Abstract
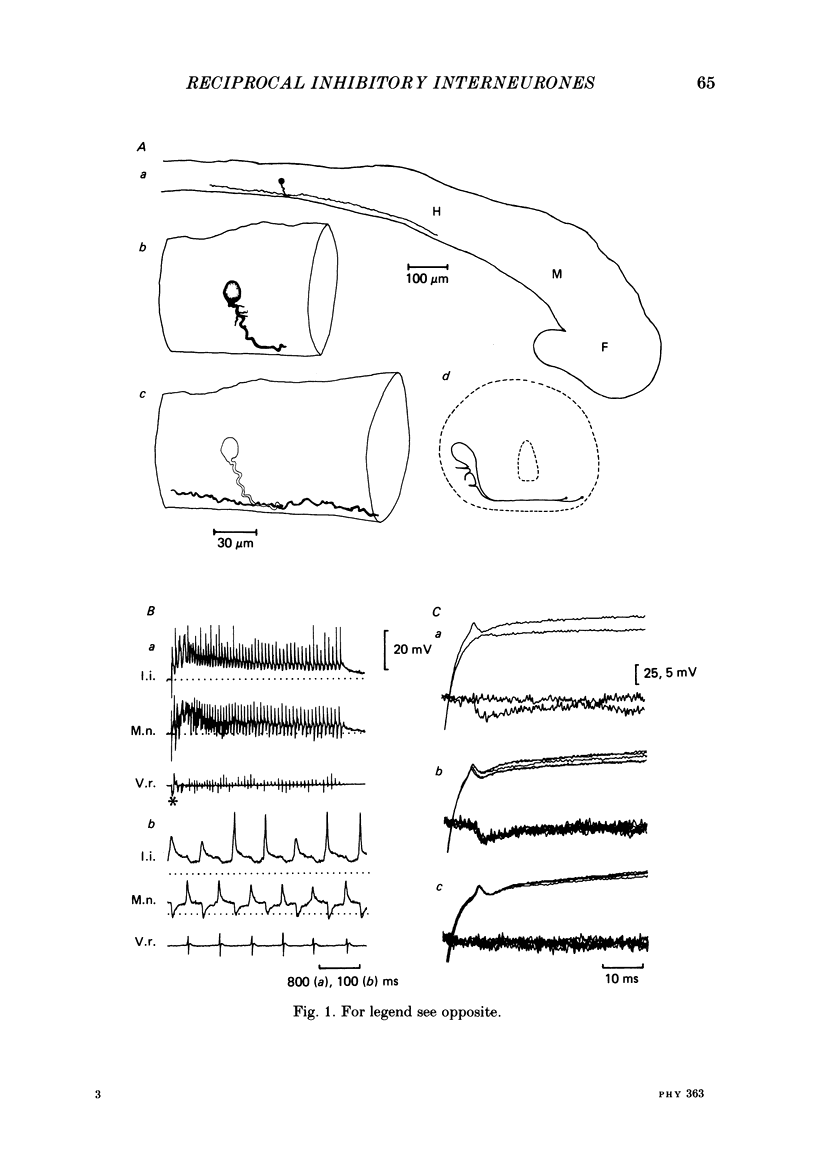
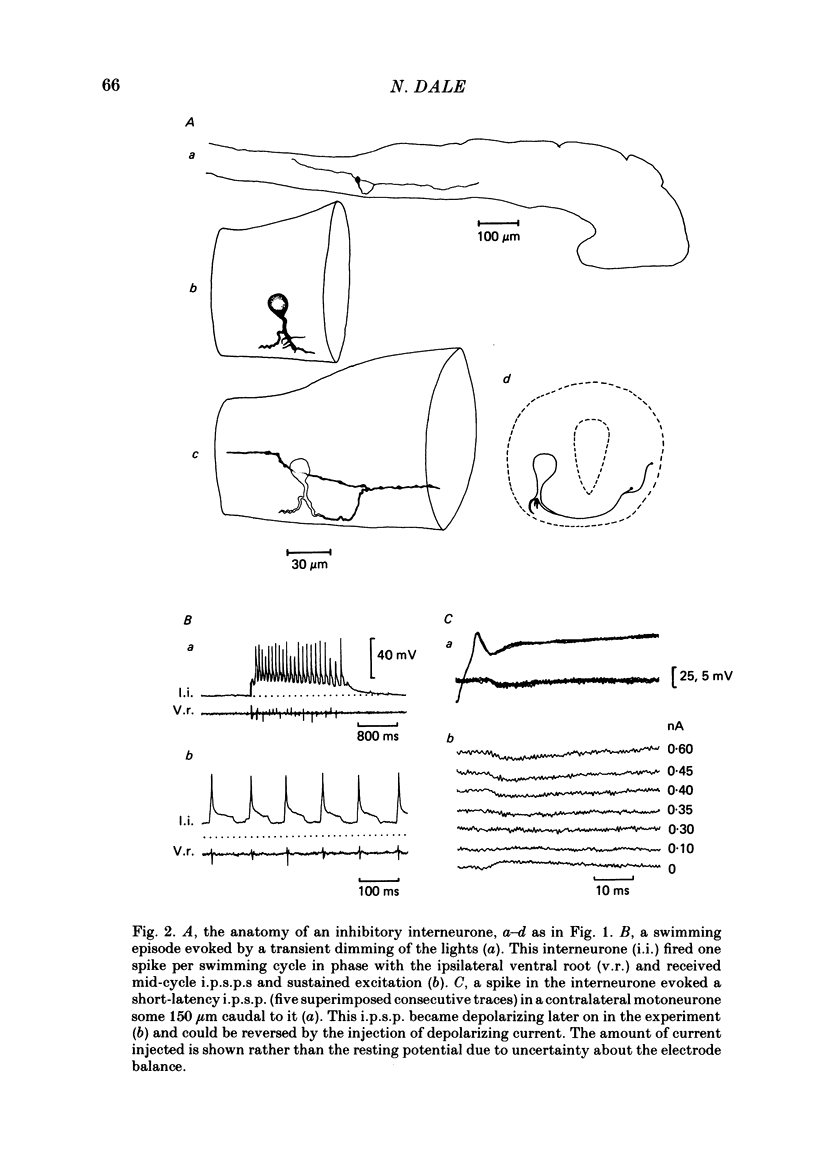
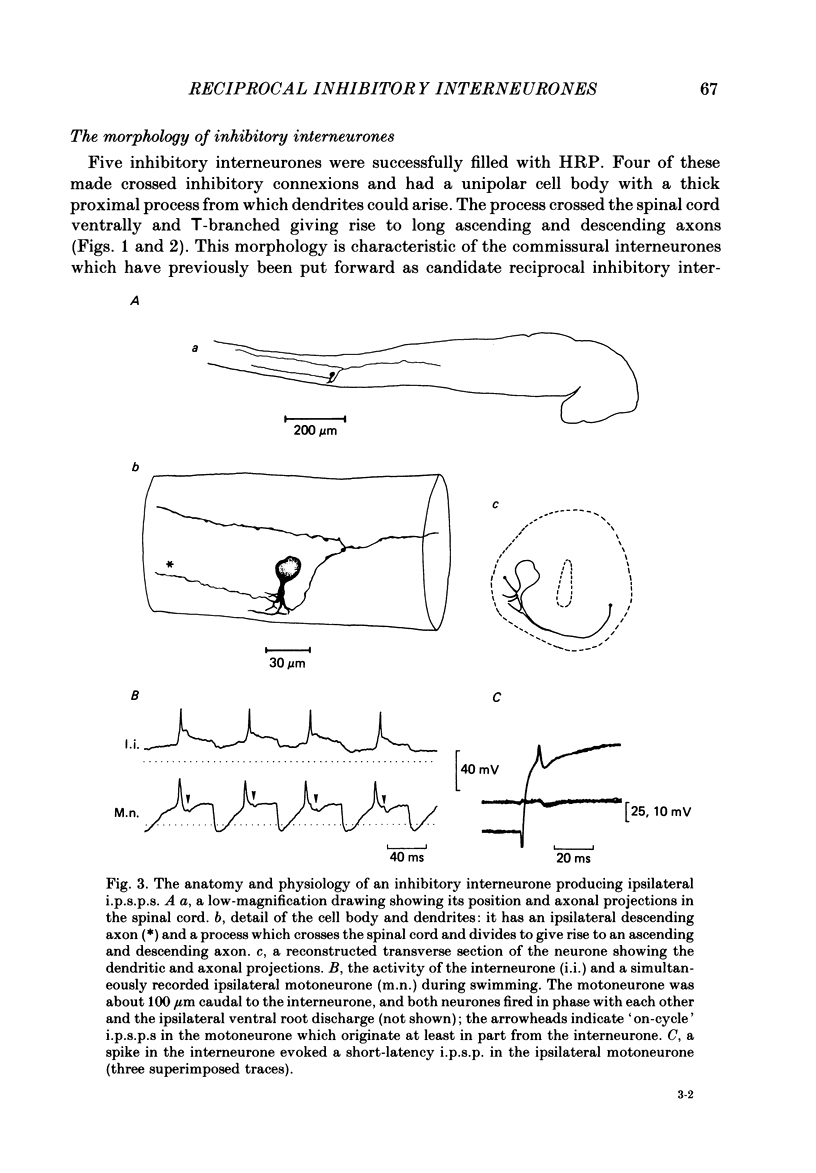
The mechanism of reciprocal inhibition between antagonistic motor centres during swimming in the paralysed Xenopus embryo has been investigated further. Paired intracellular recordings have been made from interneurones and motoneurones in an attempt to identify neurones which make direct inhibitory synapses onto motoneurones on the opposite side of the spinal cord. A physiological class of inhibitory interneurones is described which, when stimulated by intracellular current passage, evoke short-latency, probably monosynaptic, strychnine-sensitive inhibitory potentials in contralateral motoneurones. These inhibitory interneurones fire once per swimming cycle in phase with the ipsilateral motor root discharge. They therefore have a pattern of activity which would cause them to inhibit motoneurones of the antagonistic motor centre at an appropriate part of the swimming cycle. The intracellular injection of horseradish peroxidase (HRP) has allowed the morphology of these inhibitory interneurones to be characterized. They have unipolar cell bodies with a thick proximal process with short dendrites which crosses the spinal cord ventrally and then bifurcates with one axonal branch ascending into the hind brain and the other descending the spinal cord. These anatomical features are typical of the 'commissural interneurones' first described by Roberts & Clarke (1982). There are also some inhibitory interneurones which can inhibit motoneurones on the same side of the spinal cord. At least some of these interneurones may be commissural interneurones with ipsilateral axons and they may play a role in the generation of the swimming rhythm.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buchanan J. T. Identification of interneurons with contralateral, caudal axons in the lamprey spinal cord: synaptic interactions and morphology. J Neurophysiol. 1982 May;47(5):961–975. doi: 10.1152/jn.1982.47.5.961. [DOI] [PubMed] [Google Scholar]
- CURTIS D. R., ECCLES J. C. The time courses of excitatory and inhibitory synaptic actions. J Physiol. 1959 Mar 12;145(3):529–546. doi: 10.1113/jphysiol.1959.sp006159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese R. L., Peterson E. Neural control of heartbeat in the leech, Hirudo medicinalis. Symp Soc Exp Biol. 1983;37:195–221. [PubMed] [Google Scholar]
- Clarke J. D., Hayes B. P., Hunt S. P., Roberts A. Sensory physiology, anatomy and immunohistochemistry of Rohon-Beard neurones in embryos of Xenopus laevis. J Physiol. 1984 Mar;348:511–525. doi: 10.1113/jphysiol.1984.sp015122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke J. D., Roberts A. Interneurones in the Xenopus embryo spinal cord: sensory excitation and activity during swimming. J Physiol. 1984 Sep;354:345–362. doi: 10.1113/jphysiol.1984.sp015380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A. H., Harris-Warrick R. M. Strychnine eliminates alternating motor output during fictive locomotion in the lamprey. Brain Res. 1984 Feb 13;293(1):164–167. doi: 10.1016/0006-8993(84)91464-1. [DOI] [PubMed] [Google Scholar]
- Dale N., Roberts A. Dual-component amino-acid-mediated synaptic potentials: excitatory drive for swimming in Xenopus embryos. J Physiol. 1985 Jun;363:35–59. doi: 10.1113/jphysiol.1985.sp015694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N., Roberts A. Excitatory amino acid receptors in Xenopus embryo spinal cord and their role in the activation of swimming. J Physiol. 1984 Mar;348:527–543. doi: 10.1113/jphysiol.1984.sp015123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman A. G., Orlovsky G. N. Activity of interneurons mediating reciprocal 1a inhibition during locomotion. Brain Res. 1975 Feb 7;84(2):181–194. doi: 10.1016/0006-8993(75)90974-9. [DOI] [PubMed] [Google Scholar]
- Friesen W. O., Stent G. S. Generation of a locomotory rhythm by a neural network with reccurrent cyclic inhibition. Biol Cybern. 1977 Dec 16;28(1):27–40. doi: 10.1007/BF00360911. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Roberts W. J. Synaptic actions of single interneurones mediating reciprocal Ia inhibition of motoneurones. J Physiol. 1972 May;222(3):623–642. doi: 10.1113/jphysiol.1972.sp009818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn J. A., Roberts A. Experiments on the central pattern generator for swimming in amphibian embryos. Philos Trans R Soc Lond B Biol Sci. 1982 Jan 27;296(1081):229–243. doi: 10.1098/rstb.1982.0004. [DOI] [PubMed] [Google Scholar]
- Kling U., Székely G. Simulation of rhythmic nervous activities. I. Function of networks with cyclic inhibitions. Kybernetik. 1968 Sep;5(3):89–103. doi: 10.1007/BF00288899. [DOI] [PubMed] [Google Scholar]
- Perkel D. H., Mulloney B. Motor pattern production in reciprocally inhibitory neurons exhibiting postinhibitory rebound. Science. 1974 Jul 12;185(4146):181–183. doi: 10.1126/science.185.4146.181. [DOI] [PubMed] [Google Scholar]
- Roberts A., Clarke J. D. The neuroanatomy of an amphibian embryo spinal cord. Philos Trans R Soc Lond B Biol Sci. 1982 Jan 27;296(1081):195–212. doi: 10.1098/rstb.1982.0002. [DOI] [PubMed] [Google Scholar]
- SZEKELY G. LOGICAL NETWORK FOR CONTROLLING LIMB MOVEMENTS IN URODELA. Acta Physiol Acad Sci Hung. 1965;27:285–289. [PubMed] [Google Scholar]
- Selverston A. I., Miller J. P., Wadepuhl M. Cooperative mechanisms for the production of rhythmic movements. Symp Soc Exp Biol. 1983;37:55–87. [PubMed] [Google Scholar]
- Soffe S. R., Clarke J. D., Roberts A. Activity of commissural interneurons in spinal cord of Xenopus embryos. J Neurophysiol. 1984 Jun;51(6):1257–1267. doi: 10.1152/jn.1984.51.6.1257. [DOI] [PubMed] [Google Scholar]
- Soffe S. R., Roberts A. Activity of myotomal motoneurons during fictive swimming in frog embryos. J Neurophysiol. 1982 Dec;48(6):1274–1278. doi: 10.1152/jn.1982.48.6.1274. [DOI] [PubMed] [Google Scholar]
- Soffe S. R., Roberts A. Tonic and phasic synaptic input to spinal cord motoneurons during fictive locomotion in frog embryos. J Neurophysiol. 1982 Dec;48(6):1279–1288. doi: 10.1152/jn.1982.48.6.1279. [DOI] [PubMed] [Google Scholar]


