Abstract
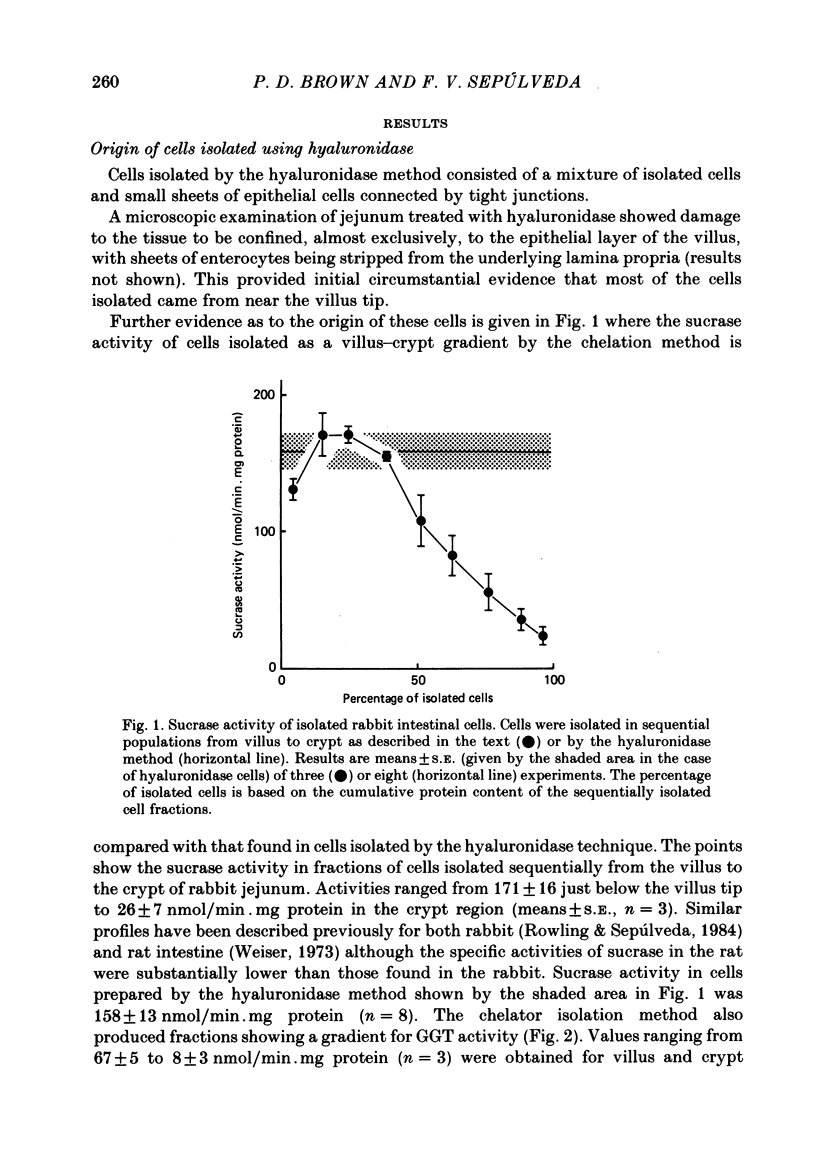
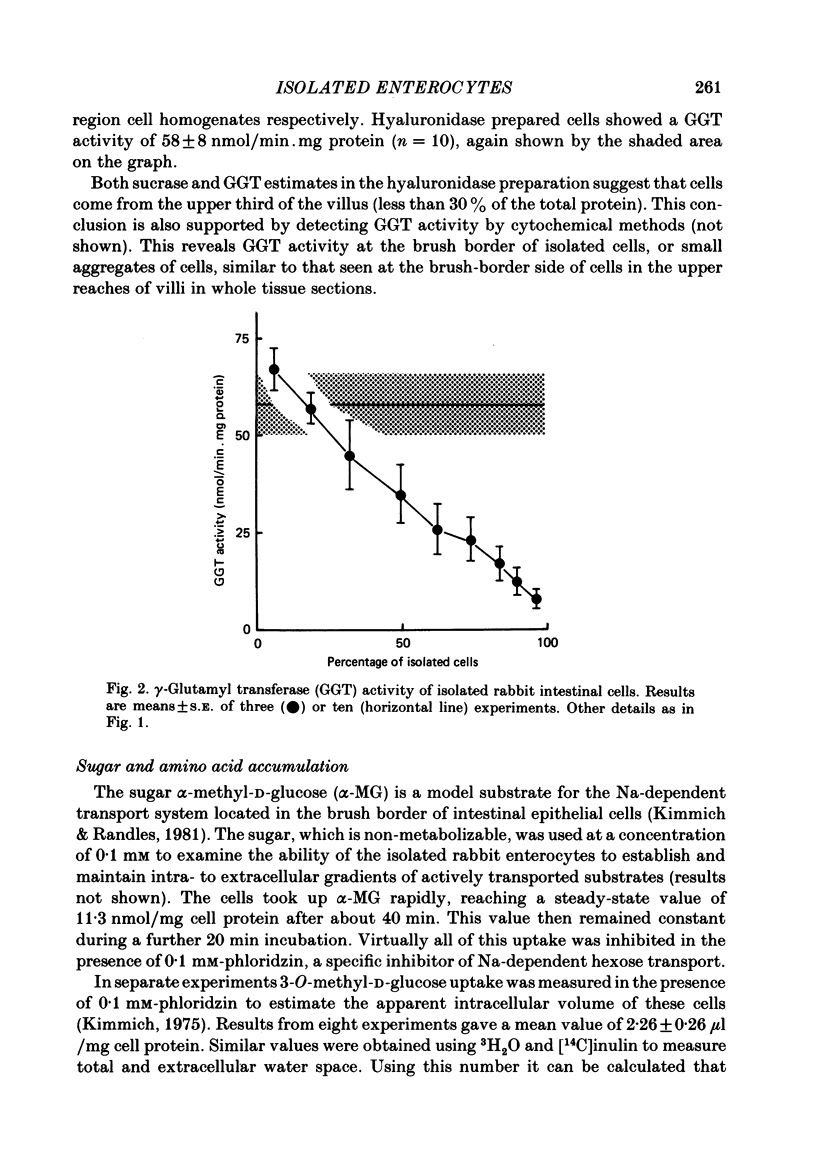
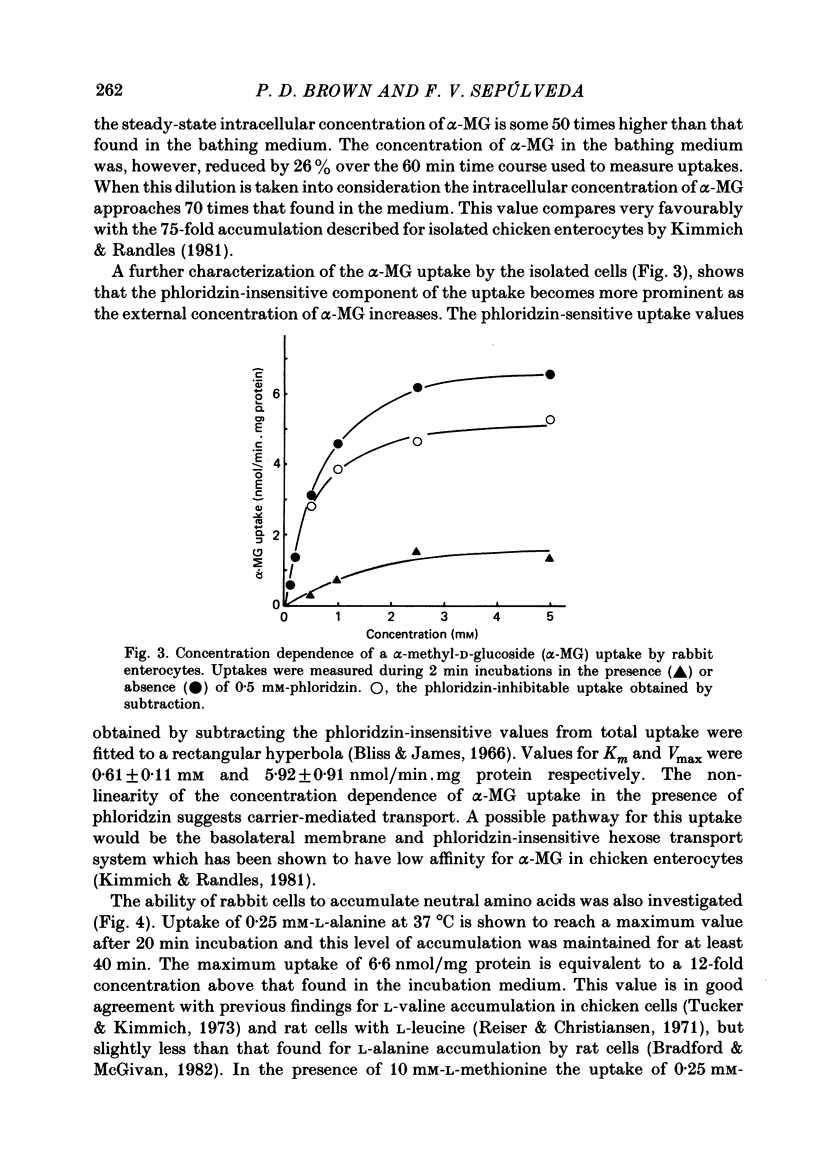
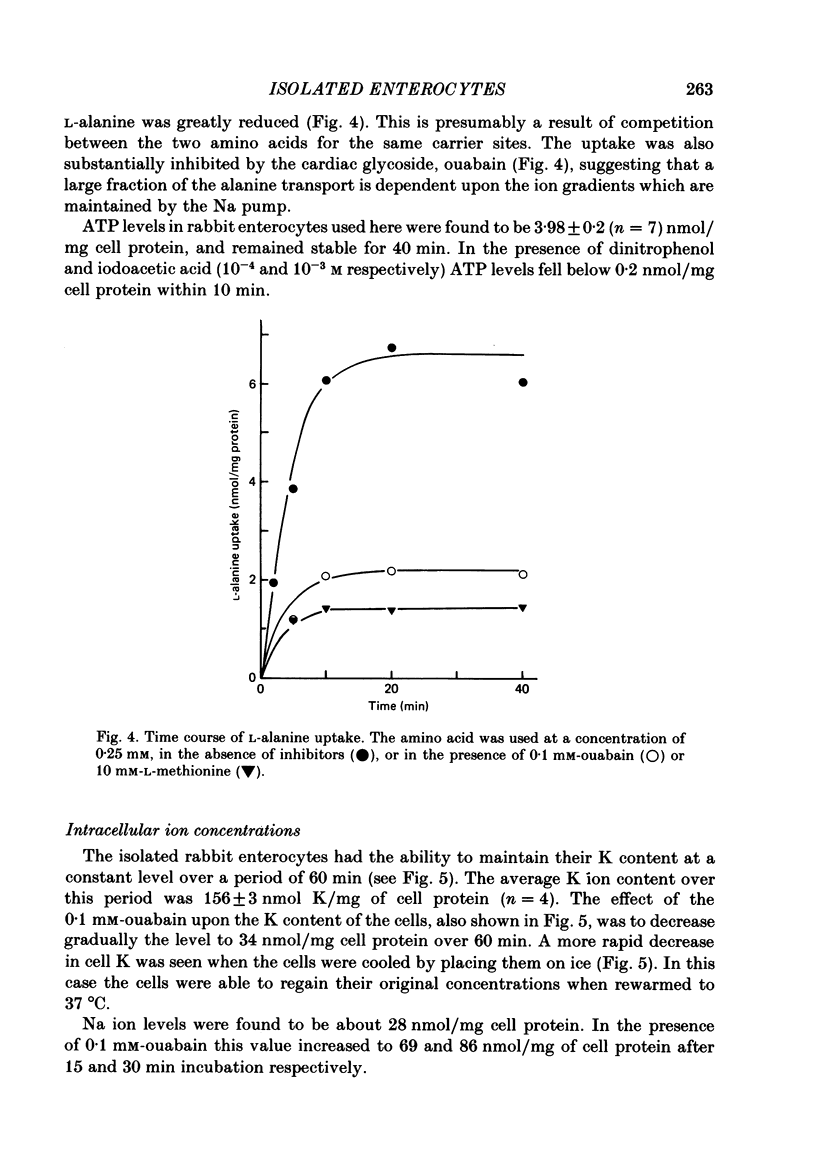
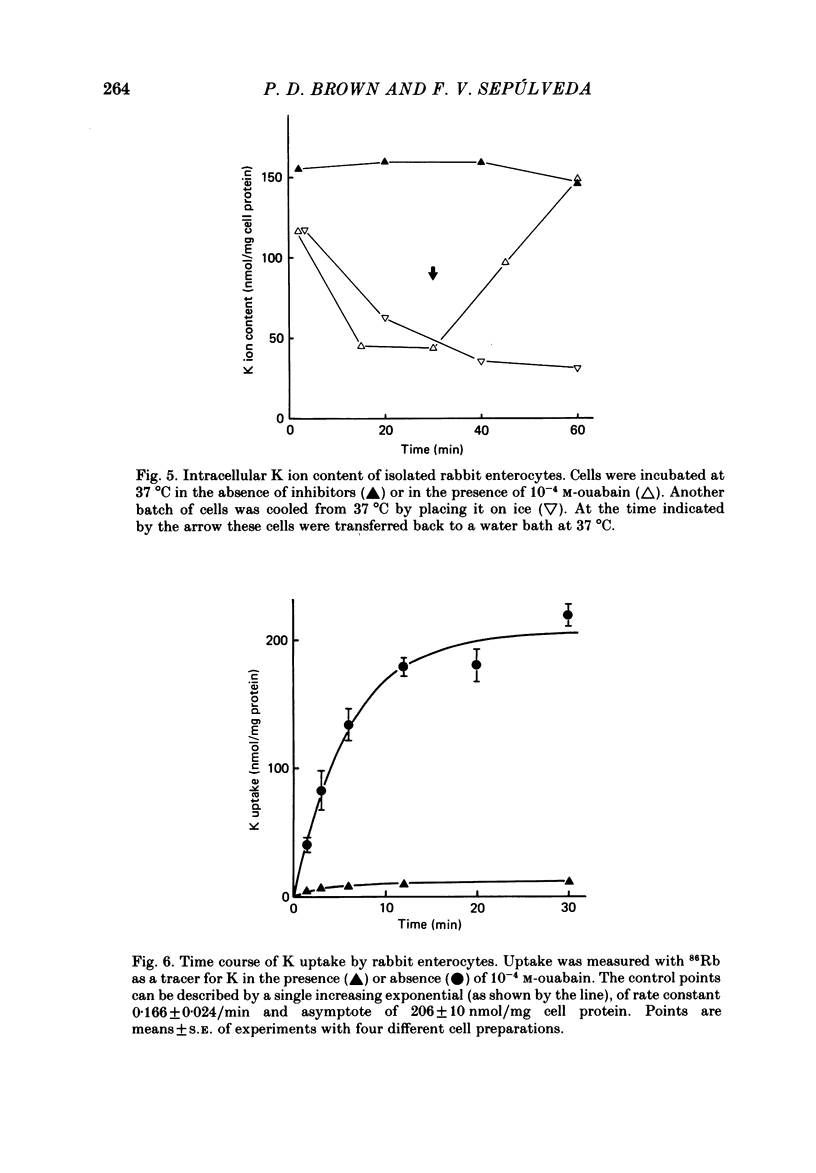
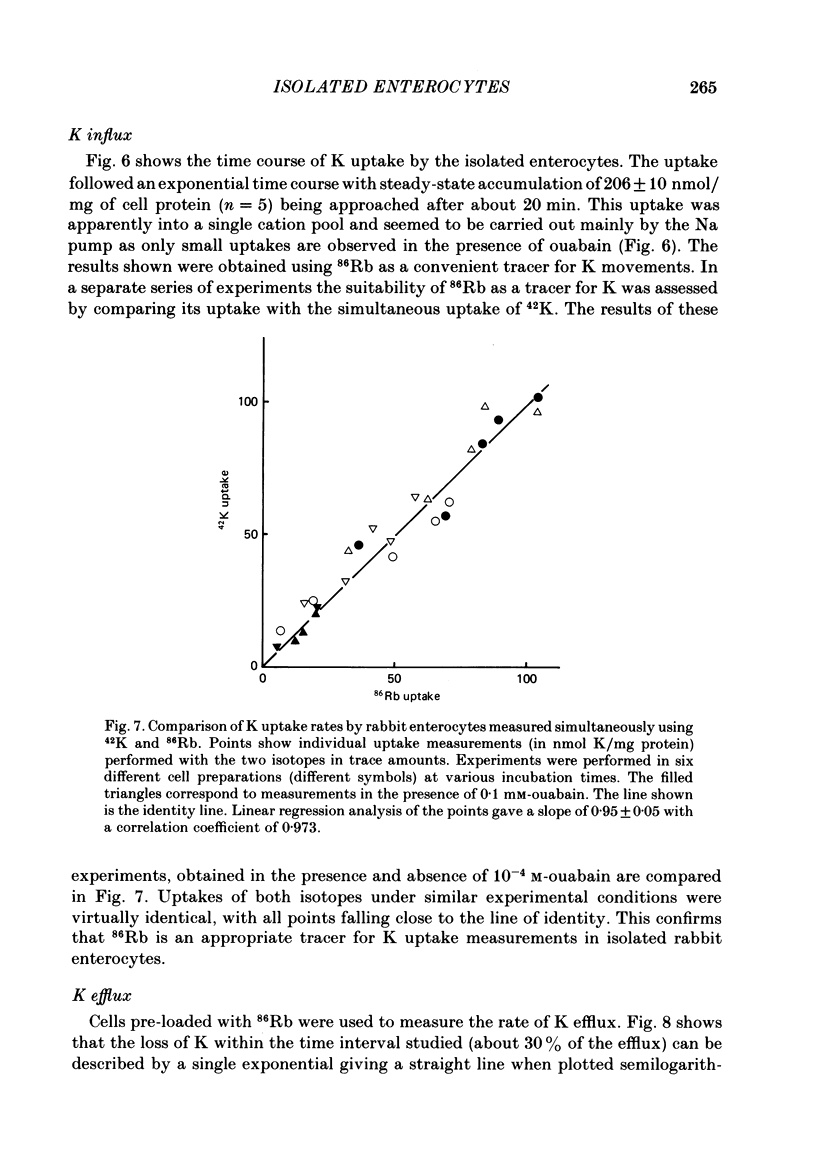
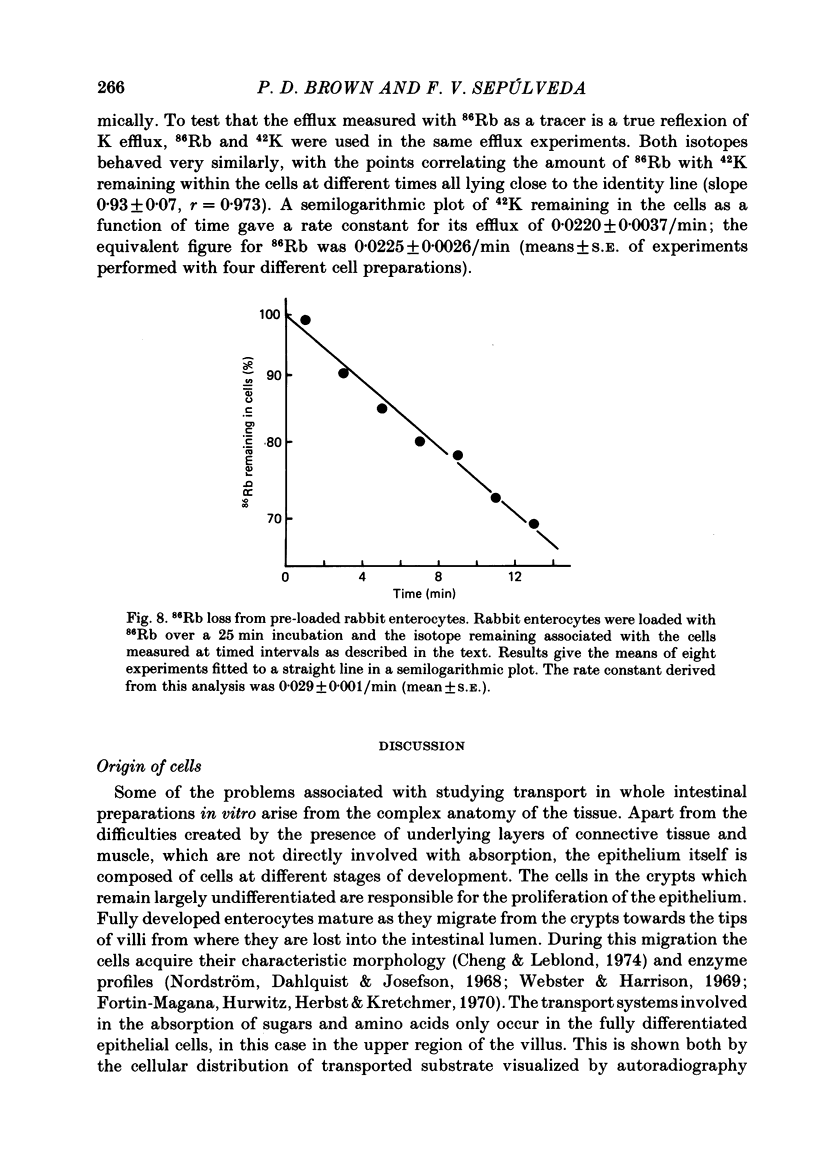
A method is described for isolating viable enterocytes from rabbit jejunum. Estimates of sucrase and gamma-glutamyl transferase activities in cells isolated by this method suggest that they originate from the upper villus only. Isolated cells accumulate both alpha-methyl-D-glucoside and alanine, maintaining high intracellular concentrations for at least 60 and 40 min respectively. Accumulation of alpha-methyl-D-glucoside is inhibited by the presence of phloridzin. The cells accumulate 42K and 86Rb in an identical manner. This uptake, which is maintained for at least 60 min, is inhibited in the presence of ouabain. Passive efflux of 42K and 86Rb occurs with rate constants which are virtually identical. The efflux follows a single exponential suggesting that it originates from only one intracellular compartment. It is suggested that the preparation can be used to study the effect of sugars and amino acids on K efflux. The advantages of using such a preparation are discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bliss C. I., James A. T. Fitting the rectangular hyperbola. Biometrics. 1966 Sep;22(3):573–602. [PubMed] [Google Scholar]
- Boyd C. A., Perring V. S. Amino acid inhibition and stimulation of 2-aminoisobutyric acid exit from anuran small intestine. J Physiol. 1982 Jun;327:53–64. doi: 10.1113/jphysiol.1982.sp014219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford N. M., McGivan J. D. The transport of alanine and glutamine into isolated rat intestinal epithelial cells. Biochim Biophys Acta. 1982 Jul 14;689(1):55–62. doi: 10.1016/0005-2736(82)90188-2. [DOI] [PubMed] [Google Scholar]
- Brown P. D., Sepúlveda F. V. Potassium movements associated with amino acid and sugar transport in enterocytes isolated from rabbit jejunum. J Physiol. 1985 Jun;363:271–285. doi: 10.1113/jphysiol.1985.sp015709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman C. I. The mechanism of transfer for L-leucine into the vascular bed of the Anuran small intestine. J Physiol. 1981 Aug;317:91–102. doi: 10.1113/jphysiol.1981.sp013815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Leblond C. P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. I. Columnar cell. Am J Anat. 1974 Dec;141(4):461–479. doi: 10.1002/aja.1001410403. [DOI] [PubMed] [Google Scholar]
- Cremaschi D., James P. S., Meyer G., Rossetti C., Smith M. W. Developmental changes in intra-enterocyte cation activities in hamster terminal ileum. J Physiol. 1984 Sep;354:363–373. doi: 10.1113/jphysiol.1984.sp015381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. M., Wrigglesworth J. M., Burdett K., Pover W. F. Studies on epithelial cells isolated from guinea pig small intestine. J Cell Biol. 1971 Nov;51(21):452–464. doi: 10.1083/jcb.51.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin-Magana R., Hurwitz R., Herbst J. J., Kretchmer N. Intestinal enzymes: indicators of proliferation and differentiation in the jejunum. Science. 1970 Mar 20;167(3925):1627–1628. doi: 10.1126/science.167.3925.1627. [DOI] [PubMed] [Google Scholar]
- Gonzalez C., Garcia-Sancho J. A sensitive radioenzymatic assay for ATP. Anal Biochem. 1981 Jul 1;114(2):285–287. doi: 10.1016/0003-2697(81)90482-6. [DOI] [PubMed] [Google Scholar]
- Grasset E., Gunter-Smith P., Schultz S. G. Effects of Na-coupled alanine transport on intracellular K activities and the K conductance of the basolateral membranes of Necturus small intestine. J Membr Biol. 1983;71(1-2):89–94. doi: 10.1007/BF01870677. [DOI] [PubMed] [Google Scholar]
- Inui K., Quaroni A., Tillotson L. G., Isselbacher K. J. Amino acid and hexose transport by cultured crypt cells from rat small intestine. Am J Physiol. 1980 Nov;239(5):C190–C196. doi: 10.1152/ajpcell.1980.239.5.C190. [DOI] [PubMed] [Google Scholar]
- Kimmich G. A. Preparation and properties of mucosl epithelial cells isolated frmsmall intestine of the chicken. Biochemistry. 1970 Sep 15;9(19):3659–3668. doi: 10.1021/bi00821a003. [DOI] [PubMed] [Google Scholar]
- Kimmich G. A., Randles J. alpha-Methylglucoside satisfies only Na+-dependent transport system of intestinal epithelium. Am J Physiol. 1981 Nov;241(5):C227–C232. doi: 10.1152/ajpcell.1981.241.5.C227. [DOI] [PubMed] [Google Scholar]
- King I. S., Sepúlveda F. V., Smith M. W. Cellular distribution of neutral and basic amino acid transport systems in rabbit ileal mucosa. J Physiol. 1981;319:355–368. doi: 10.1113/jphysiol.1981.sp013913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen L. O. Energization of alanine transport in isolated rat hepatocytes. Electrogenic Na+-alanine co-transport leading to increased K+ permeability. J Biol Chem. 1980 Jun 10;255(11):5236–5243. [PubMed] [Google Scholar]
- Lee C. O., Armstrong W. M. Activities of sodium and potassium ions in epithelial cells of small intestine. Science. 1972 Mar 17;175(4027):1261–1264. doi: 10.1126/science.175.4027.1261. [DOI] [PubMed] [Google Scholar]
- Mahmood A., Alvarado F. The activation of intestinal brush border sucrase by alkali metal ions: an allosteric mechanism similar to that for the Na+-activation of nonelectrolyte transport systems in intestine. Arch Biochem Biophys. 1975 Jun;168(2):585–593. doi: 10.1016/0003-9861(75)90290-8. [DOI] [PubMed] [Google Scholar]
- Mircheff A. K., van Os C. H., Wright E. M. Pathways for alanine transport in intestinal basal lateral membrane vesicles. J Membr Biol. 1980 Jan 31;52(1):83–92. doi: 10.1007/BF01869009. [DOI] [PubMed] [Google Scholar]
- Nordström C., Dahlqvist A., Josefsson L. Quantitative determination of enzymes in different parts of the villi and crypts of rat small intestine. Comparison of alkaline phosphatase, disaccharidases and dipepeptidases. J Histochem Cytochem. 1967 Dec;15(12):713–721. doi: 10.1177/15.12.713. [DOI] [PubMed] [Google Scholar]
- Paterson J. Y., Sepúlveda F. V., Smith M. W. Amino acid efflux from rabbit ileal enterocytes. J Physiol. 1982 Oct;331:537–546. doi: 10.1113/jphysiol.1982.sp014389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson J. Y., Sepúlveda F. V., Smith M. W. Distribution of transported amino acid within rabbit ileal mucosa. J Physiol. 1982 Oct;331:523–535. doi: 10.1113/jphysiol.1982.sp014388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowling P. J., Sepúlveda F. V. The distribution of (Na+ + K+)-ATPase along the villus crypt-axis in the rabbit small intestine. Biochim Biophys Acta. 1984 Mar 28;771(1):35–41. doi: 10.1016/0005-2736(84)90107-x. [DOI] [PubMed] [Google Scholar]
- Rutenburg A. M., Kim H., Fischbein J. W., Hanker J. S., Wasserkrug H. L., Seligman A. M. Histochemical and ultrastructural demonstration of gamma-glutamyl transpeptidase activity. J Histochem Cytochem. 1969 Aug;17(8):517–526. doi: 10.1177/17.8.517. [DOI] [PubMed] [Google Scholar]
- Sepúlveda F. V., Burton K. A., Brown P. D. Relation between sodium-coupled amino acid and sugar transport and sodium/potassium pump activity in isolated intestinal epithelial cells. J Cell Physiol. 1982 Jun;111(3):303–308. doi: 10.1002/jcp.1041110312. [DOI] [PubMed] [Google Scholar]
- Sepúlveda F. V., Burton K. A. gamma-Glutamyl transferase activity in the pig proximal colon during early postnatal development. FEBS Lett. 1982 Mar 22;139(2):171–173. doi: 10.1016/0014-5793(82)80843-0. [DOI] [PubMed] [Google Scholar]
- Sepúlveda F. V., Smith M. W. Discrimination between different entry mechanisms for neutral amino acids in rabbit ileal mucosa. J Physiol. 1978 Sep;282:73–90. doi: 10.1113/jphysiol.1978.sp012449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towler C. M., Pugh-Humphreys G. P., Porteous J. W. Characterization of columnar absorptive epithelial cells isolated from rat jejunum. J Cell Sci. 1978 Feb;29:53–75. doi: 10.1242/jcs.29.1.53. [DOI] [PubMed] [Google Scholar]
- Tucker A. M., Kimmich G. A. Characteristics of amino acid accumulation by isolated intestinal epithelial cells. J Membr Biol. 1973;12(1):1–22. doi: 10.1007/BF01869989. [DOI] [PubMed] [Google Scholar]
- Valdeolmillos M., García-Sancho J., Herreros B. Ca2+-dependent K+ transport in the Ehrlich ascites tumor cell. Biochim Biophys Acta. 1982 Mar 8;685(3):273–278. doi: 10.1016/0005-2736(82)90067-0. [DOI] [PubMed] [Google Scholar]
- Webster H. L., Harrison D. D. Enzymic activities during the transformation of crypt to columnar intestinal cells. Exp Cell Res. 1969 Aug;56(2):245–253. doi: 10.1016/0014-4827(69)90009-3. [DOI] [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem. 1973 Apr 10;248(7):2536–2541. [PubMed] [Google Scholar]
- Ziomek C. A., Schulman S., Edidin M. Redistribution of membrane proteins in isolated mouse intestinal epithelial cells. J Cell Biol. 1980 Sep;86(3):849–857. doi: 10.1083/jcb.86.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylber E. A., Rotummo C. A., Cereijido M. Ionic fluxes in isolated epithelial cells of the abdominal skin of the frog Leptodactylus ocellatus. J Membr Biol. 1975 Jul 24;22(3-4):265–284. doi: 10.1007/BF01868175. [DOI] [PubMed] [Google Scholar]


