actions. J Physiol. 1959 Mar 12;145(3):529–546. doi: 10.1113/jphysiol.1959.sp006159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke J. D., Hayes B. P., Hunt S. P., Roberts A. Sensory physiology, anatomy and immunohistochemistry of Rohon-Beard neurones in embryos of Xenopus laevis. J Physiol. 1984 Mar;348:511–525. doi: 10.1113/jphysiol.1984.sp015122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983 Jan;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. The antagonism of amino acid-induced excitations of rat hippocampal CA1 neurones in vitro. J Physiol. 1983 Jan;334:19–31. doi: 10.1113/jphysiol.1983.sp014477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V., Forda S., Collingridge G. L., Kelly J. S. Intracellular recorded synaptic antagonism in the rat dentate gyrus. Nature. 1982 Dec 2;300(5891):450–452. doi: 10.1038/300450a0. [DOI] [PubMed] [Google Scholar]
- Dale N., Roberts A. Excitatory amino acid receptors in Xenopus embryo spinal cord and their role in the activation of swimming. J Physiol. 1984 Mar;348:527–543. doi: 10.1113/jphysiol.1984.sp015123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Evans R. H., Jones A. W., Smith D. A., Watkins J. C. Differential activation and blockade of excitatory amino acid receptors in the mammalian and amphibian central nervous systems. Comp Biochem Physiol C. 1982;72(2):211–224. doi: 10.1016/0306-4492(82)90086-7. [DOI] [PubMed] [Google Scholar]
- Davies J., Watkins J. C. Differentiation of kainate and quisqualate receptors in the cat spinal cord by selective antagonism with gamma-D(and L)-glutamylglycine. Brain Res. 1981 Feb 9;206(1):172–177. doi: 10.1016/0006-8993(81)90111-6. [DOI] [PubMed] [Google Scholar]
- Davies J., Watkins J. C. Role of excitatory amino acid receptors in mono- and polysynaptic excitation in the cat spinal cord. Exp Brain Res. 1983;49(2):280–290. doi: 10.1007/BF00238587. [DOI] [PubMed] [Google Scholar]
- Davies J., Watkins J. C. Selective antagonism of amino acid-induced and synaptic excitation in the cat spinal cord. J Physiol. 1979 Dec;297(0):621–635. doi: 10.1113/jphysiol.1979.sp013060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. H., Francis A. A., Hunt K., Oakes D. J., Watkins J. C. Antagonism of excitatory amino acid-induced responses and of synaptic excitation in the isolated spinal cord of the frog. Br J Pharmacol. 1979 Dec;67(4):591–603. doi: 10.1111/j.1476-5381.1979.tb08706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S., McClellan A., Sigvardt K., Wallén P., Wilén M. Activation of NMDA-receptors elicits "fictive locomotion" in lamprey spinal cord in vitro. Acta Physiol Scand. 1981 Dec;113(4):549–551. doi: 10.1111/j.1748-1716.1981.tb06937.x. [DOI] [PubMed] [Google Scholar]
- Harth E., Lewis N. S. The escape of Tritonia: dynamics of a neuromuscular control mechanism. J Theor Biol. 1975 Nov;55(1):201–228. doi: 10.1016/s0022-5193(75)80116-0. [DOI] [PubMed] [Google Scholar]
- Herrling P. L., Morris R., Salt T. E. Effects of excitatory amino acids and their antagonists on membrane and action potentials of cat caudate neurones. J Physiol. 1983 Jun;339:207–222. doi: 10.1113/jphysiol.1983.sp014712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack J. J., Redman S. J., Wong K. The components of synaptic potentials evoked in cat spinal motoneurones by impulses in single group Ia afferents. J Physiol. 1981 Dec;321:65–96. doi: 10.1113/jphysiol.1981.sp013972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn J. A., Roberts A. Experiments on the central pattern generator for swimming in amphibian embryos. Philos Trans R Soc Lond B Biol Sci. 1982 Jan 27;296(1081):229–243. doi: 10.1098/rstb.1982.0004. [DOI] [PubMed] [Google Scholar]
- Kahn J. A., Roberts A., Kashin S. M. The neuromuscular basis of swimming movements in embryos of the amphibian Xenopus laevis. J Exp Biol. 1982 Aug;99:175–184. doi: 10.1242/jeb.99.1.175. [DOI] [PubMed] [Google Scholar]
- Kahn J. A., Roberts A. The central nervous origin of the swimming motor pattern in embryos of Xenopus laevis. J Exp Biol. 1982 Aug;99:185–196. doi: 10.1242/jeb.99.1.185. [DOI] [PubMed] [Google Scholar]
- LIBET B. SLOW SYNAPTIC RESPONSES AND EXCITATORY CHANGES IN SYMPATHETIC GANGLIA. J Physiol. 1964 Oct;174:1–25. doi: 10.1113/jphysiol.1964.sp007471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libet B., Chichibu S., Tosaka T. Slow synaptic responses and excitability in sympathetic ganglia of the bullfrog. J Neurophysiol. 1968 May;31(3):383–395. doi: 10.1152/jn.1968.31.3.383. [DOI] [PubMed] [Google Scholar]
- Libet B. Long latent periods and further analysis of slow synaptic responses in sympathetic ganglia. J Neurophysiol. 1967 May;30(3):494–514. doi: 10.1152/jn.1967.30.3.494. [DOI] [PubMed] [Google Scholar]
- McLennan H. Receptors for the excitatory amino acids in the mammalian central nervous system. Prog Neurobiol. 1983;20(3-4):251–271. doi: 10.1016/0301-0082(83)90004-7. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Ogden T. E., Citron M. C., Pierantoni R. The jet stream microbeveler: an inexpensive way to bevel ultrafine glass micropipettes. Science. 1978 Aug 4;201(4354):469–470. doi: 10.1126/science.663670. [DOI] [PubMed] [Google Scholar]
- Redman S., Walmsley B. Amplitude fluctuations in synaptic potentials evoked in cat spinal motoneurones at identified group Ia synapses. J Physiol. 1983 Oct;343:135–145. doi: 10.1113/jphysiol.1983.sp014885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman S., Walmsley B. The time course of synaptic potentials evoked in cat spinal motoneurones at identified group Ia synapses. J Physiol. 1983 Oct;343:117–133. doi: 10.1113/jphysiol.1983.sp014884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A., Clarke J. D. The neuroanatomy of an amphibian embryo spinal cord. Philos Trans R Soc Lond B Biol Sci. 1982 Jan 27;296(1081):195–212. doi: 10.1098/rstb.1982.0002. [DOI] [PubMed] [Google Scholar]
- Roberts A., Khan J. A. Intracellular recordings from spinal neurons during 'swimming' in paralysed amphibian embryos. Philos Trans R Soc Lond B Biol Sci. 1982 Jan 27;296(1081):213–228. doi: 10.1098/rstb.1982.0003. [DOI] [PubMed] [Google Scholar]
- Roberts A., Soffe S. R., Clarke J. D., Dale N. Initiation and control of swimming in amphibian embryos. Symp Soc Exp Biol. 1983;37:261–284. [PubMed] [Google Scholar]
- Shik M. L., Orlovsky G. N. Neurophysiology of locomotor automatism. Physiol Rev. 1976 Jul;56(3):465–501. doi: 10.1152/physrev.1976.56.3.465. [DOI] [PubMed] [Google Scholar]
- Slaughter M. M., Miller R. F. An excitatory amino acid antagonist blocks cone input to sign-conserving second-order retinal neurons. Science. 1983 Mar 11;219(4589):1230–1232. doi: 10.1126/science.6131536. [DOI] [PubMed] [Google Scholar]
- Soffe S. R., Clarke J. D., Roberts A. Activity of commissural interneurons in spinal cord of Xenopus embryos. J Neurophysiol. 1984 Jun;51(6):1257–1267. doi: 10.1152/jn.1984.51.6.1257. [DOI] [PubMed] [Google Scholar]
- Soffe S. R., Roberts A. Activity of myotomal motoneurons during fictive swimming in frog embryos. J Neurophysiol. 1982 Dec;48(6):1274–1278. doi: 10.1152/jn.1982.48.6.1274. [DOI] [PubMed] [Google Scholar]
- Soffe S. R., Roberts A. Tonic and phasic synaptic input to spinal cord motoneurons during fictive locomotion in frog embryos. J Neurophysiol. 1982 Dec;48(6):1279–1288. doi: 10.1152/jn.1982.48.6.1279. [DOI] [PubMed] [Google Scholar]
- Westbrook G. L., Mayer M. L. Glutamate currents in mammalian spinal neurons: resolution of a paradox. Brain Res. 1984 Jun 3;301(2):375–379. doi: 10.1016/0006-8993(84)91107-7. [DOI] [PubMed] [Google Scholar]



