Abstract
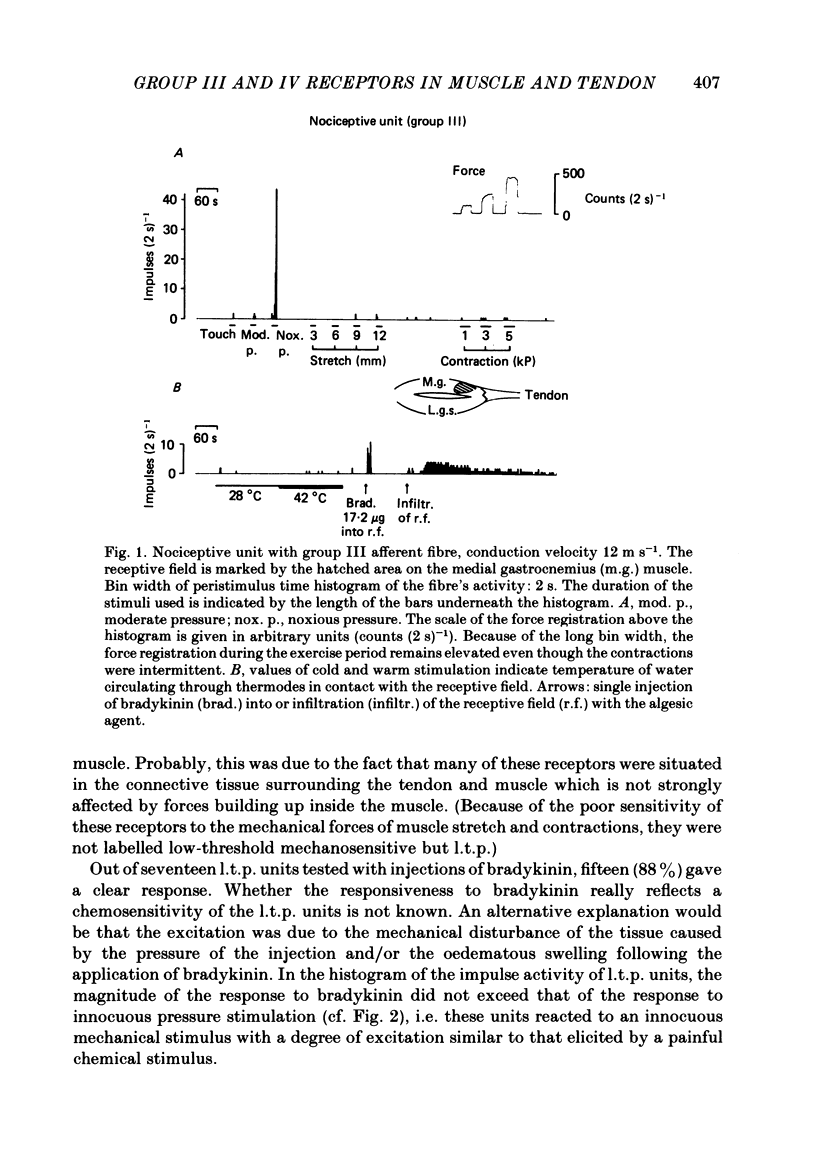
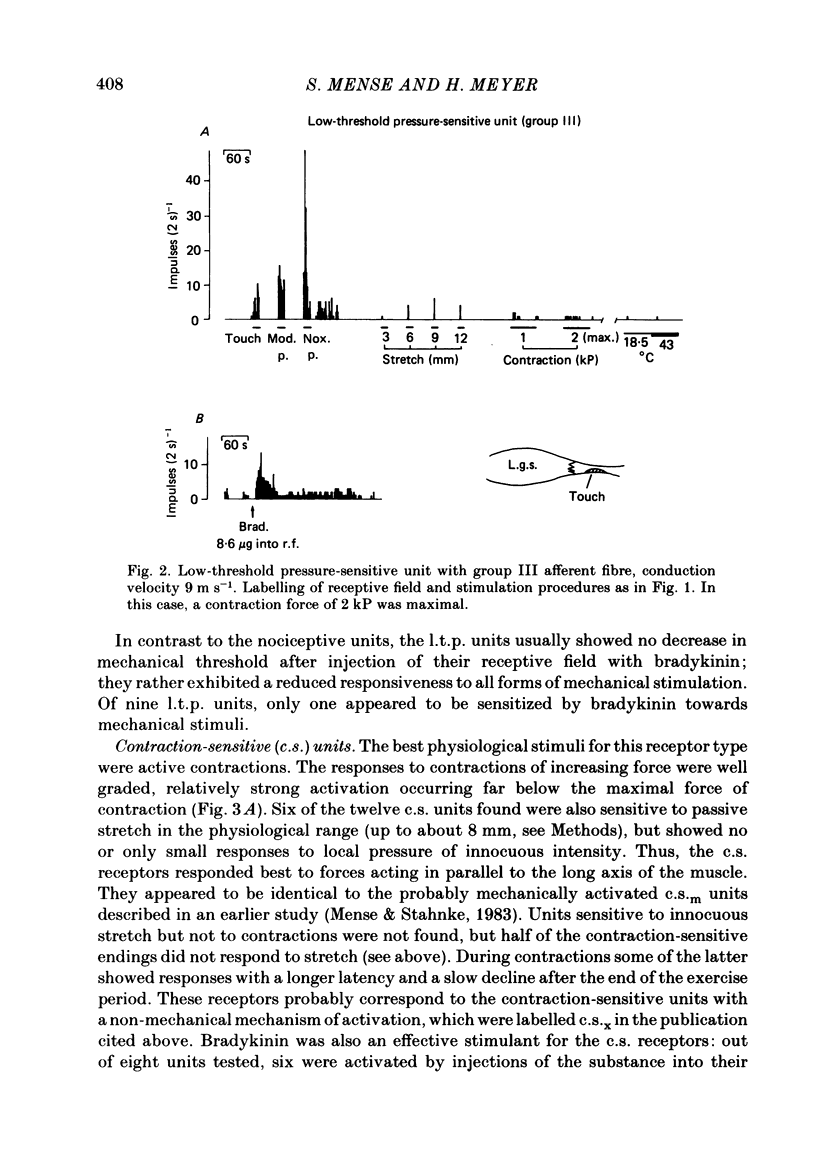
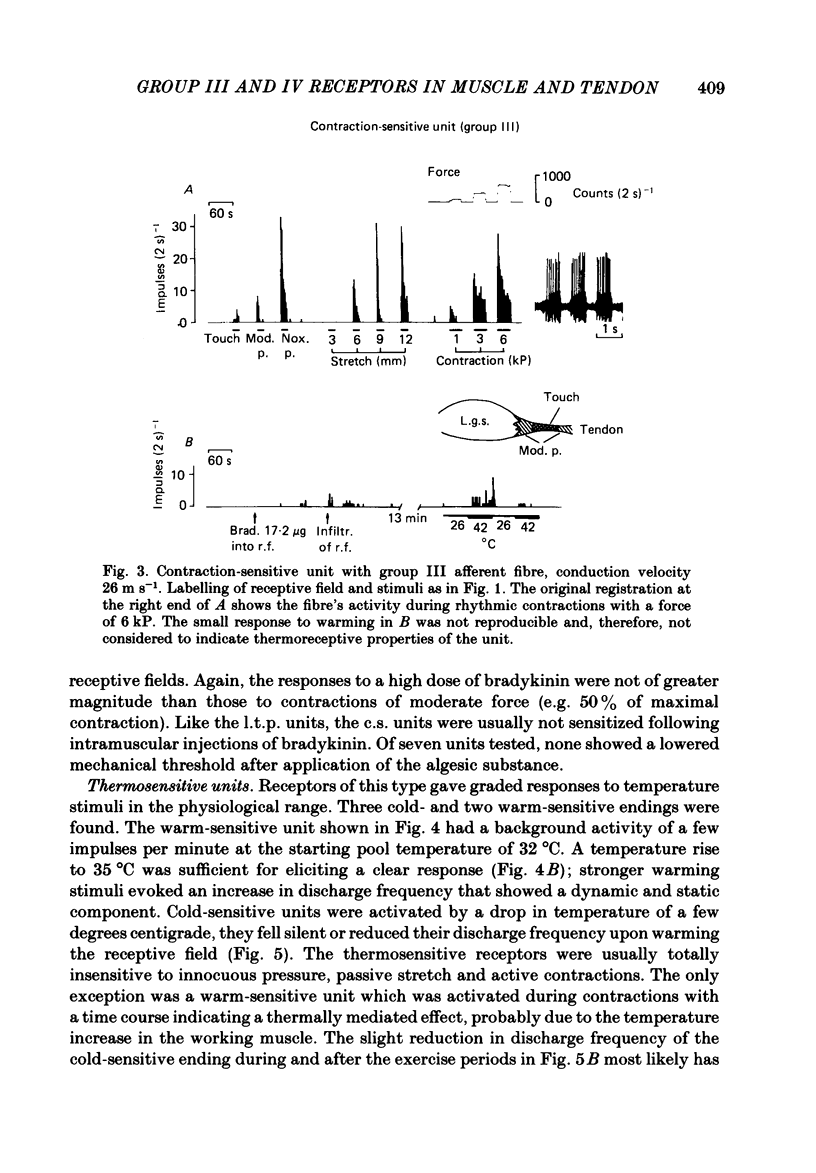
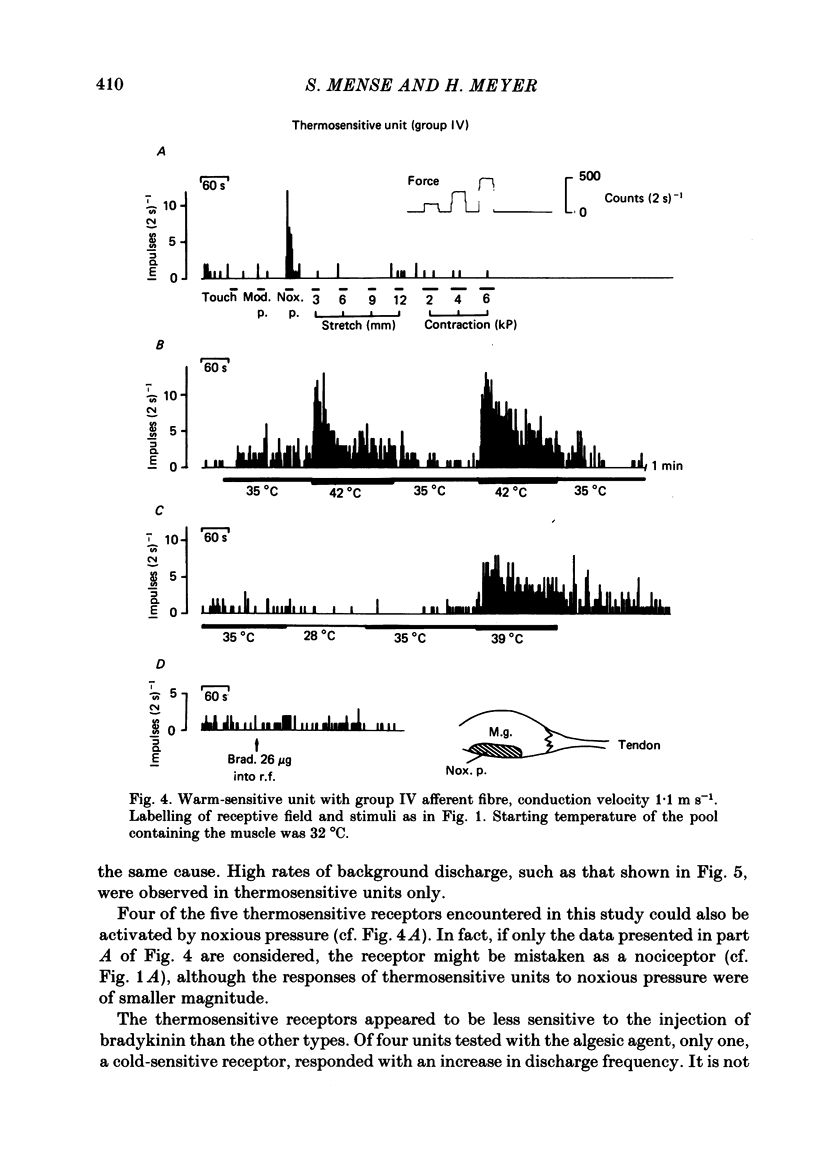
In chloralose-anaesthetized cats, the impulse activity of single afferent units conducting at less than 30 m s-1 and having receptive fields in the triceps surae muscle or the calcaneal tendon, was recorded from thin filaments of the dorsal roots L7 and S1. The receptive fields of the units were tested with a variety of graded natural stimuli (local pressure, stretch, contractions, temperature changes). In addition, the algesic agent bradykinin was injected into the receptive fields, but the sensitivity of the receptors to this substance was not used for classification purposes. Four types of receptors could be distinguished using the strongest response to innocuous natural stimulation as the criterion for characterizing a given ending: (a) nociceptors showing no response to innocuous forms of stimulation and requiring noxious (tissue-threatening) stimuli to be clearly activated; (b) low-threshold pressure-sensitive receptors responding to innocuous indentation of the tissue but being relatively insensitive to stretch and contractions; (c) contraction-sensitive receptors reaching high discharge frequencies during active contractions of moderate force and innocuous stretch, but being relatively insensitive to local pressure stimulation; (d) thermosensitive receptors responding strongly to small changes in temperature without reacting to innocuous mechanical stimulation. The possible involvement of the different receptor types in central nervous functions (nociception, mechanoreception, ergoreception, thermoregulation) is discussed.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahams V. C., Lynn B., Richmond F. J. Organization and sensory properties of small myelinated fibres in the dorsal cervical rami of the cat. J Physiol. 1984 Feb;347:177–187. doi: 10.1113/jphysiol.1984.sp015060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck P. W., Handwerker H. O. Bradykinin and serotonin effects on various types of cutaneous nerve fibers. Pflugers Arch. 1974 Mar 11;347(3):209–222. doi: 10.1007/BF00592598. [DOI] [PubMed] [Google Scholar]
- Burgess P. R., Perl E. R. Myelinated afferent fibres responding specifically to noxious stimulation of the skin. J Physiol. 1967 Jun;190(3):541–562. doi: 10.1113/jphysiol.1967.sp008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H., Murphy P. R., Tripathi A. Closely coupled excitation of gamma-motoneurones by group III Muscle afferents with low mechanical threshold in the cat. J Physiol. 1982 Oct;331:481–498. doi: 10.1113/jphysiol.1982.sp014385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FJALLBRANT N., IGGO A. The effect of histamine, 5-hydroxytryptamine and acetylcholine on cutaneous afferent fibres. J Physiol. 1961 May;156:578–590. doi: 10.1113/jphysiol.1961.sp006694. [DOI] [PMC free article] [PubMed] [Google Scholar]
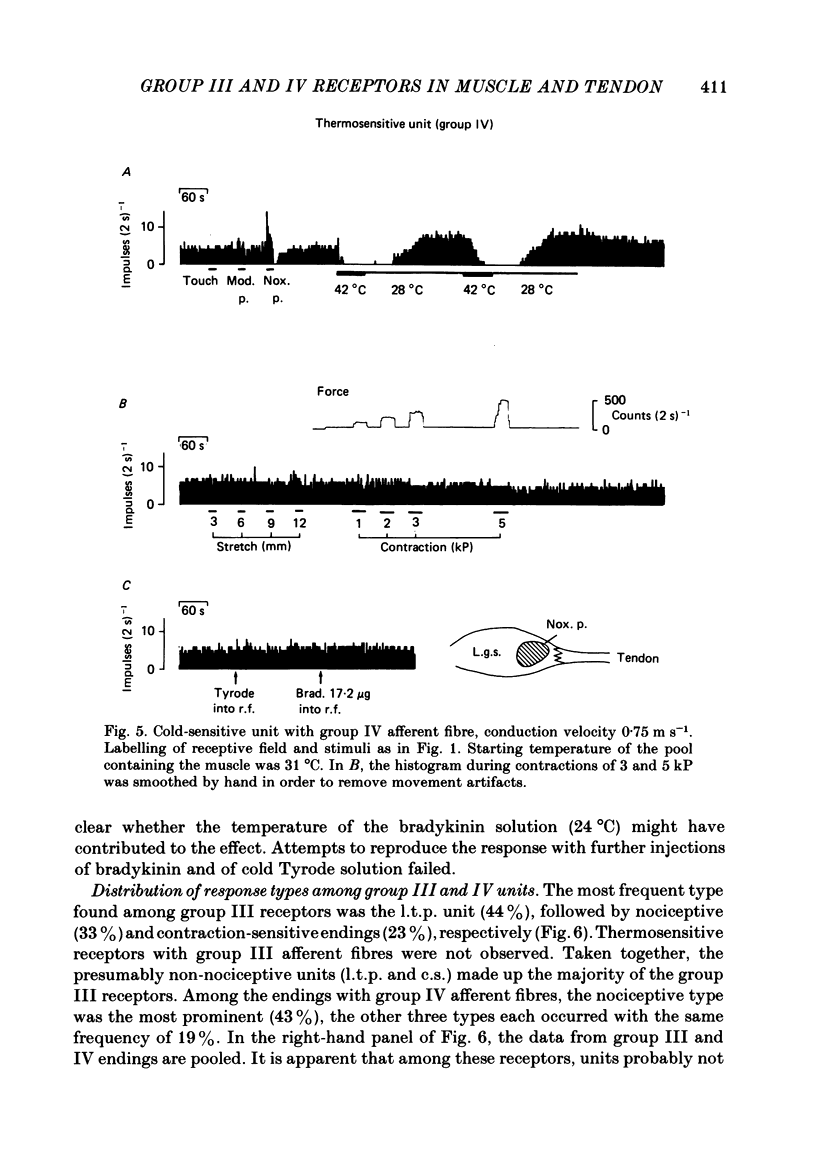
- Hertel H. C., Howaldt B., Mense S. Responses of group IV and group III muscle afferents to thermal stimuli. Brain Res. 1976 Aug 20;113(1):201–205. doi: 10.1016/0006-8993(76)90020-2. [DOI] [PubMed] [Google Scholar]
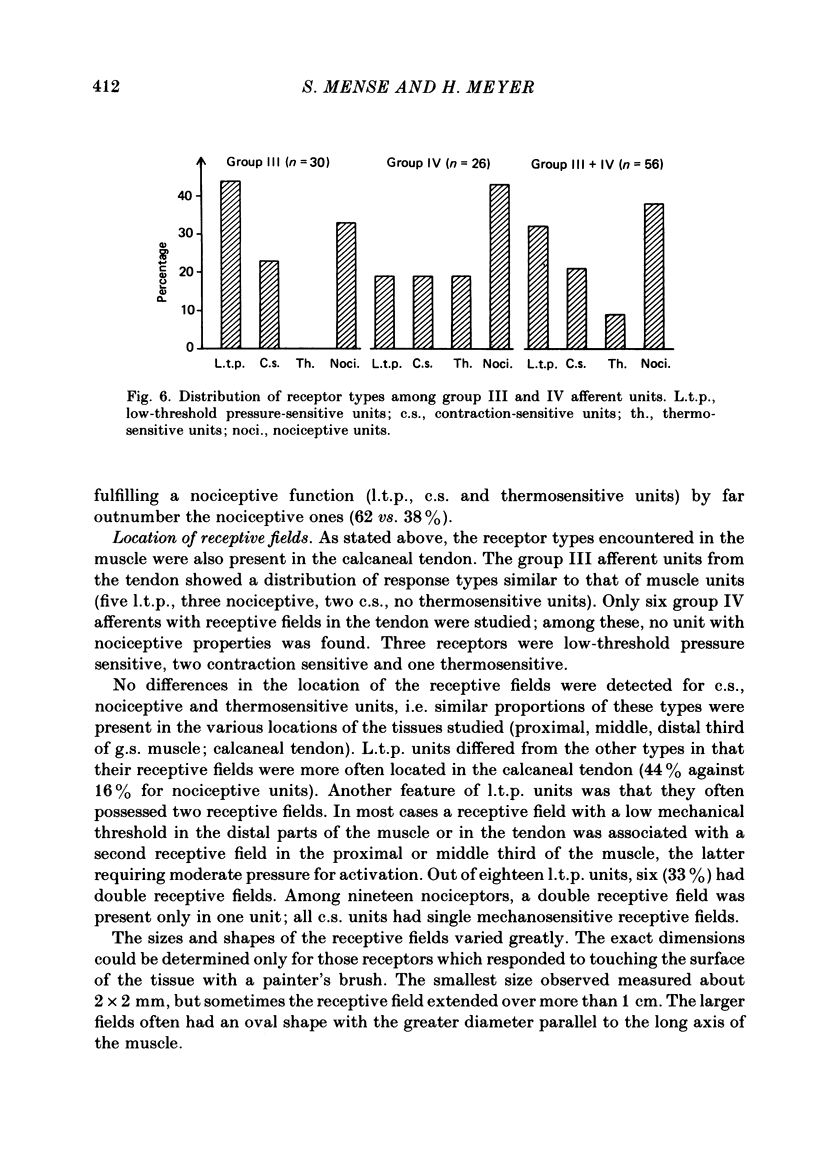
- Houk J., Henneman E. Responses of Golgi tendon organs to active contractions of the soleus muscle of the cat. J Neurophysiol. 1967 May;30(3):466–481. doi: 10.1152/jn.1967.30.3.466. [DOI] [PubMed] [Google Scholar]
- Iggo A. Cutaneous thermoreceptors in primates and sub-primates. J Physiol. 1969 Feb;200(2):403–430. doi: 10.1113/jphysiol.1969.sp008701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen C., Feistkorn G., Nagel A. Temperature sensitivity of skeletal muscle in the conscious goat. J Appl Physiol Respir Environ Exerc Physiol. 1983 Apr;54(4):880–886. doi: 10.1152/jappl.1983.54.4.880. [DOI] [PubMed] [Google Scholar]
- Kniffki K. D., Mense S., Schmidt R. F. Responses of group IV afferent units from skeletal muscle to stretch, contraction and chemical stimulation. Exp Brain Res. 1978 Apr 14;31(4):511–522. doi: 10.1007/BF00239809. [DOI] [PubMed] [Google Scholar]
- Kumazawa T., Mizumura K. The polymodal C-fiber receptor in the muscle of the dog. Brain Res. 1976 Jan 23;101(3):589–593. doi: 10.1016/0006-8993(76)90483-2. [DOI] [PubMed] [Google Scholar]
- Kumazawa T., Mizumura K. Thin-fibre receptors responding to mechanical, chemical, and thermal stimulation in the skeletal muscle of the dog. J Physiol. 1977 Dec;273(1):179–194. doi: 10.1113/jphysiol.1977.sp012088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey D. I., Mitchell J. H. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol. 1972 Jul;224(1):173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense S. Nervous outflow from skeletal muscle following chemical noxious stimulation. J Physiol. 1977 May;267(1):75–88. doi: 10.1113/jphysiol.1977.sp011802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense S., Stahnke M. Responses in muscle afferent fibres of slow conduction velocity to contractions and ischaemia in the cat. J Physiol. 1983 Sep;342:383–397. doi: 10.1113/jphysiol.1983.sp014857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAINTAL A. S. Functional analysis of group III afferent fibres of mammalian muscles. J Physiol. 1960 Jul;152:250–270. doi: 10.1113/jphysiol.1960.sp006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl E. R., Kumazawa T., Lynn B., Kenins P. Sensitization of high threshold receptors with unmyelinated (C) afferent fibers. Prog Brain Res. 1976;43:263–277. doi: 10.1016/S0079-6123(08)64359-9. [DOI] [PubMed] [Google Scholar]
- Sherrington C. S. Flexion-reflex of the limb, crossed extension-reflex, and reflex stepping and standing. J Physiol. 1910 Apr 26;40(1-2):28–121. doi: 10.1113/jphysiol.1910.sp001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey M. J. Free nerve endings in skeletal muscle of the cat. J Anat. 1969 Sep;105(Pt 2):231–254. [PMC free article] [PubMed] [Google Scholar]


